Comparison of Properties among Dendritic and Hyperbranched Poly(ether ether ketone)s and Linear Poly(ether ketone)s
Abstract
:1. Introduction
2. Results and Discussion
2.1. Poly(ether ether ketone)s Dendrimers
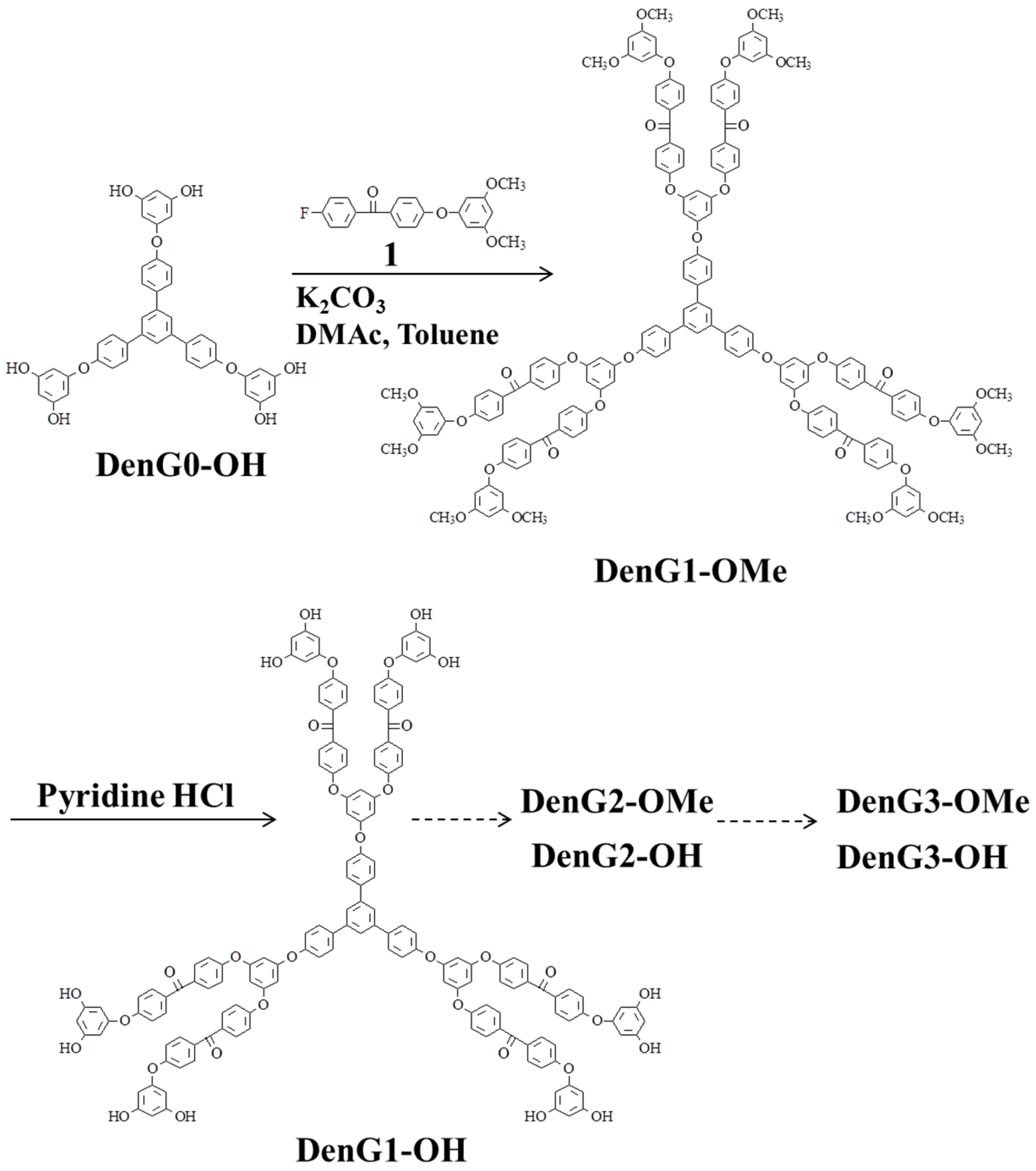
2.2. Hyperbranched Poly(ether ether ketone)s
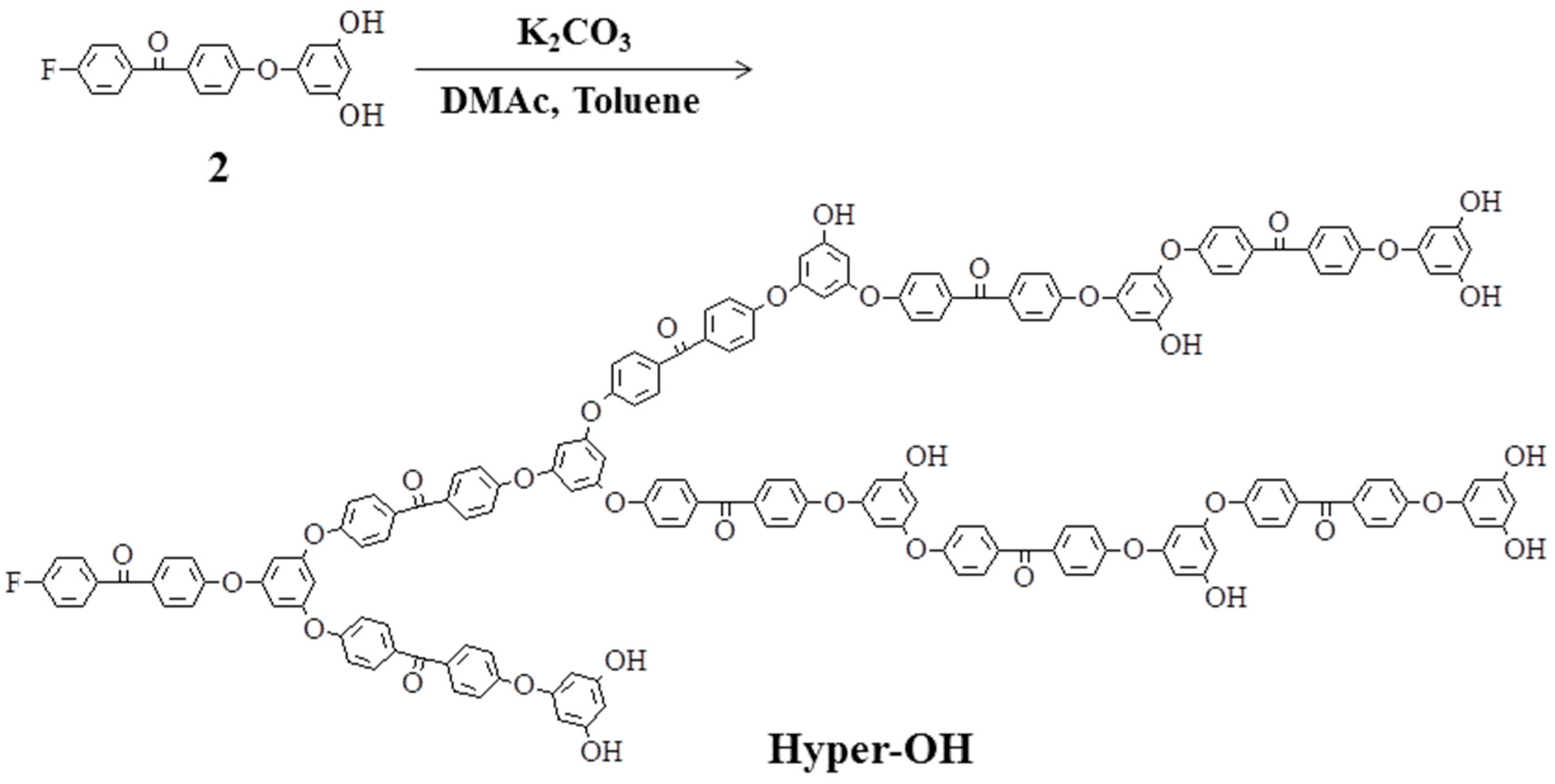
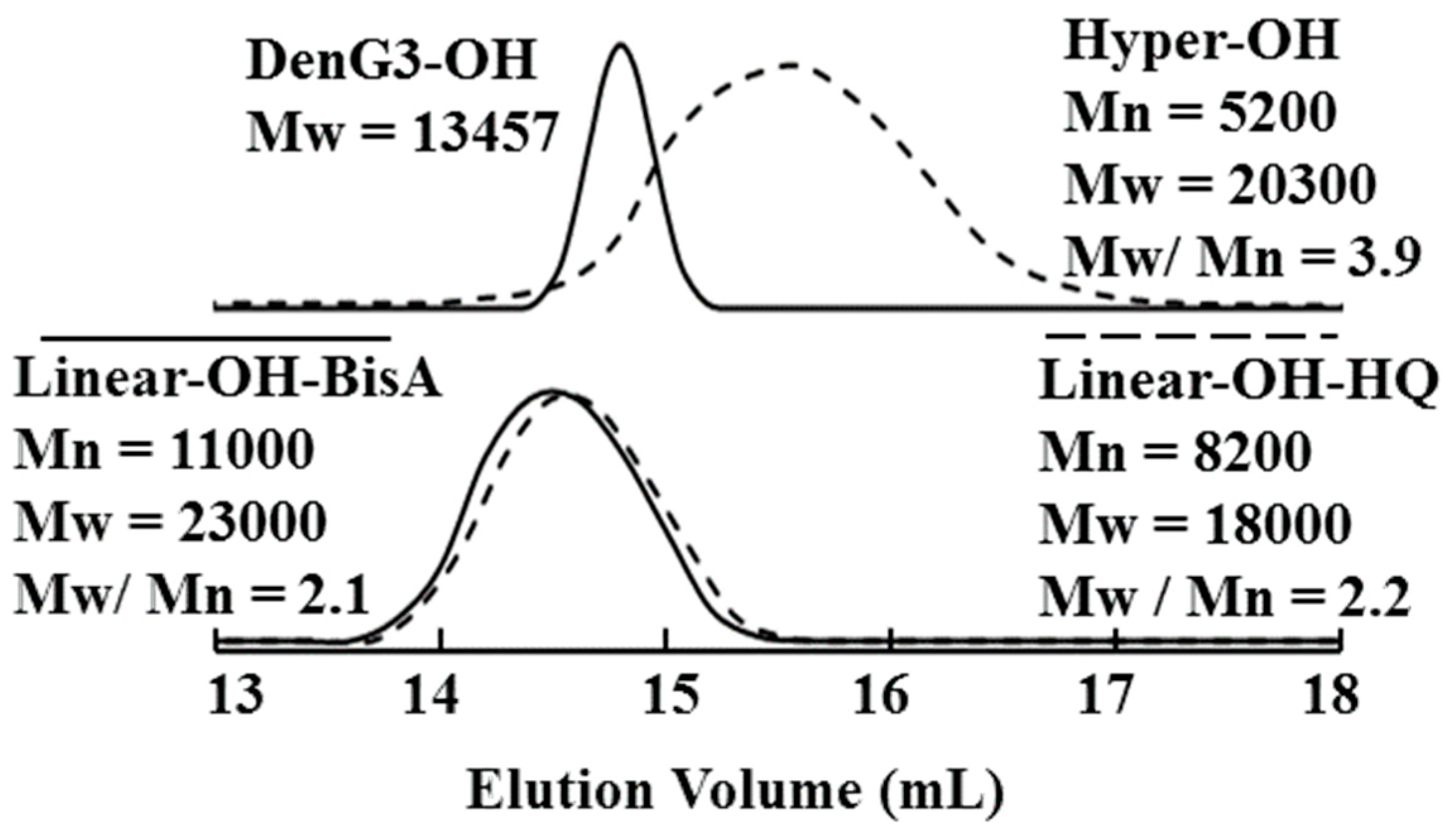
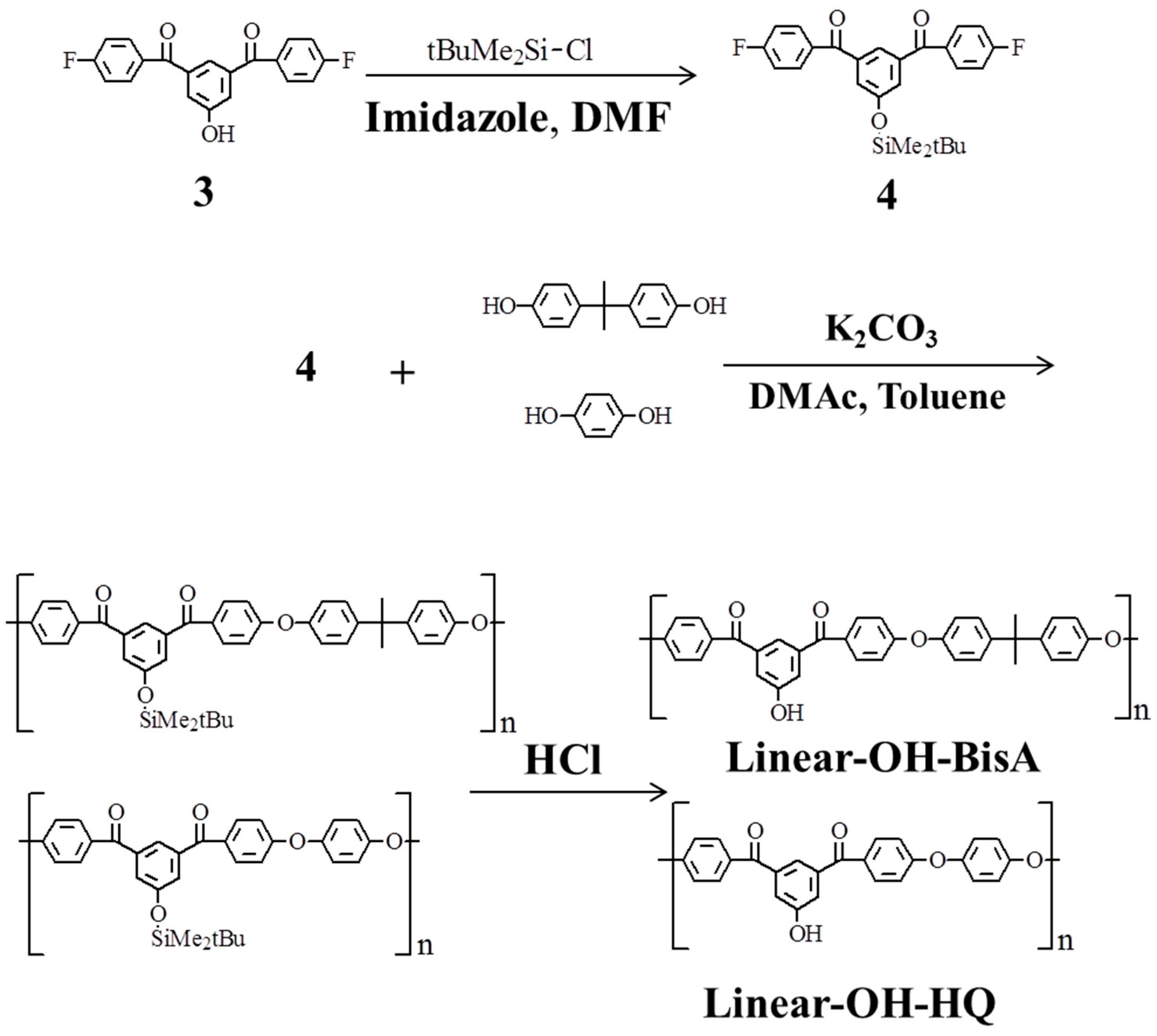
2.3. Linear Poly(ether ketone)s Having Hydroxyl Groups in the Side Chains
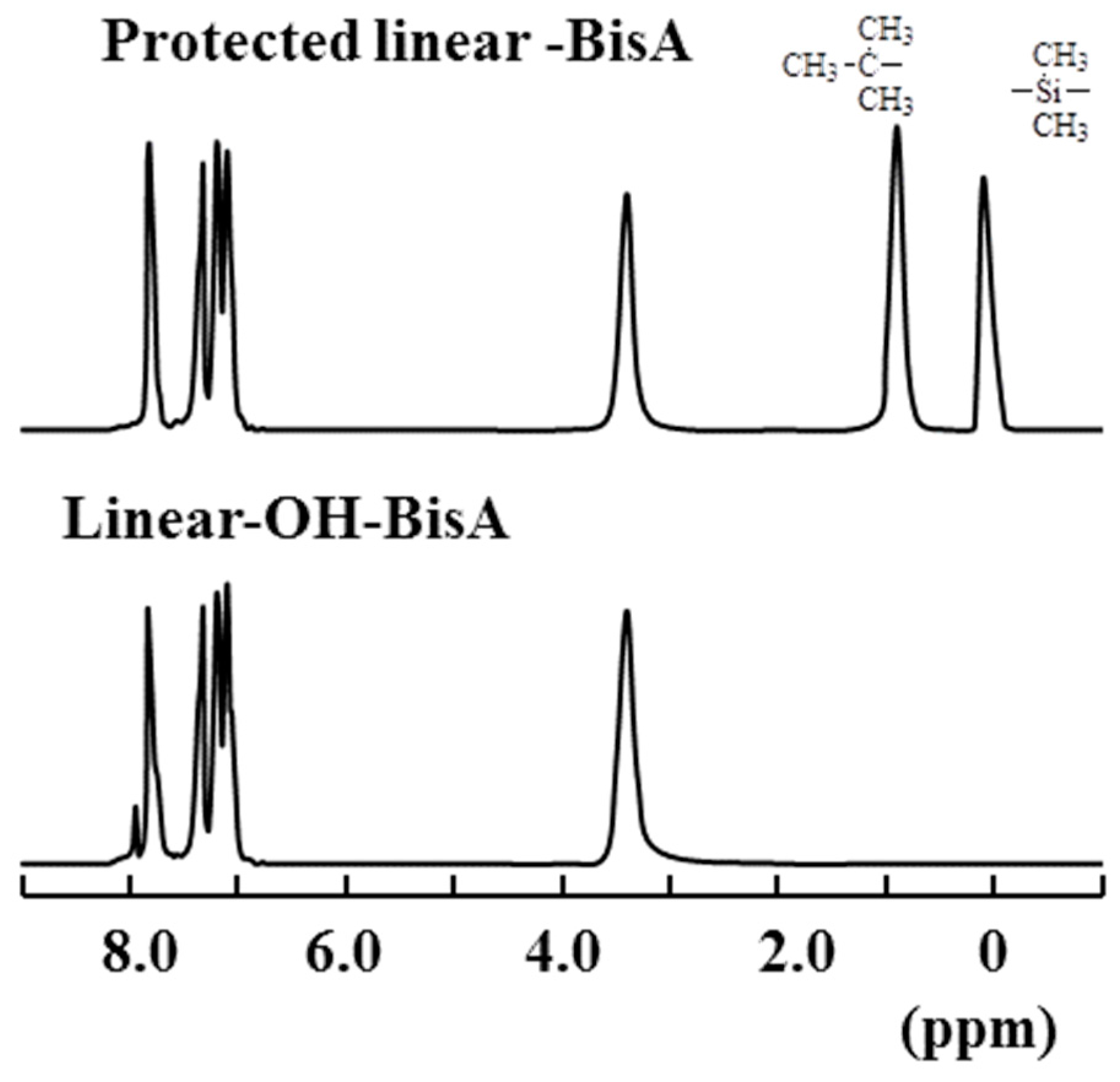
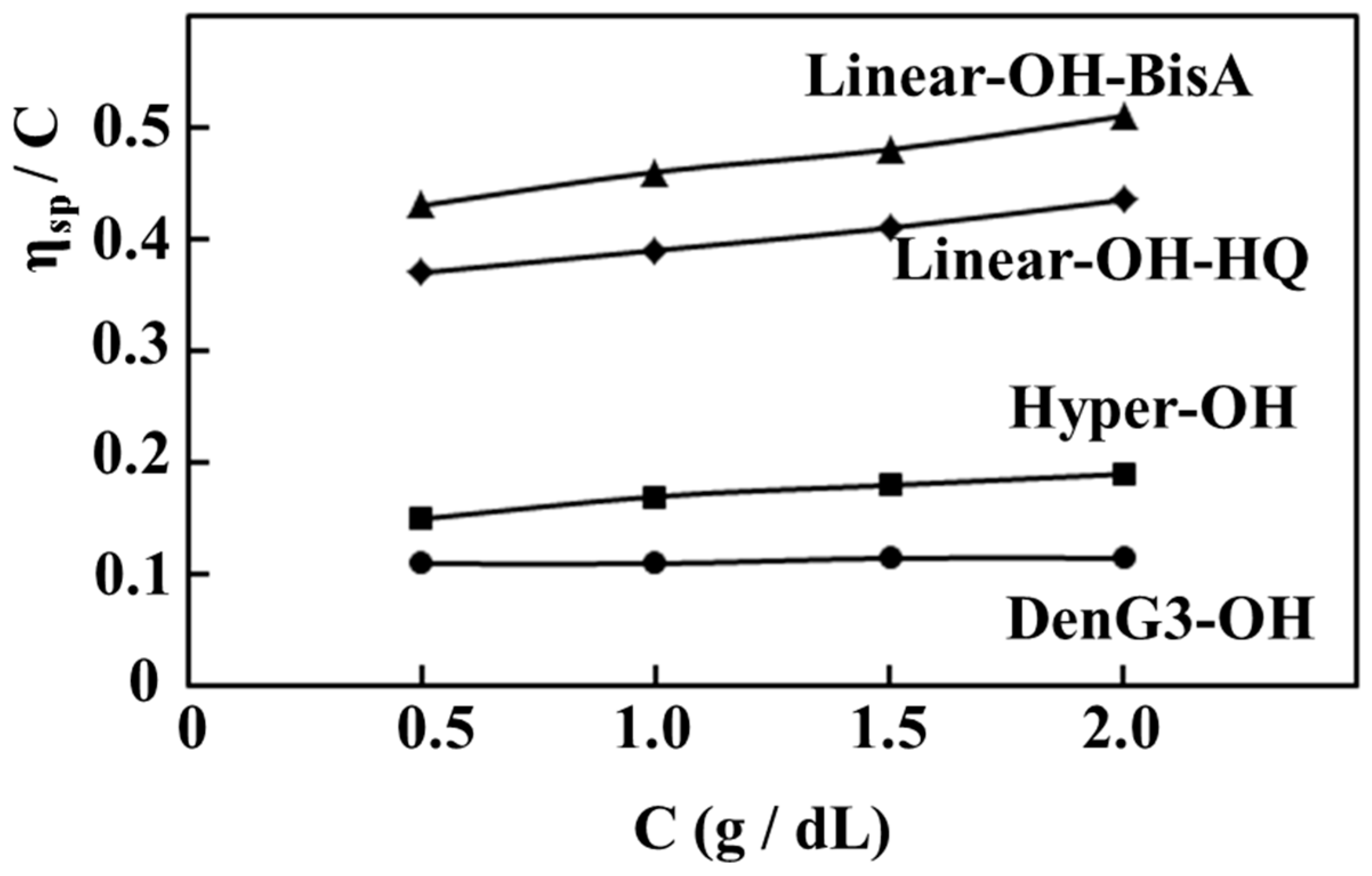
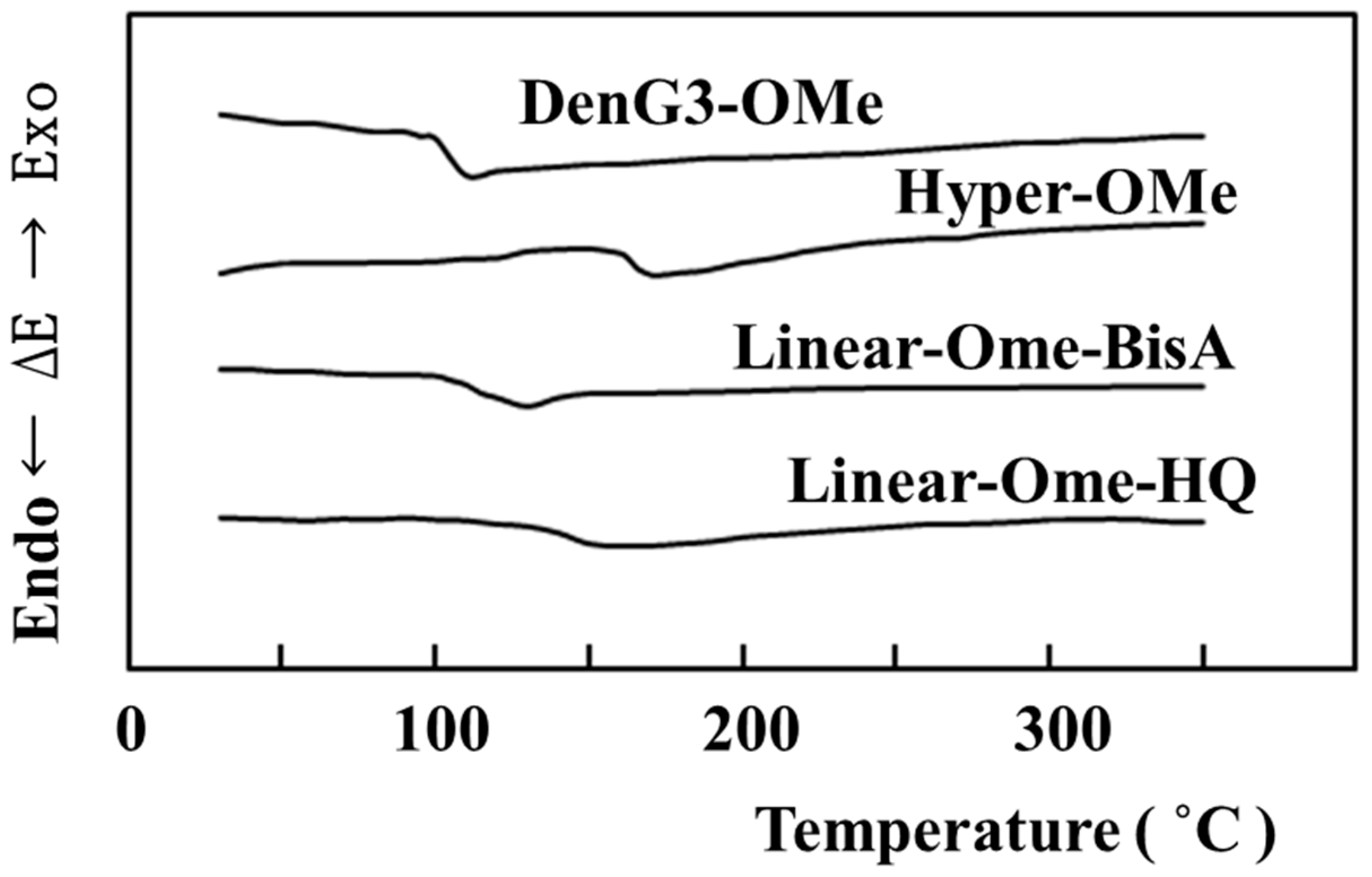
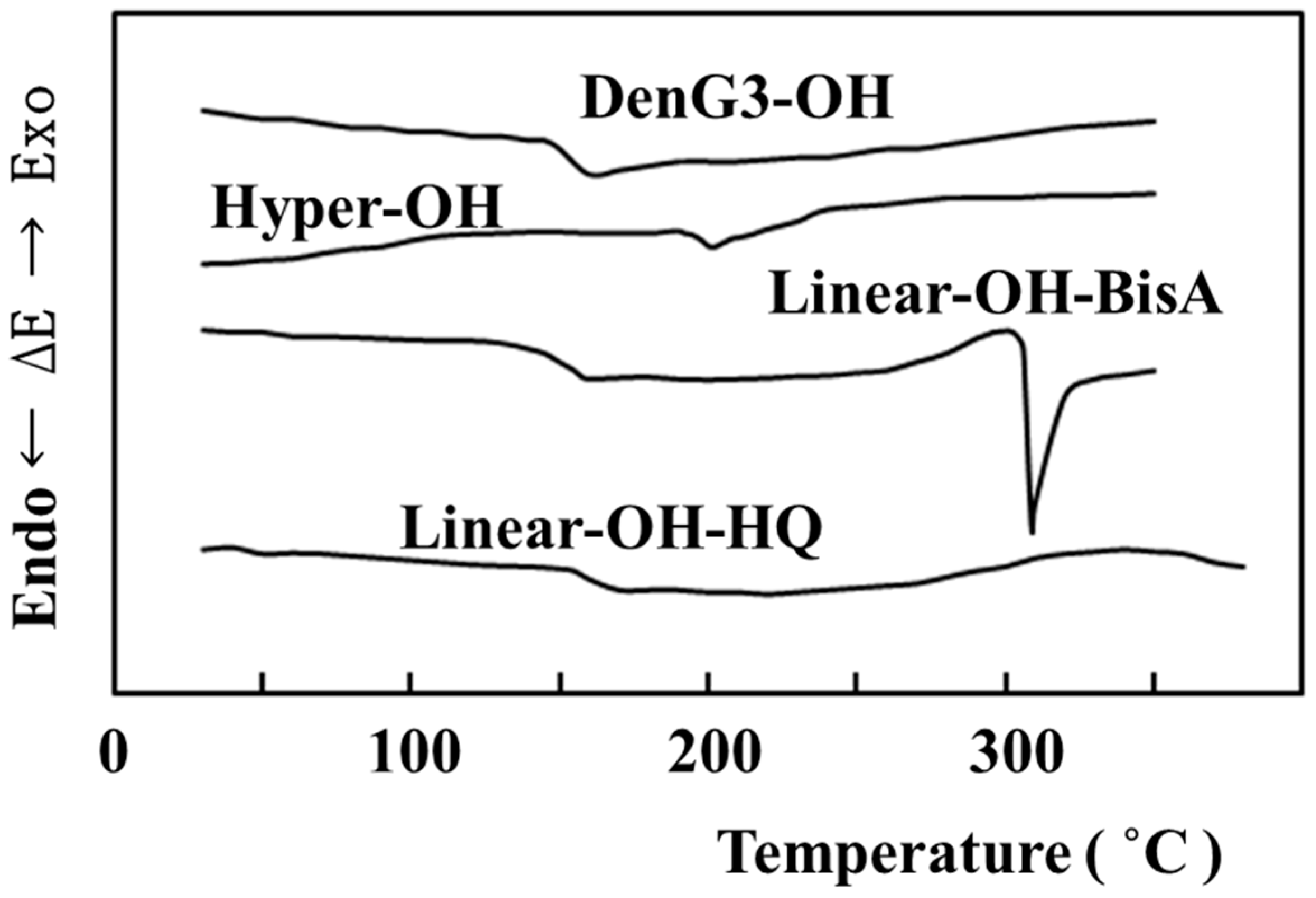
2.4. Comparison of Properties
| Methoxy Group Polymer | NMP | DMAc | CHCl3 | Pyridine | THF | Toluene | CH3OH | NaOH Aquation |
|---|---|---|---|---|---|---|---|---|
| DenG3-OMe | ++ | ++ | ++ | ++ | ++ | ++ | -- | -- |
| Hyper-OMe | ++ | ++ | ++ | ++ | ++ | ++ | -- | -- |
| Linear-OMe-BisA | ++ | ++ | ++ | -- | ++ | -- | -- | -- |
| Linear-OMe-HQ | ++ | ++ | -- | -- | ++ | -- | -- | -- |
| Hydroxy Group Polymer | NMP | DMAc | CHCl3 | Pyridine | THF | Toluene | CH3OH | NaOH Aquation |
|---|---|---|---|---|---|---|---|---|
| DenG3-OH | ++ | ++ | -- | ++ | ++ | -- | ++ | ++ |
| Hyper-OH | ++ | ++ | -- | ++ | ++ | -- | -- | + |
| Linear-OH-BisA | ++ | ++ | -- | ++ | ++ | -- | -- | -- |
| Linear-OH-HQ | ++ | ++ | -- | ++ | ++ | -- | -- | -- |

3. Experimental Section
3.1. General Information
3.2. Conventional Synthesis
3.2.1. Third Generation Dendrimer (DenG3-OMe)
3.2.2. Hydroxyl Terminal Third Generation (DenG3-OH)
3.2.3. Hyperbranched Poly(ether ether ketone) (Hyper-OH)
3.2.4. Hyperbranched Poly(ether ether ketone) (Hyper-OMe)
3.2.5. 1-(tert-Butyldimethylsilyloxy)-3,5-bis(4-fluorobenzoyl)benzene (4)
3.2.6. Linear poly(ether ketone) Having Hydroxyl Groups in the Side Chains (Linear-OH-BisA)
