The Inhibitory Mechanisms Study of 5,6,4′-Trihydroxy-7,3′-Dimethoxyflavone against the LPS-Induced Macrophage Inflammatory Responses through the Antioxidant Ability
Abstract
:1. Introduction
2. Results
2.1. 5-TDMF Possesses Weak or no Cytotoxicity toward the Experimental Cell Lines

2.2. 5-TDMF Demonstrates Potent Inhibitory Activity of Lipid Peroxidation
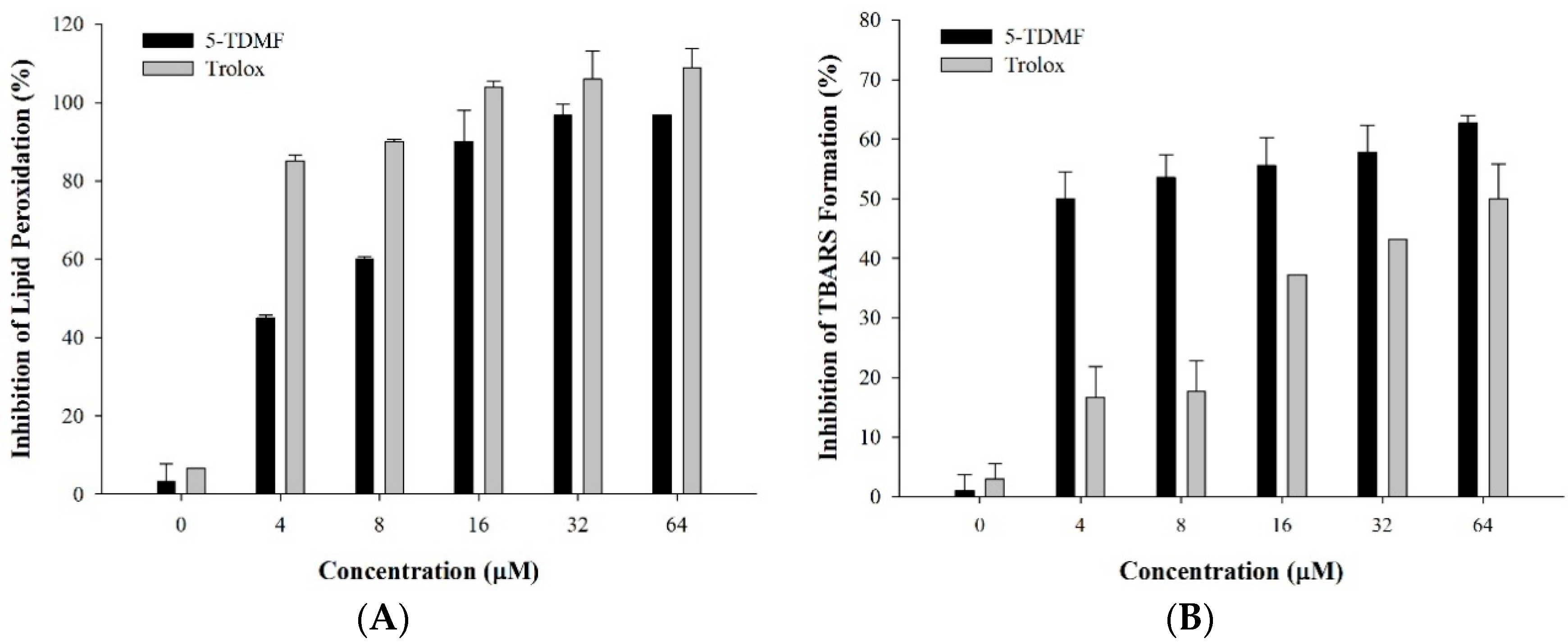
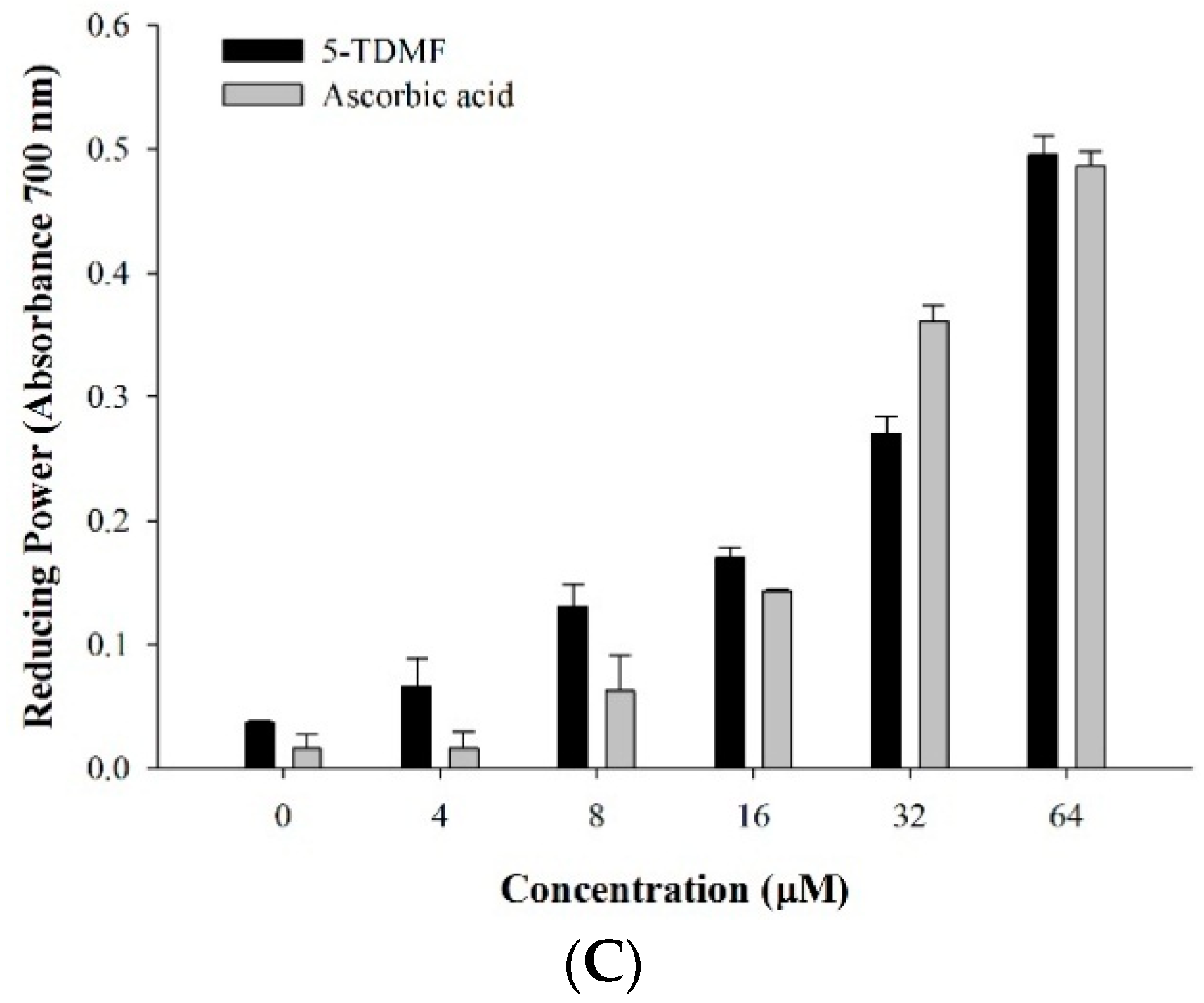
2.3. 5-TDMF is a Potent Free Radical Scavenger and Anti-Oxidant

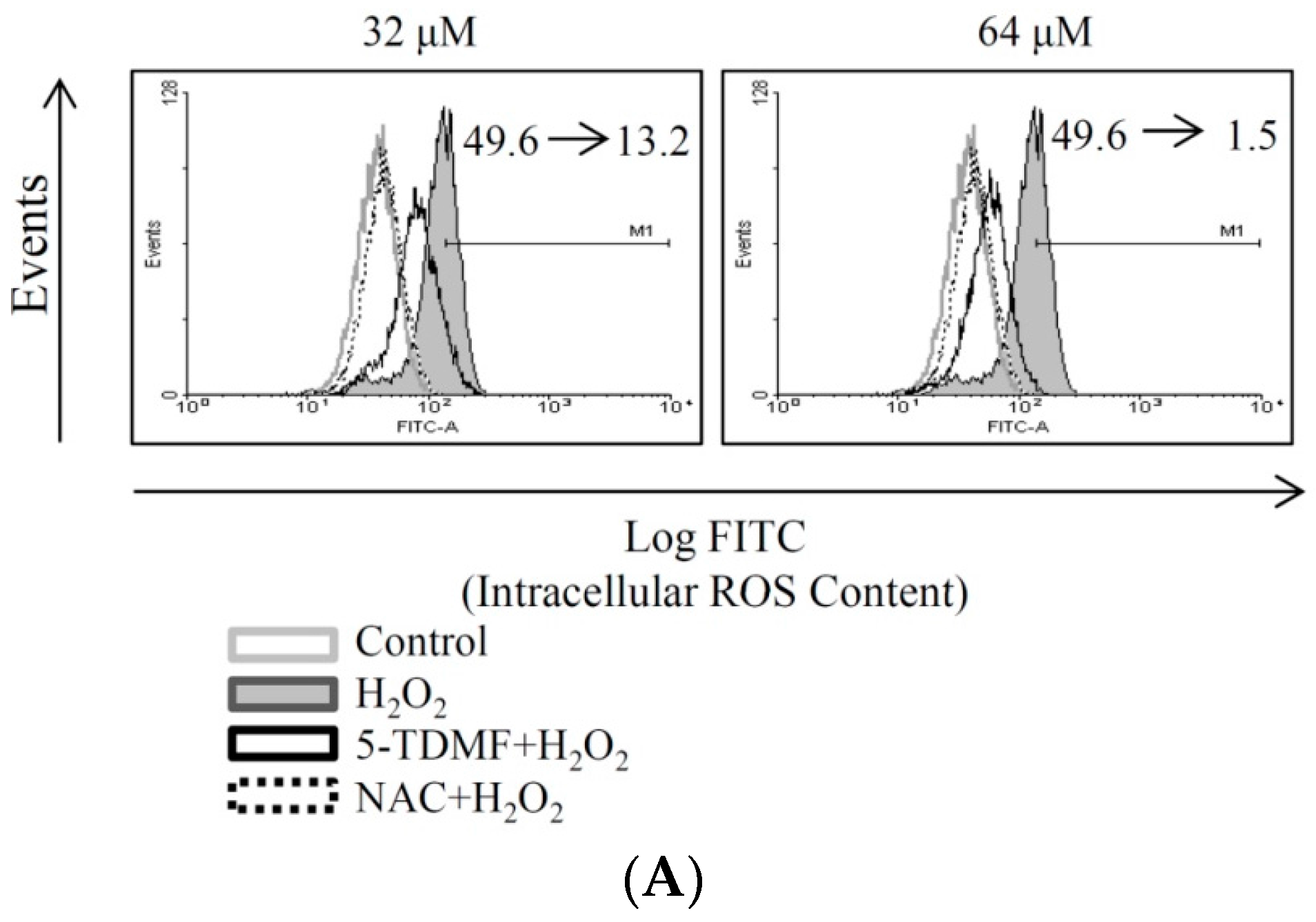
