Abstract
Zanthoxylum schinifolium Sieb. et Zucc. (Rutaceae), a dioecious shrub with hooked prickly branches, has been used as folk medicine for the treatment of the common cold, stomach ache, diarrhea, and jaundice in China, Korea, and Japan. In our phytochemical investigations on this genus, a new megastigmane sesquiterpenoid, which is referred to as schinifolenol A (1), was isolated from Z. schinifolium. The stereochemistry was characterized via the analyses of extensive spectra. The absolute configuration was established by the application of a modified Mosher’s experiment and assisted by a time-dependent density functional theory (TD-DFT) on calculated electronic circular dichroism (ECD). Bioactivity screenings showed that compound 1 exhibited a safe hypotoxicity and a better selectivity on anti-Kaposi’s sarcoma associated herpes virus (KSHV).
1. Introduction
Zanthoxylum schinifolium Sieb. et Zucc. is a dioecious shrub with hooked prickly branches from the genus Zanthoxylum (family Rutaceae), which was termed Qinghuajiao, Yajiao, Tianjiao, and Xiaohuajiao, etc., and prosperously distributed from the south of the Yangtze River to the southwest provinces in China [1]. Clinical application in folk medicines of Z. schinifolium mainly included cures for the common cold, stomach ache, diarrhea, and jaundice in China, Korea, and Japan [2]. Recently, phytochemical studies on Zanthoxylum have resulted in the isolation of diverse chemical constituents such as alkaloids, amides, lignans, coumarins, essential oils and aliphatic acids [1,3,4,5]. In our continuous research for structurally unique and biologically active metabolites from traditional Chinese medicine [6,7,8], a megastigmane sesquiterpenoid termed schinifolenol A (1) (Figure 1) was obtained from the dried rhizomes of Z. schinifolium. Herein, we elucidated the isolation procedures and the stereochemistry structure establishment of compound 1, as well as its inhibitory effect on Kaposi’s sarcoma associated herpes virus (KSHV) infection.
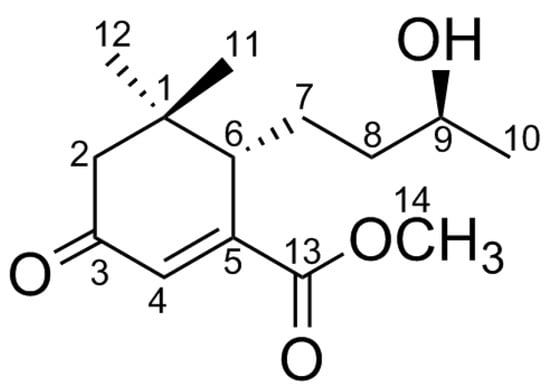
Figure 1.
Structure of compound 1.
2. Results
The dried rhizomes of Z. schinifolium (30 kg) were exhaustively extracted with 95% EtOH to furnish a syrup (1.5 kg), which was successively partitioned by petroleum ether, CH2Cl2, and EtOAc against water. The petroleum ether fraction (300 g) was repeatedly subjected to silica gel column chromatography (silica gel CC), Sephadex LH-20, and semi-preparative High Performance Liquid Chromatography (HPLC) to afford a new megastigmane sesquiterpenoid, viz., compound 1, which is named schinifolenol A (Figure 1).