3.2.7. Linear Poly(ether ketone) with Methoxy Groups in the Side Chains (Linear-OMe-BisA)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tomallia, D.A.; Naylor, A.M.; Goddard, W.A. Starburst Dendrimers: Molecular-Level Control of Size, Shape, Surface Chemistry, Topology, and Flexibility from Atoms to Macroscopic Matter. Angew. Chem. Int. Ed. 1990, 29, 138–175. [Google Scholar] [CrossRef]
- Hawke, C.J.; Frechet, J.M.J. Control of Surface Functionality in the Synthesis of Dendritic Macromolecules Using the Convergent-Growth Approach. Macromolecules 1990, 23, 4726–4729. [Google Scholar] [CrossRef]
- Morikawa, A.; Kakimoto, M.; Imai, Y. Convergent Synthesis of Starburst Poly(ether ketone) Dendrons. Macromolecules 1993, 26, 6324–6329. [Google Scholar] [CrossRef]
- Trollsås, M.; Hedrick, J. De drimer-likt Star Polymers. J. Am. Chem. Soc. 1998, 120, 4644–4651. [Google Scholar] [CrossRef]
- Morikawa, A.; Ono, K. Preparation of Poly(ether ketone) Dendrons with Graded Structures. Macromolecules 1999, 32, 1062–1068. [Google Scholar] [CrossRef]
- Morikawa, A.; Ono, K. Preparation of poly(ether ether ketone) dendrimers by the divergent method. Polym. J. 2000, 32, 234–242. [Google Scholar] [CrossRef]
- Morikawa, A.; Ono, K. Preparation of Poly(ether)-(ether ether ketone) Dendrimers by the Convergent Method. Polym. J. 2000, 32, 255–262. [Google Scholar] [CrossRef]
- Ishida, Y.; Jikei, M.; Kakimoto, M. Rapid Synthesis of Aromatic Polyamide Dendrimers by an Orthogonal and a Double-Stage Convergent Approach. Macromolecules 2000, 33, 3202–3211. [Google Scholar] [CrossRef]
- In, I.; Kim, S.Y. Orthogonal Synthesis of Poly(aryl ether amide) Dendrons. Macromolecules 2005, 38, 9399–9401. [Google Scholar] [CrossRef]
- Albrecht, K.; Kasai, Y.; Kimoto, A.; Yamamoto, K. The Synthesis and Properties of Carbazole-Phenylazomethine Double Layer-Type Dendrimers. Macromolecules 2008, 41, 3793–3800. [Google Scholar] [CrossRef]