2.4. 5-TDMF Showed Potent Cellular Radical-Scavenging Activity by Suppressing the Intracellular ROS Formation and Up-Regulating GSH Expression

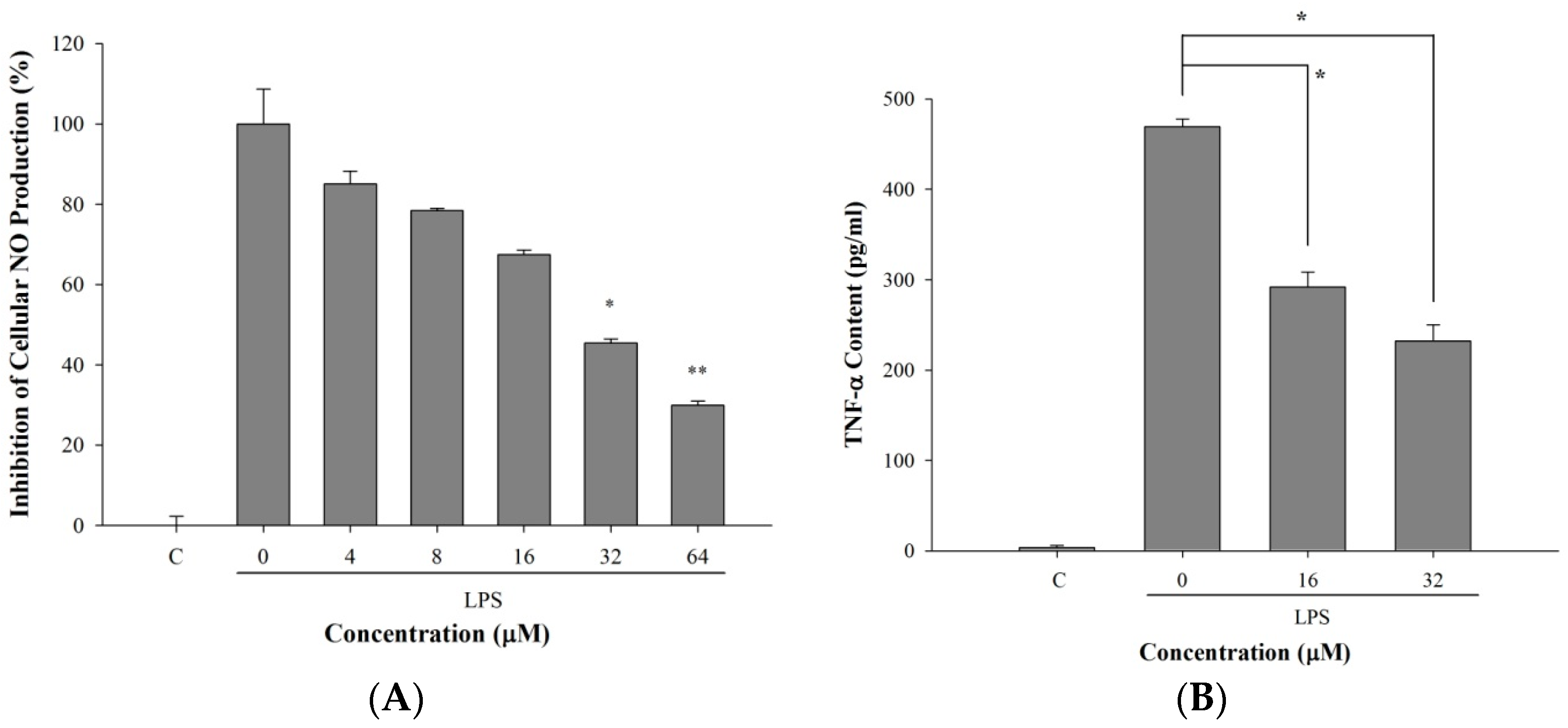
2.5. 5-TDMF Reduced LPS-Induced Inflammatory Responses in RAW 264.7 Cells
2.6. Pre-Treatment with 5-TDMF Can Suppress LPS-Induced NF-κB and MAP Signaling Pathway Resulting in the Down-Regulation of iNOS and COX-2 Expression
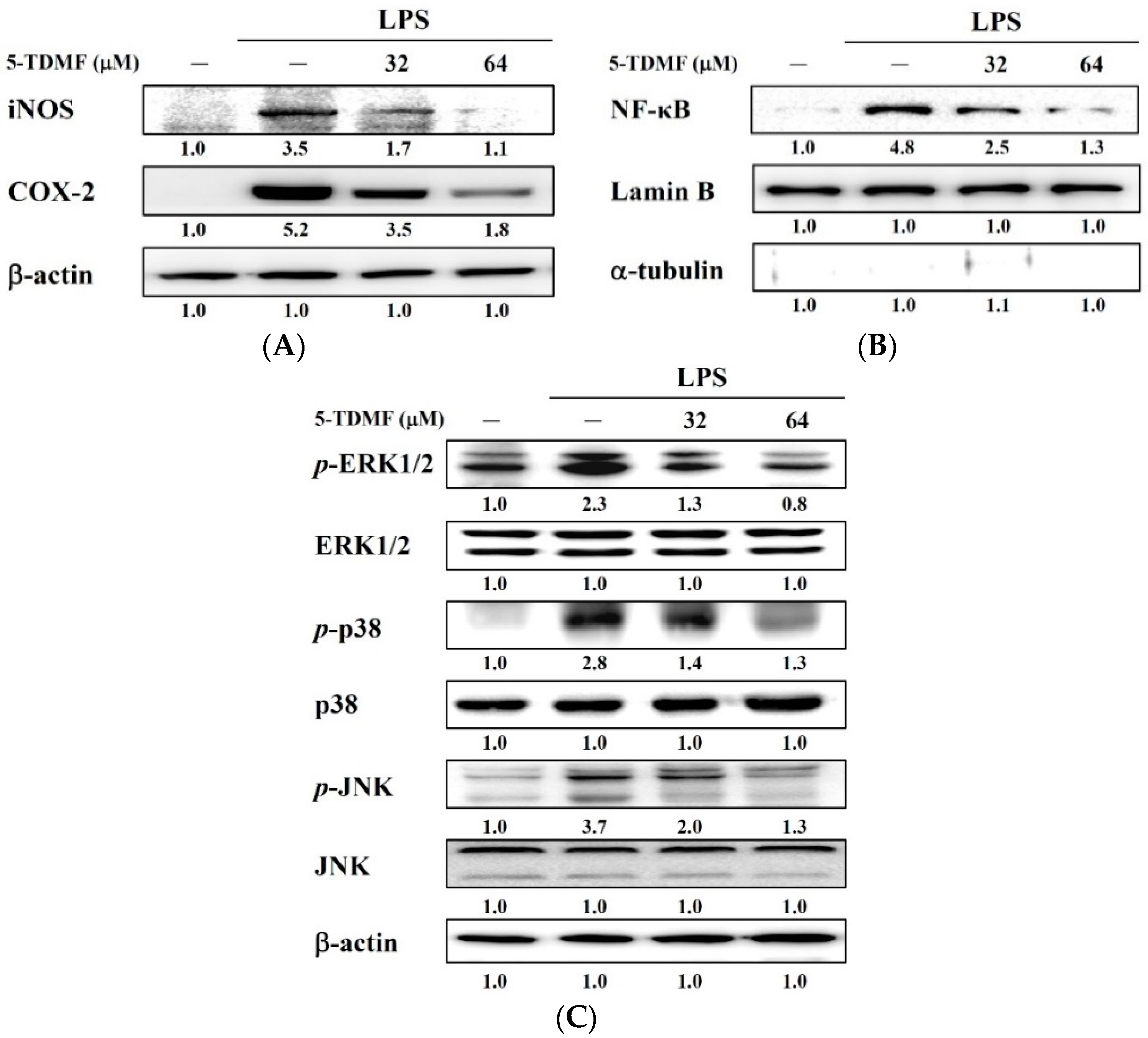
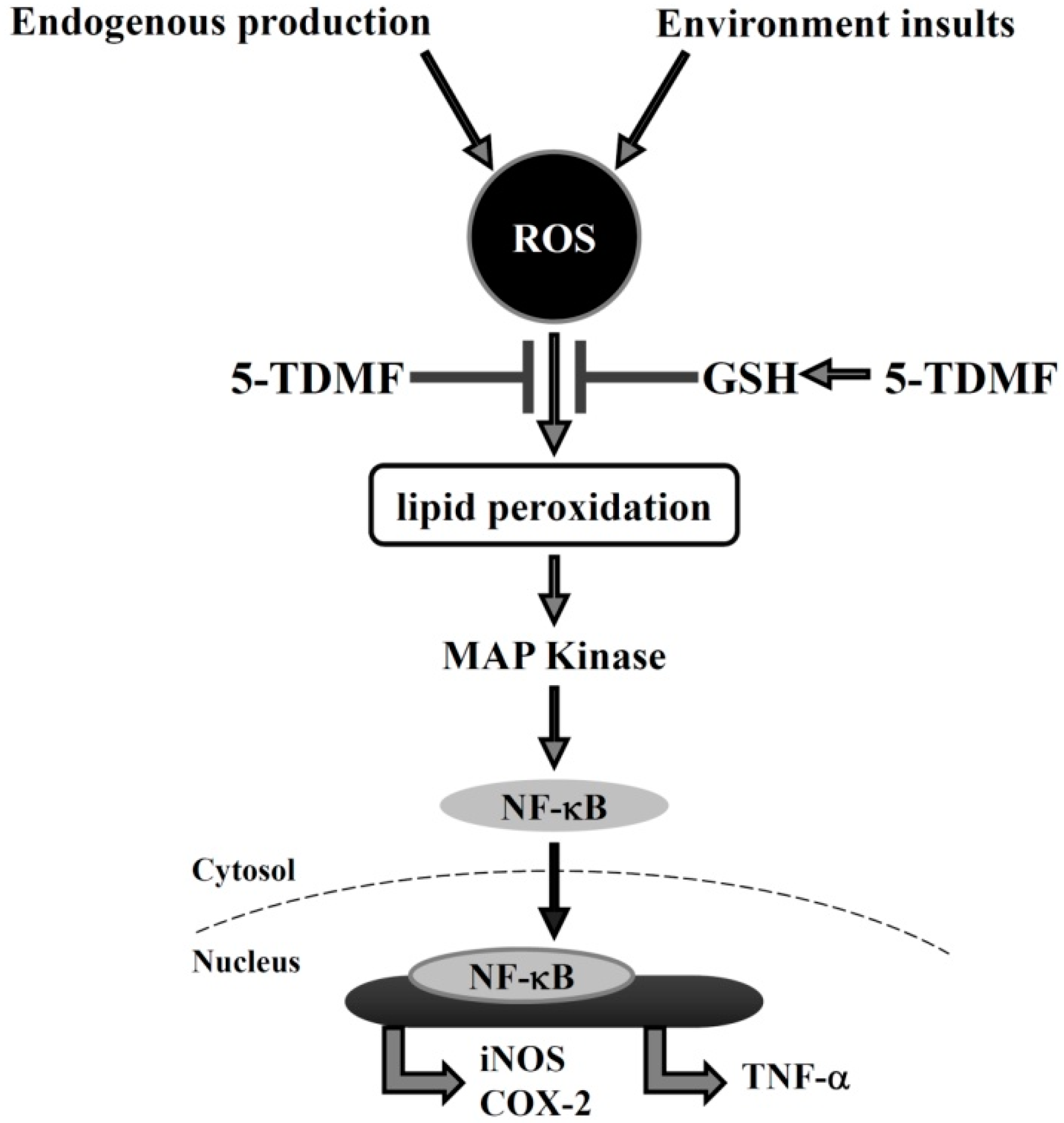
3. Discussion
| Sample | Lipid Peroxidation | TBARS Levels | DPPH• | ABTS•+ |
|---|---|---|---|---|