Schinifolenol A (1), a violet oil, has the elemental composition of C14H22O4, which corresponded to the (+)-HRESIMS peak (m/z 255.1596 [M + H]+, calcd as 255.1592). IR (KBr) spectrum showed the characteristic absorption bands for hydroxyl (3695 cm−1) and carbonyl (1724 and 1667 cm−1) functionalities along with UV (CH3OH) spectrum at λmax 244 nm, which are closely similar with those data of megastigmane sesquiterpenoids such as blumenol C in reported literatures [9,10,11]. According to the NMR data of the literature [11], the difference between compound 1 and blumenol C is that a methoxycarbonyl group at C-5 of 1 was replaced by a methyl function in blumenol C. Spectral analyses of 1D NMR (Table 1) and HSQC correlations indicated the presence of two carbonyls (δC 202.3 and 169.3), one oxygenated methyl (δH 3.84, s and δC 53.4), three methyls (δH 1.01, s; 1.12, d, J = 6.2 Hz; and 1.15, s), three methylenes, three methines (including an olefinic methine (δH 6.55, s and δC 131.4) and a hydroxylated carbon (δH 3.65, m and δC 68.8)), and two quaternary carbons (including one olefinic carbon (δC 155.2)). Referring to the literature data [9,10,11], the aforementioned analyses illustrate that compound 1 belongs to a class chemicals of megastigmane sesquiterpenoid.

Table 1.
1H-NMR (400 MHz) and 13C-NMR (100 MHz) Spectral Data of Compound 1 in Methanol-d4 (δ in ppm, J in Hz).
Combining with the signals of HSQC, iterative analyses of HMBC and 1H-1H COSY experiments could elucidate the planar constitution of 1. HMBC correlations from Me-12 and Me-13 to C-1, C-2, and C-6, from H-2 to C-3 and C-4, and from H-4 to C-5 and C-6 indicated the presence of a 5,5-dimethyl-cyclohex-3-one entity. Meanwhile, the HMBC cross peak of H-4 to the ester carbonyl C-13 implied that the ester carbonyl function was located at C-5. Moreover, HMBC correlations from Me-10 to C-8 and C-9, and from H-6 to C-7 and C-8, as well as the 1H-1H COSY spin systems of H-6/H-7/H-8/H-9/H-10 revealed that a 3-hydroxybutyl functionality was connected to the 3,3-dimethyl-cyclohexanone at C-6 (Figure 2). No diagnostic NOESY could be applied to determine the relative configurations of C-6 and C-9, since both carbons were located on a rotational aliphatic chain. Thus, the aforementioned spectral analyses allowed us to assign compound 1 as a member of megastigmane sesquiterpenoid.
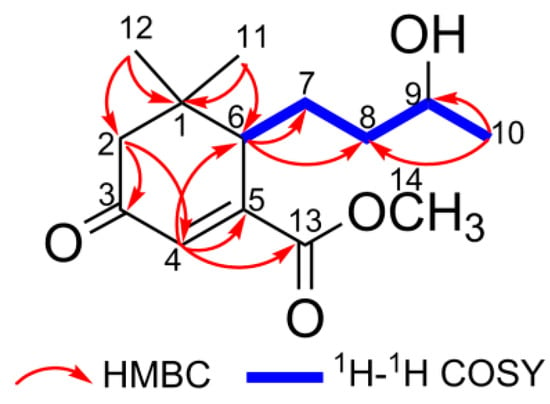
Figure 2.
Key HMBC and 1H-1H COSY correlations of compound 1.
In order to determine the absolute configuration of compound 1, a modified Mosher’s method was carried out to establish the stereochemistry characteristic of secondary alcohol carbon C-9. (S)- and (R)-MTPA esters of 1 were prepared as previously reported [12,13], then analyzed via 1H-NMR chemical shifts study. The distinguishable values (∆δ = δS-MTPA-ester − δR-MTPA-ester) were calculated for proton chemical shifts adjacent to C-9, as shown in Figure 3. Correspondingly, the absolute configuration at C-9 was confirmed to be S. Furthermore, a time-dependent density functional theory (TD-DFT) method on ECD calculation was performed to determine the absolute configuration of the other chiral carbon, viz., C-6. Based on the ascertained absolute configuration of C-9, the calculated ECD curve of (6S,9S)-1 showed a good consistency with the experimental ECD curve (Figure 4), which unequivocally established the absolute configuration of 1 as 6S,9S.