- Van der Poll, D.G.; Kieler-Ferguson, H.M.; Floyd, W.C.; Guillaudeu, S.J.; Jerger, K.; Szoka, F.C.; Fréchet, J.M. Design, Synthesis, and Biological Evaluation of a Robust, Biodegradable Dendrimer. Bioconjugate Chem. 2010, 21, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, K.; Higashimura, H.; Yamamoto, K. Synthesis and Properties of Nitrogen-Introduced Phenylazomethine Dendrimers. Synth. Commun. 2014, 44, 2239–2247. [Google Scholar] [CrossRef]
- Hawker, C.J.; Lee, R.; Frechet, J.M.J. One-pot synthesis of hyperbranched polyesters. J. Am. Chem. Soc. 1991, 113, 4583–4588. [Google Scholar] [CrossRef]
- Miller, T.M.; Neenan, T.X.; Kwock, E.W.; Stein, S.M. Dendritic analogs of engineering plastics: A general one-step synthesis of dendritic polyaryl ethers. J. Am. Chem. Soc. 1993, 115, 356–357. [Google Scholar] [CrossRef]
- Wooly, K.L.; Frechet, J.M.J.; Hawker, C.J. Influence of shape on the reactivity and properties of dendritic, hyperbranched and linear aromatic polyesters. Polymer 1994, 35, 4489–4495. [Google Scholar] [CrossRef]
- Hawker, C.J.; Chu, F. Hyperbranched Poly(ether ketones): Manipulation of Structure and Physical Properties. Macromolecules 1996, 29, 4370–4380. [Google Scholar] [CrossRef]
- Jikei, M.; Hu, Z.; Kakimoto, M.; Imai, Y. Synthesis of Hyperbranched Poly(phenylene sulfide) via a Poly(sulfonium cation) Precursor. Macromolecules 1996, 29, 1062–1106. [Google Scholar] [CrossRef]
- Morikawa, A. Preparation and Properties of Hyperbranched Poly(ether ketones) with a Various Number of Phenylene Units. Macromolecules 1998, 31, 5999–6009. [Google Scholar] [CrossRef]
- Yang, G.; Jikei, M.; Kakimoto, M. Successful Thermal Self-Polycondensation of AB2 Monomer to Form Hyperbranched Aromatic Polyamide. Macromolecules 1998, 31, 5964–5966. [Google Scholar] [CrossRef]
- Yamanaka, K.; Jikei, M.; Kakimoto, M. Preparation and Properties of Hyperbranched Aromatic Polyimides via Polyamic Acid Methyl Ester Precursors. Macromolecules 2000, 33, 6937–6944. [Google Scholar] [CrossRef]