| EC50 (μM) | EC50 (μM) | EC50 (μM) | EC50 (μM) | |
| 5-TDMF | 4.5 | 3.0 | 26.9 | 47.2 |
| Trolox | 2.4 | 80.0 | 14 | 21.5 |
4. Materials and Methods
4.1. Isolation of 5-TDMF from the Whole Plant of Anisomeles Ovata
4.2. Cell Culture
4.3. Chemicals and Reagents
4.4. Assessment of Cell Viability
4.5. Free radical Scavenging Activity
4.6. Reducing Power
4.7. Inhibition of Lipid Peroxidation Formation
4.8. Inhibition of Thiobarbituric Acid-Reactive Substances Formation
4.9. Measurement of Reactive Oxygen Species and Glutathione Production
4.10. Measurements of LPS-Induced NO Production and Inflammatory Cytokines Production
4.11. Preparation of Cell Lysates and Western Blot Analysis
4.12. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gao, Y.; Jiang, W.; Dong, C.; Li, C.; Fu, X.; Min, L.; Tian, J.; Jin, H.; Shen, J. Anti-inflammatory effects of sophocarpine in LPS-induced RAW 264.7 cells via NF-kappab and mapks signaling pathways. Toxicol. In Vitro 2012, 26, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.W.; Yu, Q.; Liao, L.X.; Song, F.J.; Lv, H.N.; Jiang, Y.; Tu, P.F. Anti-neuroinflammatory effect of MC13, a novel