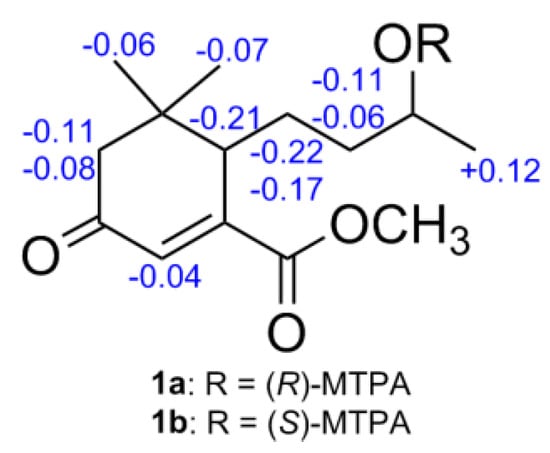
Figure 3.
∆δ values (in ppm) = δS-MTPA-ester − δR-MTPA-ester for 1a and 1b.
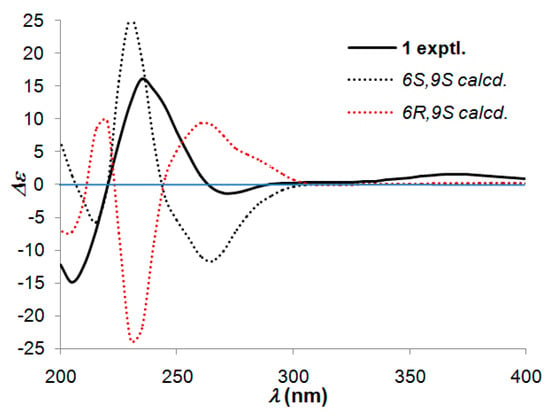
Figure 4.
Experimental and calculated ECD spectra of 1.
Since previous literatures reported that dictamnine (one type of alkaloids was isolated from Z. schinifolium) exhibited activity towards anti-Epstein–Barr virus (EBV) [14] and sesquiterpenes/sesquiiterpene lactones exhibited moderately activities towards anti-HIV [15], these two assays were also assessed for compound 1 and neither of the results showed obvious activities (1 towards both assays with CC50 > 300 μM and EC50 > 300 μM). Besides the aforementioned assays of anti-EBV and anti-HIV, some other bioactive screenings for 1 were performed, which included inhibitory activities on β-site amyloid precursor protein cleaving enzyme 1 (BACE1) (1 towards this assay with IC50 > 40 μM), inhibitory activities on NO production (1 towards this assay with IC50 > 25 μM), and cytotoxic activities against five human cancer cell lines (HL-60, SMMC-7721, A-549, MCF-7, and SW480) (1 towards all these cytotoxicity assays with IC50 > 40 μM). Disappointingly, compound 1 did not show any activities in the above assays.
Kaposi’s sarcoma associated herpes virus (KSHV) belongs to the gamma 2-herpesvirus subfamily, which is the etiological agent of all types of Kaposi’s sarcoma, like primary effusion lymphoma, multicentric Castleman’s disease, and posttransplant [16]. Currently, typical antiviral drugs, like acyclovir or ganciclovir, do not show a satisfactory potency to extinguish gamma herpes viruses in human bodies [17]. In this case, it is a challenge to discover more effective drugs or lead compounds against KSHV. Compound 1 was used to carry out an anti-KSHV assay referring to the procedure of previous literature [18]. The inhibitory activity of compound 1 against KSHV lytic replication was measured. The results indicated that 1 exhibited potency with a safe hypotoxicity and a definite selectivity (i.e., EC50 of 501.3 μM and selectivity index of 1.99, respectively).