- Hao, J.; Jikei, M.; Kakimoto, M. Synthesis and Comparison of Hyperbranched Aromatic Polyimides Having the Same Repeating Unit by AB2 Self-Polymerization and A2 + B3 Polymerization. Macromolecules 2003, 36, 3519–3528. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Tong, Y.; Shi, L.; Liu, X. Synthesis and Characterization of Hyperbranched Aromatic Poly(ether imide)s. Macromolecules 2003, 36, 5537–5544. [Google Scholar] [CrossRef]
- Li, X.; Zhan, J.; Lin, Y.; Li, Y.; Li, Y. Facile Synthesis and Characterization of Aromatic and Semiaromatic Hyperbranched Poly(ester-amide)s. Macromolecules 2005, 38, 8235–8243. [Google Scholar] [CrossRef]
- Seino, M.; Hayakawa, T.; Ishida, Y.; Kakimoto, M. Synthesis and Characterization of Crystalline Hyperbranched Polysiloxane with POSS Groups at the Terminal Position. Macromolecules 2006, 39, 8892–8894. [Google Scholar] [CrossRef]
- Baek, J.B.; Simko, S.R.; Tan, L.S. Synthesis and Chain-End Modification of a Novel Hyperbranched Polymer Containing Alternating Quinoxaline and Benzoxazole Repeat Units. Macromolecules 2006, 39, 7959–7966. [Google Scholar] [CrossRef]
- Xue, Z.; Finke, A.D.; Moore, J.S. Synthesis of Hyperbranched Poly(m-phenylene)s via Suzuki Polycondensation of a Branched AB2 Monomer. Macromolecules 2010, 43, 9277–9282. [Google Scholar] [CrossRef]
- Fu, Y.; Vandendriessche, A.; Dehaen, W.; Smet, M. Effective Acid-Catalyzed Synthesis of 100% Hyperbranched Polyacenaphthenones. Macromolecules 2006, 39, 5183–5186. [Google Scholar] [CrossRef]
- Fu, Y.; Oosterwijck, C.W.; Vandendriessche, A.; Kowalczuk-Bleja, A.; Zhang, X.; Dworak, A.; Dehaen, W.; Smet, M. Hyperbranched Poly(arylene oxindole)s with a Degree of Branching of 100% for the Construction of Nanocontainers by Orthogonal Modification. Macromolecules 2008, 41, 2388–2393. [Google Scholar] [CrossRef]
- Jikei, M.; Uchida, D.; Haruta, Y.; Takahashi, Y.; Matsumoto, K. Synthesis and Properties of Hyperbranched Poly(ether sulfone)s Prepared by Self-Polycondensation of Novel AB2 Monomer. J. Polym. Sci. A Polym. Chem. 2012, 50, 3830–3839. [Google Scholar] [CrossRef]