coumarin compound from condiment murraya, through inhibiting lipopolysaccharide-induced TRAF6-TAK1-NF-kappab, p38/ERK mapks and Jak2-Stat1/Stat3 pathways. J. Cell. Biochem. 2015, 116, 1286–1299. [Google Scholar] [CrossRef] [PubMed]
- Bickers, D.R.; Athar, M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Barreira, J.C.; Ferreira, I.C.; Oliveira, M.B.; Pereira, J.A. Antioxidant activity and bioactive compounds of ten portuguese regional and commercial almond cultivars. Food Chem. Toxicol. 2008, 46, 2230–2235. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.H.; Chan, L.P.; Ding, H.Y.; So, E.C.; Lin, R.J.; Wang, H.M.; Chen, Y.G.; Chou, T.H. Free radical scavenging activity of 4-(3,4-dihydroxybenzoyloxymethyl)phenyl-O-beta-d-glucopyranoside from origanum vulgare and its protection against oxidative damage. J. Agric. Food Chem. 2012, 60, 7690–7696. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.C.; Hsieh, C.F.; Boufford, D.E.; Kuoh, C.S.; Ohashi, H.; Peng, C.I.; Tsai, J.L.; Yang, K.C.; Hsiao, A.; Tsai, J.M.E. Floraoftaiwan, 2nd ed.National Science Council of the Republic of China: Taipei, Taiwan, 2003; pp. 437–488.