3. Materials and Methods
3.1. General Experiments
TLC was carried out by silica gel 60 F254 (Shanghai Beinuo Biological Technology Co. Ltd., Shanghai, China). Sephadex LH-20 (GE Healthcare Bio-Sciences AB, Uppsala, Sweden), Silica gel (200–300 mesh; Shanghai Xibao Biological Technology Co. Ltd., Shanghai, China), and RP-18 (50 μm, Merck Co. Ltd., Darmstadt, Germany) were applied in column chromatography. Pseudomolecular ion peak was recorded by analyzing HRESIMS data through a Thermo Fisher LC-LTQ-Orbitrap XL spectrometer (Thermo Fisher Scientific Inc., Waltham, MA, USA). A Perkin-Elmer 341 polarimeter (Perkin Elmer Inc., Waltham, MA, USA) was performed to measure optical rotation. The UV and IR spectra were obtained by a Varian Cary 50 (Varian Medical Systems, Salt Lake City, UT, USA) and Bruker Vertex 70 instruments (Brucker Corporation, Karlsruhe, Germany), respectively. The NMR spectra were acquired using a Bruker AM-600/400 spectrometer (Brucker Corporation). The chemical shifts of 1H- and 13C-NMR were referenced to the solvent peaks for methanol-d4 at δH 3.31 and δC 49.2. HPLC procedures were carried out on a Dionex Ultimate 3000 (Thermo Fisher Scientific Inc.) applied with a UV detector and a semi-preparative column (5 μm, 10 × 250 mm, Welch Ultimate® XB-C18).
3.2. Plant Material
The dried rhizomes of Z. schinifolium were collected in September 2013 at Da-Bie Mountain area of Hubei Province, China and authenticated by Changgong Zhang. A voucher specimen (ID 20131011) has been preserved in Herbarium of Material Medicine, School of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
3.3. Extraction and Isolation
The air-dried rhizomes of Z. schinifolium (30 kg) were exhaustively extracted with 95% EtOH at 25 °C and to furnish a syrup (1.5 kg) after vacuum distillation. The syrup was sequentially partitioned by petroleum ether, CH2Cl2, and EtOAc against water. Based on the TLC analyses, the petroleum ether extracts (300 g) were chromatographed and silica gel CC eluted with petroleum ether-acetone (100:1–1:1) to afford seven fractions (Fr.1–Fr.10). Fr.6 was further subjected to silica gel CC by gradient elution with petroleum ether–acetone (50:1–1:1), which afforded seven subfractions (Fr.6.1–Fr.6.7). Then, Fr.6.5 was subjected to MPLC (RP-18, CH3OH–H2O, 30%–60%) to yield three subfractions of Fr.6.5.1–Fr.6.5.3. Next, Fr.6.5.2 was subjected to Sephadex LH-20, repurified by a silica gel CC eluting with CH2Cl2–CH3OH 25/1, and isolated via semi-preparative HPLC (CH3OH-H2O 30%) to obtain 1 (9.2 mg).
3.4. Experimental Procedures of the Derivatives of (S)-MTPA and (R)-MTPA Esters of 1
MTPA ester derivatives of 1 were obtained referring to the previously reported procedure [12,13]. A solvent of 1 (1.0 mg) in anhydrous CH2Cl2 (2.5 mL) was successively added with (R)-MTPA (25.0 mg), dimethylaminopyridine (17 mg), and trimethylamine (25 µL). Then, the mixed solution was agitated and refluxed at 25 °C until 2 h and extinguished whereby adding with 40 µL anhydrous CH3OH. Next, the reaction mixture underwent a vacuum evaporation to yield a residue, which was purified via a small silica gel CC (1.6 g, hexane-IPA (75:1–40:1), v/v)) to provide the (S)-MTPA ester of 1 (1a, 1.5 mg). The (R)-MTPA derivative (1b, 1.6 mg) was obtained using (S)-MTPA chloride and experienced via the same procedure.
Schinifolenol A (1): violet oil; + 64.8 (c 0.03, CH3OH); UV (CH3OH) λmax (log ε) 244 (3.84) nm; IR (KBr) νmax 3695, 2965, 2361, 2337, 1724, 1667 cm−1; ECD (c 1.09 × 10−3 M, CH3OH) λmax nm (∆ε) 235 (+16.04), 270 (−1.35); 1H- and 13C-NMR data, see Table 1; positive HRESIMS: m/z 255.1596 [M + H]+ (calcd for C14H23O4, 255.1592).