- Jikei, M.; Suzuki, M.; Itoh, K.; Matsumoto, K.; Saito, Y.; Kawaguchi, S. Synthesis of Hyperbranched Poly(l-lactide)s by Self-Polycondensation of AB2 Macromonomers and Their Structural Characterization by Light Scattering Measurements. Macromolecules 2012, 45, 8237–8244. [Google Scholar] [CrossRef]
- Morikawa, A.; Akagi, M. Hyperbranched poly(ether ether ketone)s: Preparation and comparison of properties the corresponding dendrimers. Polym. J. 2013, 45, 614–621. [Google Scholar] [CrossRef]
- Shi, Y.; Nabae, Y.; Hayakawa, T.; Kobayashi, H.; Yabishita, M.; Fukuoka, A.; Kakimoto, M. Synthesis and characterization of hyperbranched aromatic poly(ether ketone)s functionalized with carboxylic acid terminal groups. Polym. J. 2014, 46, 722–727. [Google Scholar] [CrossRef]
- Tian, W.; Fan, X.; Kong, J.; Liu, Y.; Hung, Y. Novel supramolecular system of amphiphilic hyperbranched polymer with β-cyclodextrine and hyperbranched topography cavites: Synthesis and selective encapsulation. Polymer 2010, 51, 2556–2564. [Google Scholar] [CrossRef]
- Herrero, M.A.; Guerra, J.; Myers, V.S.; Gómez, M.V.; Crooks, R.M.; Prato, M. Gold Dendrimer Encapsulated Nanoparticles as Labeling Agents for Multiwalled Carbon Nanotubes. ACS Nano 2010, 4, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, R.; Jikei, M.; Kakimoto, M. Preparation and properties of composite materials composed aromatic polyamides and vinyl polymers. High Perform. Polym. 2002, 14, 41–51. [Google Scholar] [CrossRef]
- Gao, H.; Yorifuji, D.; Wakita, J.; Jiang, Z.H.; Ando, S. In situ preparation of nano ZnO/hyperbranched polyimide hybrid film and their optical properties. Polymer 2010, 51, 3173–3180. [Google Scholar] [CrossRef]
- Park, B.R.; Nabae, Y.; Surapati, M.; Hayakawa, T.; Kakimoto, M. Poly(N-isopropylacrylamide)-modified silica beads with hyperbranched polysiloxane for three-dimensional cell cultivation. Polym. J. 2013, 45, 210–215. [Google Scholar] [CrossRef]