- Hsieh, S.C.; Fang, S.H.; Rao, Y.K.; Tzeng, Y.M. Inhibition of pro-inflammatory mediators and tumor cell proliferation by anisomeles indica extracts. J. Ethnopharmacol. 2008, 118, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.Y.; Wu, M.L.; Hwang, Y.C.; Chang, F.R.; Wu, Y.C.; Wu, C.C. The natural diterpenoid ovatodiolide induces cell cycle arrest and apoptosis in human oral squamous cell carcinoma Ca9-22 cells. Life Sci. 2009, 85, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.K.; Fang, S.H.; Hsieh, S.C.; Yeh, T.H.; Tzeng, Y.M. The constituents of anisomeles indica and their anti-inflammatory activities. J. Ethnopharmacol. 2009, 121, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Lien, H.M.; Ke, H.J.; Chang, L.L.; Chen, C.C.; Chang, T.M. Antioxidative characteristics of anisomeles indica extract and inhibitory effect of ovatodiolide on melanogenesis. Int. J. Mol. Sci. 2012, 13, 6220–6235. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.F.; Rao, Y.K.; Tzeng, Y.M. Aqueous extract of anisomeles indica and its purified compound exerts anti-metastatic activity through inhibition of NF-kappab/AP-1-dependent MMP-9 activation in human breast cancer MCF-7 cells. Food Chem. Toxicol. 2012, 50, 2930–2936. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Lan, Y.H.; Hsieh, P.W.; Wu, C.C.; Chen, S.L.; Yen, C.T.; Chang, F.R.; Hung, W.C.; Wu, Y.C. Bioactive cembrane diterpenoids of anisomeles indica. J. Nat. Prod. 2008, 71, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Lien, H.M.; Wang, C.Y.; Chang, H.Y.; Huang, C.L.; Peng, M.T.; Sing, Y.T.; Chen, C.C.; Lai, C.H. Bioevaluation of anisomeles indica extracts and their inhibitory effects on helicobacter pylori-mediated inflammation. J. Ethnopharmacol. 2013, 145, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.L.; Ding, H.Y.; Teng, K.N.; Fang, Y.C. 5,6,3′,4′-Tetrahydroxy-7-methoxyflavone as a novel potential proteasome inhibitor. Planta Med. 2010, 76, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, P.; Sudhandiran, G. Protective role of luteolin on the status of lipid peroxidation and antioxidant defense against azoxymethane-induced experimental colon carcinogenesis. Biomed. Pharmacother. 2008, 62, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Mimica-Dukic, N.; Bozin, B.; Sokovic, M.; Simin, N. Antimicrobial and antioxidant activities of melissa officinalis l. (lamiaceae) essential oil. J. Agric. Food Chem. 2004, 52, 2485–2489. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.H.; Ding, H.Y.; Hung, W.J.; Liang, C.H. Antioxidative characteristics and inhibition of alpha-melanocyte-stimulating hormone-stimulated melanogenesis of vanillin and vanillic acid from origanum vulgare. Exp. Dermatol. 2010, 19, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on products of browning reaction—Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric oxide production and signaling in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Gwon, W.G.; Lee, M.S.; Kim, J.S.; Kim, J.I.; Lim, C.W.; Kim, N.G.; Kim, H.R. Hexane fraction from sargassum fulvellum inhibits lipopolysaccharide-induced inducible nitric oxide synthase expression in RAW 264.7 cells via nf-kappab pathways. Am. J. Chin. Med. 2013, 41, 565–584. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [n-15]-labeled nitrate in biological-fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Rajapakse, N.; Kim, M.M.; Mendis, E.; Kim, S.K. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 in lipopolysaccharide-stimulated RAW264.7 cells by carboxybutyrylated glucosamine takes place via down-regulation of mitogen-activated protein kinase-mediated nuclear factor-kappab signaling. Immunology 2008, 123, 348–357. [Google Scholar] [PubMed]