Compound 1a: (S)-MTPA-ester: Amorphous powder; 1H-NMR (400 MHz, in methanol-d4) δH: 7.49–7.51 (2H, m, aromatic protons), 7.39–7.41 (3H, m, aromatic protons), 6.53 (1H, s, H-4), 2.47 (1H, m, H-2a), 2.30 (1H, d, J = 17.5 Hz, H-6), 1.98 (1H, d, J = 17.6 Hz, H-2b), 1.68 (1H, m, H-7a), 1.63 (1H, m, H-8a), 1.56 (1H, m, H-8b), 1.34, (3H, d, J = 6.3 Hz, Me-10), 1.18 (1H, m, H-7b), 0.94 (3H, s, Me-11), 0.93 (3H, s, Me-12); positive HRESIMS: m/z 493.1806 [M + Na]+ (calcd for C24H29F3O6Na, 493.1814).
Compound 1b: (R)-MTPA-ester: Amorphous powder; 1H-NMR (400 MHz, in methanol-d4) δH: 7.48–7.50 (2H, m, aromatic protons), 7.41–7.45 (3H, m, aromatic protons), 6.57 (1H, s, H-4), 2.58 (1H, m, H-2a), 2.51 (1H, d, J = 17.5 Hz, H-6), 2.07 (1H, d, J = 17.6 Hz, H-2b), 1.89 (1H, m, H-7a), 1.74 (1H, m, H-8a), 1.62 (1H, m, H-8b), 1.35 (1H, m, H-7b), 1.25 (3H, d, J = 6.3 Hz, Me-10), 1.07 (3H, s, Me-11), 0.99 (3H, s, Me-12); positive HRESIMS: m/z 493.1802 [M + Na]+ (calcd for C24H29F3O6Na, 493.1814).
3.5. Anti-KSHV Assay
Anti-KSHV assay was evaluated via cytotoxicity assessments and anti-KSHV infectivity assays. Human iSLK.219 cells were adopted to assess the bioactivity of compound 1 towards anti-KSHV. The human iSLK.219 cells were embedded with the rKSHV.219 virus, which was a harbored green fluorescent protein (GFP), through control of the elongation factor 1α (EF-1α) promoter. The lytic replication of KSHV was activated by the addition with 1.2 mM sodium butyrate (NaB) (Sigma, Shanghai, China) and 1 µg/mL doxycycline (Dox) (Beyotime, Jiangsu, China) [19,20]. After cells grew to 70% confluence in 96-well culture plates, compound 1 in presence of Dox and NaB with assigned concentrations was instilled to the wells. According to AlamarBlue® Cell Viability Assay (Invitrogen, Shanghai, China), the cell viability was evaluated after 48 h post drugs-induced. The luminescent expression was measured by the Envison 2102 Multilabel Reader (Perkin Elmer). The 50% cytotoxic concentration (CC50) of compound 1 was obtained through mathematical statistics with Graphpad5.0 Prism. The result was shown in Figure S1, Supplementary Materials.
An infectivity assay, like previous reports [18], was performed to determine the anti-KSHV activity of compound 1. The supernatants were harvested from iSLK.219-treated or untreated with compound 1 containing Dox and NaB at 48 h. Then, the supernatants were added to infect the Vero cells, which were seeded in a 96-well plate. Next, the Vero cells were centrifuged by a SORVALL®Pico apparatus at 1500× g for 60 min [21]. The supernatants were replaced by fresh DMEM medium to remove superfluous viruses. At 48 h, the expression of GFP per well in Vero cells were detected via an Operetta High-Content Screening System (HCS) (Perkin Elmer). Image fields (9/well) were observed by the automated microscope based HCS. The GFP intensity of each well was afforded using the Harmony 3.5 software (Perkin Elmer). The DMSO control was used as normalized group. The 50% effective concentration (EC50) of compound 1 towards anti-KSHV infectivity was calculated by reduced quantitative expression of the intensity of GFP by 50%. The result is exhibited in Figure S1, Supplementary Materials.