- Sudo, Y.; Sakai, H.; Nabae, Y.; Hayakawa, Y.; Kakimoto, M. Preparation of hyperbranched polystyrene-g-poly(N-isopropylacrylamide) copolymers and its application to novel thermos-responsible cell culture dishes. Polymer 2015, 70, 307–314. [Google Scholar] [CrossRef]
- Kakimoto, M.; Grunzinger, S.J.; Hayakawa, T. Hyperbranched poly(ether sulfone)s: Preparation and application to ion-exchange membranes. Polym. J. 2010, 42, 697–705. [Google Scholar] [CrossRef]
- Fang, J.; Kita, H.; Okamoto, K. Hyperbranched Polyimides for Gas Separation Applications. 1. Synthesis and Characterization. Macromolecules 2000, 33, 4639–4646. [Google Scholar] [CrossRef]
- Kaneko, R.; Jikei, M.; Kakimoto, M. Preparation and properties of photosensitive polymers based on hyperbranched aromatic polyamides. High Perform. Polym. 2002, 14, 53–62. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, X.; Goodson, T. Enhanced Third-Order Nonlinear Optical Properties in Dendrimer−Metal Nanocomposites. Nano Lett. 2005, 5, 2379–2384. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Deng, X.; Cao, Z.; Shen, Q.; Zhang, W.; Shi, W. Synthesis and second-order nonlinear optical properties of hyperbranched polymers containing pendant azobenzene chromophores. Polymer 2007, 48, 5988–5993. [Google Scholar] [CrossRef]
- Wen, G.A.; Xin, Y.; Zhu, X.R.; Zeng, W.J.; Zhu, R.; Feng, J.C.; Cao, Y.; Zhao, L.; Wang, L.H.; Wei, W.; et al. Hyperbranched triazine-containing polyfluorene: Efficient blue emitters for polymer light-emitting diodes (PLEDs). Polymer 2007, 48, 1824–1829. [Google Scholar] [CrossRef]
- Oh, J.H.; Jang, J.; Lee, S.H. Curing behavior of tetrafunctional epoxy resin/hyperbranched polymer system. Polymer 2001, 42, 8339–8347. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Shirai, K.; Saitoh, H.; Yamaguchi, T.; Tubokawa, N. Postgrafting of vinyl polymers onto hyperbranched poly(amino-amido)-grafted nano-sized silica surface. Polymer 2005, 46, 2541–2547. [Google Scholar] [CrossRef]