- Kumar, K.J.S.; Hsieh, H.W.; Wang, S.Y. Anti-inflammatory effect of lucidone in mice via inhibition of NF-kappaB/MAP kinase pathway. Int. Immunopharmacol. 2010, 10, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Durackova, Z. Some current insights into oxidative stress. Physiol. Res. 2010, 59, 459–469. [Google Scholar] [PubMed]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Yadav, U.C.; Ramana, K.V. Regulation of NF-kappaB-induced inflammatory signaling by lipid peroxidation-derived aldehydes. Oxid. Med. Cell. Longev. 2013, 2013, 690545. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Lim, S.S.; Kim, J.K.; Kang, I.J.; Kim, J.S.; Lee, C.; Kim, J.; Park, J.H. Hexane-ethanol extract of glycyrrhiza uralensis containing licoricidin inhibits the metastatic capacity of DU145 human prostate cancer cells. Br. J. Nutr. 2010, 104, 1272–1282. [Google Scholar] [CrossRef] [PubMed]
- Gloire, G.; Legrand-Poels, S.; Piette, J. NF-kappaB activation by reactive oxygen species: Fifteen years later. Biochem. Pharmacol. 2006, 72, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Granado-Serrano, A.B.; Martin, M.A.; Bravo, L.; Goya, L.; Ramos, S. Quercetin attenuates tnf-induced inflammation in hepatic cells by inhibiting the NF-kappaB pathway. Nutr. Cancer 2012, 64, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Cheong, Y.K.; Kim, N.H.; Chung, H.T.; Kang, D.G.; Pae, H.O. Mitogen-activated protein kinases and reactive oxygen species: How can ros activate mapk pathways? J. Signal Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef] [PubMed]
- Voirin, B.; Viricel, M.R.; Favre-Bonvin, J.; van den Broucke, C.O.; Lemli, J. 5,6,4′-trihydroxy-7, 3′-dimethoxyflavone and other methoxylated flavonoids isolated from thymus satureiodes. Planta Med. 1985, 51, 523–525. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.B.; Nielsen, S.E.; Berg, K. Re-examination and further development of a precise and rapid dye method for measuring cell-growth cell kill. J. Immunol. Methods 1989, 119, 203–210. [Google Scholar] [CrossRef]
- Ding, H.Y.; Chou, T.H.; Liang, C.H. Antioxidant and antimelanogenic properties of rosmarinic acid methyl ester from origanum vulgare. Food Chem. 2010, 123, 254–262. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Bass, D.A.; Parce, J.W.; Szejda, P.; Seeds, M.C.; Thomas, M.; Dechatelet, L.R. Flow cytometric studies of oxidative product formation by neutrophils—A graded response to membrane stimulation. J. Reticuloendoth. Soc. 1982, 32, 79. [Google Scholar]
- Senft, A.P.; Dalton, T.P.; Shertzer, H.G. Determining glutathione and glutathione disulfide using the fluorescence probe o-phthalaldehyde. Anal. Biochem. 2000, 280, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: 20 mM stock solution of 5-TDMF in DMSO is available from authors upon request.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-H.; Liang, C.-H.; Liang, F.-P.; Ding, H.-Y.; Lin, S.-P.; Huang, G.-J.; Lin, W.-C.; Juang, S.-H. The Inhibitory Mechanisms Study of 5,6,4′-Trihydroxy-7,3′-Dimethoxyflavone against the LPS-Induced Macrophage Inflammatory Responses through the Antioxidant Ability. Molecules 2016, 21, 136. https://doi.org/10.3390/molecules21020136
Wang S-H, Liang C-H, Liang F-P, Ding H-Y, Lin S-P, Huang G-J, Lin W-C, Juang S-H. The Inhibitory Mechanisms Study of 5,6,4′-Trihydroxy-7,3′-Dimethoxyflavone against the LPS-Induced Macrophage Inflammatory Responses through the Antioxidant Ability. Molecules. 2016; 21(2):136. https://doi.org/10.3390/molecules21020136
Chicago/Turabian StyleWang, Shih-Hao, Chia-Hua Liang, Fong-Pin Liang, Hsiou-Yu Ding, Shiuan-Pey Lin, Guan-Jhong Huang, Wen-Chuan Lin, and Shin-Hun Juang. 2016. "The Inhibitory Mechanisms Study of 5,6,4′-Trihydroxy-7,3′-Dimethoxyflavone against the LPS-Induced Macrophage Inflammatory Responses through the Antioxidant Ability" Molecules 21, no. 2: 136. https://doi.org/10.3390/molecules21020136
APA StyleWang, S.-H., Liang, C.-H., Liang, F.-P., Ding, H.-Y., Lin, S.-P., Huang, G.-J., Lin, W.-C., & Juang, S.-H. (2016). The Inhibitory Mechanisms Study of 5,6,4′-Trihydroxy-7,3′-Dimethoxyflavone against the LPS-Induced Macrophage Inflammatory Responses through the Antioxidant Ability. Molecules, 21(2), 136. https://doi.org/10.3390/molecules21020136