4. Conclusions
A new megastigmane sesquiterpenoid, methyl (S)-6-((S)-3-hydroxybutyl)-5,5-dimethyl-3-oxocyclohex-1-ene-1-carboxylate (1), termed schinifolenol A, was discovered from the rhizomes of Zanthoxylum schinifolium. The absolute configuration was established by the analyses of the extensive spectra including HRESIMS, NMR, UV, and IR spectra, the application of the modified Mosher’s method, and the method of calculated ECD spectra using the time-dependent density functional theory. Bioactivity screenings suggested that compound 1 had potential activity on anti-KSHV infection with a safe hypotoxicity and a definite selectivity.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/21/3/383/s1.
Acknowledgments
This work was financially supported by the Program for New Century Excellent Talents in University, the State Education Ministry of China (2008-0224), and the National Natural Science Foundation of China (Nos. 81573316, 31200258, 21502057, and 31500281). We express our appreciation to Xulin Chen, Jungang Chen, and Wei Tang (State Key Laboratory of Virology, Wuhan Institute of Virology, Chinese Academy of Sciences, Wuhan, China) for their anti-KSHV work.
Author Contributions
Yonghui Zhang and Yu Zhang conceived and designed the experiments; Linzhen Hu performed the experiments, analyzed the data, and wrote the manuscript; Junjun Liu undertook the tasks of ECD calculations, Kongchao Wang and Zhenzhen Wang carried out the biological assay; Kaiping Wang, Jinwen Zhang, Zengwei Luo and Yongbo Xue contributed reagents, materials, and analysis tools. All authors reviewed the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Z. schinifolium | Zanthoxylum schinifolium |
| TD-DFT | time-dependent density functional theory |
| ECD | electronic circular dichroism |
| KSHV | Kaposi’s sarcoma associated herpesvirus |
| HRESIMS | High-resolution electrospray ionization mass spectra |
| CC | column chromatography |
| HPLC | High Performance Liquid Chromatography |
| EtOH | ethanol |
| EtOAc | ethyl acetate |
| MTPA | α-Methoxy-α-Trifluoromethylphenylacetic acid |
| IPA | isopropanol |
References
- Sun, X.; Duan, Z. Research progress of medicinal plants of Zanthoxylum linn. Acta Pharm. Sin. 1996, 31, 231–240. [Google Scholar]
- Liu, Z.L.; Chu, S.S.; Jiang, G.H. Feeding deterrents from Zanthoxylum schinifolium against two stored-product insects. J. Agric. Food Chem. 2009, 57, 10130–10133. [Google Scholar] [CrossRef] [PubMed]
- Tsai, I.L.; Lin, W.Y.; Chang, C.T.; Chen, I.S. Peroxycoumarins from the root bark of Zanthoxylum schinifolium. J. Chin. Chem. Soc. 1998, 45, 99–101. [Google Scholar] [CrossRef]
- Iseli, V.; Hagmann, L.; Egli, J.; Hamburger, M. Characterization of the pungent principles and the essential oil of Zanthoxylum schinifolium pericarp. Pharmazie 2007, 62, 396–400. [Google Scholar] [PubMed]
- He, W.; Van Puyvelde, L.; De Kimpe, N.; Verbruggen, L.; Anthonissen, K.; Van der Flaas, M.; Bosselaers, J.; Mathenge, S.G.; Mudida, F.P. Chemical constituents and biological activities of Zanthoxylum usambarense. Phytother. Res. 2002, 16, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chen, C.; Yang, J.; Li, X.N.; Liu, J.; Sun, B.; Huang, S.X.; Li, D.; Yao, G.; Luo, Z.; et al. Bioactive acylphloroglucinols with adamantyl skeleton from Hypericum sampsonii. Org. Lett. 2014, 16, 6322–6325. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chen, C.; Liu, J.; Sun, B.; Wei, G.; Li, Y.; Zhang, J.; Yao, G.; Luo, Z.; Xue, Y.; et al. Hyperascyrones A–H, polyprenylated spirocyclic acylphloroglucinol derivatives from Hypericum ascyron Linn. Phytochemistry 2015, 115, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xue, Y.; Zhu, H.; Li, Y.; Sun, B.; Liu, J.; Yao, G.; Zhang, J.; Du, G.; Zhang, Y. Hyperattenins A–I, bioactive polyprenylated acylphloroglucinols from Hypericum attenuatum Choisy. RSC. Adv. 2015, 5, 5277–5287. [Google Scholar] [CrossRef]