- Maruyama, K.; Kudo, H.; Ikehara, T.; Nishikubo, T.; Nishimura, I.; Shishido, A.; Ikeda, T. Synthesis and Properties of Photo-Cross-Linkable Hyperbranched Poly(urethane)s Containing Both Terminal Methacryloyl Groups and Carboxyl Groups. Macromolecules 2007, 40, 4895–4900. [Google Scholar] [CrossRef]
- Maity, P.; Yamazoe, S.; Tsukuda, T. Dendrimer-Encapsulated Copper Cluster as a Chemoselective and Regenerable Hydrogenation Catalyst. ACS Catal. 2013, 3, 182–185. [Google Scholar] [CrossRef]
- Nabae, Y.; Mikuni, M.; Hayakawa, T.; Kakimoto, M. Synthesis of TEMPO Functionalized Polyimides by A2 + B3 polymerization. J. Photopolym. Sci. Technol. 2014, 27, 139–144. [Google Scholar] [CrossRef]
- Nabae, Y.; Liang, J.; Huang, X.; Hayakawa, T.; Kakimoto, M. Sulfonic acid functionalized hyperbranched poly(ether sulfone) as a solid acid catalyst. Green Chem. 2014, 16, 3596–3602. [Google Scholar] [CrossRef]
- Shi, Y.; Nabae, Y.; Hayakawa, T.; Kakimoto, M. Hyperbranched aromatic poly(ether ketone) functionalized with TEMPO as heterogeneous catalyst for aerobic oxidation of alkohols. R. Soc. Chem. 2015, 5, 1923–1928. [Google Scholar]
- Albrecht, K.; Kasai, Y.; Kuramoto, Y.; Yamamoto, K. A-fouth-generation carbazole-phenylazomethyne dendrimer as a size-sensitive host for fullerenes. Chem. Commun. 2013, 49, 865–867. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Imaoka, T. Precision Synthesis of Subnanoparticles Using Dendrimers as a Superatom Synthesizer. Acc. Chem. Res. 2014, 47, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Taton, D.; Borsali, R.; Chaikof, E.L.; Gnanou, Y. pH Responsiveness of Dendrimer-like Poly(ethylene oxide)s. J. Am. Chem. Soc. 2006, 128, 11551–11562. [Google Scholar] [CrossRef] [PubMed]
- Minard-Basquin, C.; Weil, T.; Hohner, A.; Rädler, J.O.; Müllen, K.A. Polyphenylene Dendrimer-Detergent Complex as a Highly Fluorescent Probe for Bioassays. J. Am. Chem. Soc. 2003, 125, 5832–5838. [Google Scholar] [CrossRef] [PubMed]
- Nwe, K.; Milenic, D.E.; Ray, G.L.; Kim, Y.S.; Brechbiel, M.W. Preparation of Cystamine Core Dendrimer and Antibody-Dendrimer Conjugates for MRI Angiography. Mol. Pharm. 2012, 9, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, K.; Matsuoka, K.; Fujita, K.; Yamamoto, K. Carbazole Dendrimers as Solution-Processable Thermally Actived Delayed Fliorescence Materials. Angew. Chem. Int. Ed. 2015, 54, 5677–5682. [Google Scholar] [CrossRef] [PubMed]
- Kesharwari, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Viswanathan, R.; Johnson, B.C.; McGrath, J.E. Synthesis, kinetic observations and characterizations of polyarylene ether sulphones prepared via a potassium carbonate DMAC process. Polymer 1984, 25, 1827–1836. [Google Scholar] [CrossRef]
- Kendal, P.M.; Johson, J.V.; Cook, C.E. Synthetic route to an aromatic analog of strigol. J. Org. Chem. 1979, 44, 1421–1424. [Google Scholar] [CrossRef]
- Sinhababu, A.K.; Kawase, M.; Borchardt, R.T. Desilylation of tert-Butyldimethylsilyl Ethers of Phenols. Synthesis 1988, 9, 710–712. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morikawa, A. Comparison of Properties among Dendritic and Hyperbranched Poly(ether ether ketone)s and Linear Poly(ether ketone)s. Molecules 2016, 21, 219. https://doi.org/10.3390/molecules21020219
Morikawa A. Comparison of Properties among Dendritic and Hyperbranched Poly(ether ether ketone)s and Linear Poly(ether ketone)s. Molecules. 2016; 21(2):219. https://doi.org/10.3390/molecules21020219
Chicago/Turabian StyleMorikawa, Atsushi. 2016. "Comparison of Properties among Dendritic and Hyperbranched Poly(ether ether ketone)s and Linear Poly(ether ketone)s" Molecules 21, no. 2: 219. https://doi.org/10.3390/molecules21020219
APA StyleMorikawa, A. (2016). Comparison of Properties among Dendritic and Hyperbranched Poly(ether ether ketone)s and Linear Poly(ether ketone)s. Molecules, 21(2), 219. https://doi.org/10.3390/molecules21020219