- Matsunami, K.; Otsuka, H.; Takeda, Y. Structural Revisions of Blumenol C Glucoside and Byzantionoside B. Chem. Pharm. Bull. 2010, 58, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Morikawa, T.; Zhang, Y.; Nakamura, S.; Muraoka, O.; Matsuda, H. Megastigmanes and their glucosides from the whole plant of Sedum sarmentosum. J. Nat. Prod. 2007, 70, 575–583. [Google Scholar] [PubMed]
- Zhao, A.H.; Peng, L.Y.; Wang, Z.Y.; Sun, H.D. An ionone derivative from Isodon leucophyllus. Acta Bot. Yunnan 2003, 25, 503–506. [Google Scholar]
- Nakanishi, T.; Iida, N.; Inatomi, Y.; Murata, H.; Inada, A.; Murata, J.; Lang, F.A.; Iinuma, M.; Tanaka, T.; Sakagami, Y. A Monoterpene glucoside and three megastigmane glycosides from Juniperus communis var. depressa. Chem. Pharm. Bull. 2005, 53, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, I.; Kusumi, T.; Kashman, Y.; Kakisawa, H. High-field FT NMR application of Mosher’s method. The absolute configurations of marine terpenoids. J. Am. Chem. Soc. 1991, 113, 4092–4096. [Google Scholar] [CrossRef]
- Michael, J.P. Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 2002, 19, 742–760. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.T.; Pezzoto, J.M.; Douglas Kinghorn, A. Evaluation of natural products as inhibitors of human immunodeficiency virus type 1 (HIV-1) reverse transcriptase. J. Nat. Prod. 1991, 54, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Cesarman, E.; Pessin, M.S.; Lee, F.; Culpepper, J.; Knowles, D.M.; Moore, P.S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s Sarcoma. Science 1994, 266, 1865–1869. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Jeong, S.G.; Park, J.E.; Han, J.A.; Kang, H.R.; Lee, D.; Song, M.J. Antiviral activity of angelicin against gammaherpesviruses. Antivir. Res. 2013, 100, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, L.; Lan, K.; Chen, X. Celecoxib inhibits the lytic activation of Kaposi’s Sarcoma-Associated Herpesvirus through down-regulation of RTA expression by inhibiting the activation of p38 MAPK. Viruses 2015, 7, 2268–2287. [Google Scholar] [CrossRef] [PubMed]
- Myoung, J.; Ganem, D. Generation of a doxycycline-inducible KSHV producer cell line of endothelial origin: Maintenance of tight latency with efficient reactivation upon induction. J. Virol. Methods 2011, 174, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Vieira, J.; O’Hearn, P.M. Use of the red fluorescent protein as a marker of Kaposi’s sarcoma-associated herpesvirus lytic gene expression. Virology 2004, 325, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.M.; Ahn, A.K.; Seo, T.; Hong, H.B.; Chung, M.A.; Jung, S.D.; Cho, H.; Lee, M.S. Centrifugal enhancement of Kaposi’s sarcoma-associated virus infection of human endothelial cells in vitro. J. Virol. Methods 2008, 154, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compound 1 are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).