The Cooperative Effect of Genistein and Protein Hydrolysates on the Proliferation and Survival of Osteoblastic Cells (hFOB 1.19)
Abstract
:1. Introduction
2. Results
2.1. Degrees of Hydrolysis and Amino Acid Compositions of the Prepared Hydrolysates
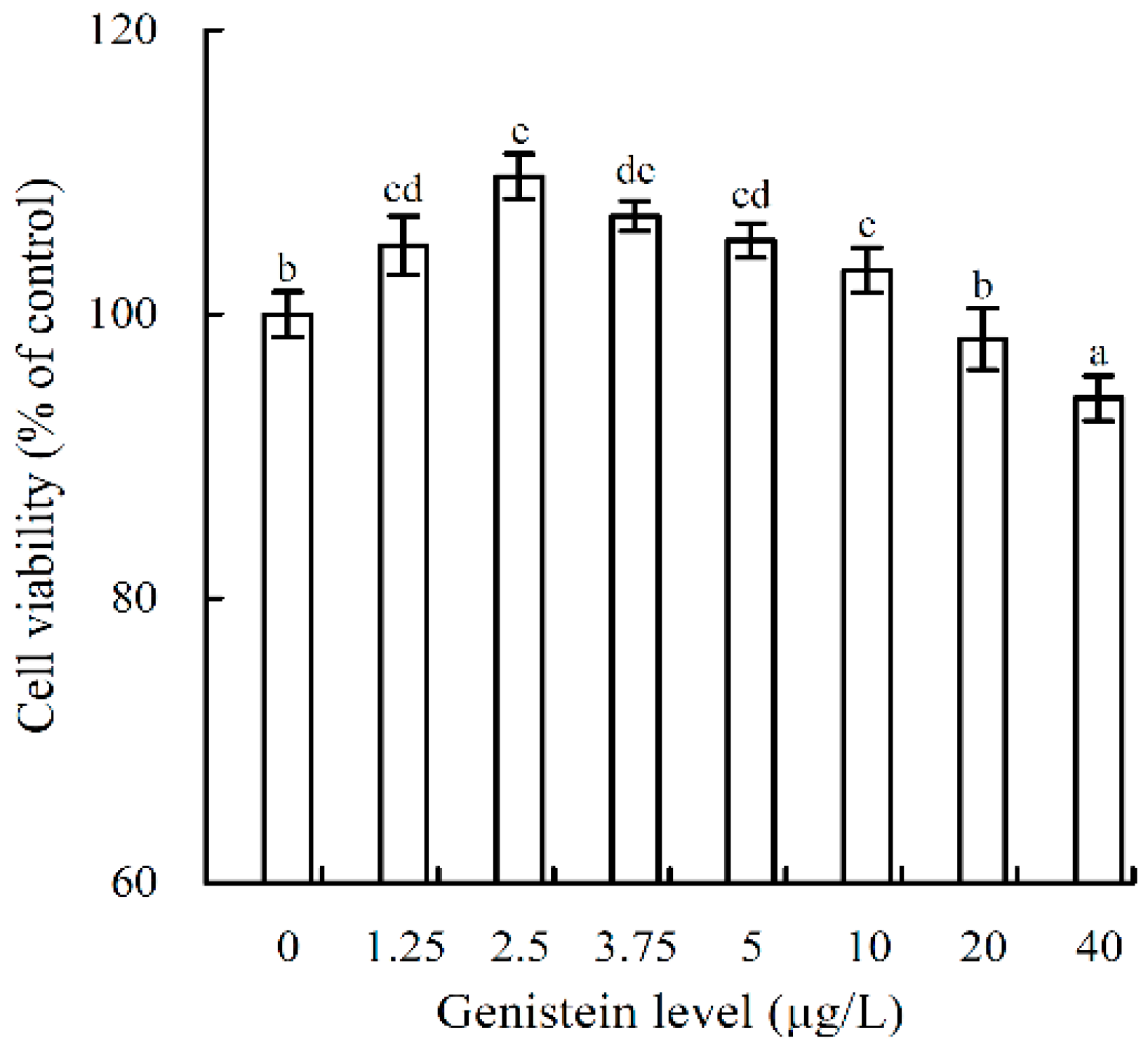
2.2. Growth Proliferation and Inhibition of Genistein on the Osteoblasts
2.3. Cooperation between Genistein and the Hydrolysates in Osteoblast Proliferation
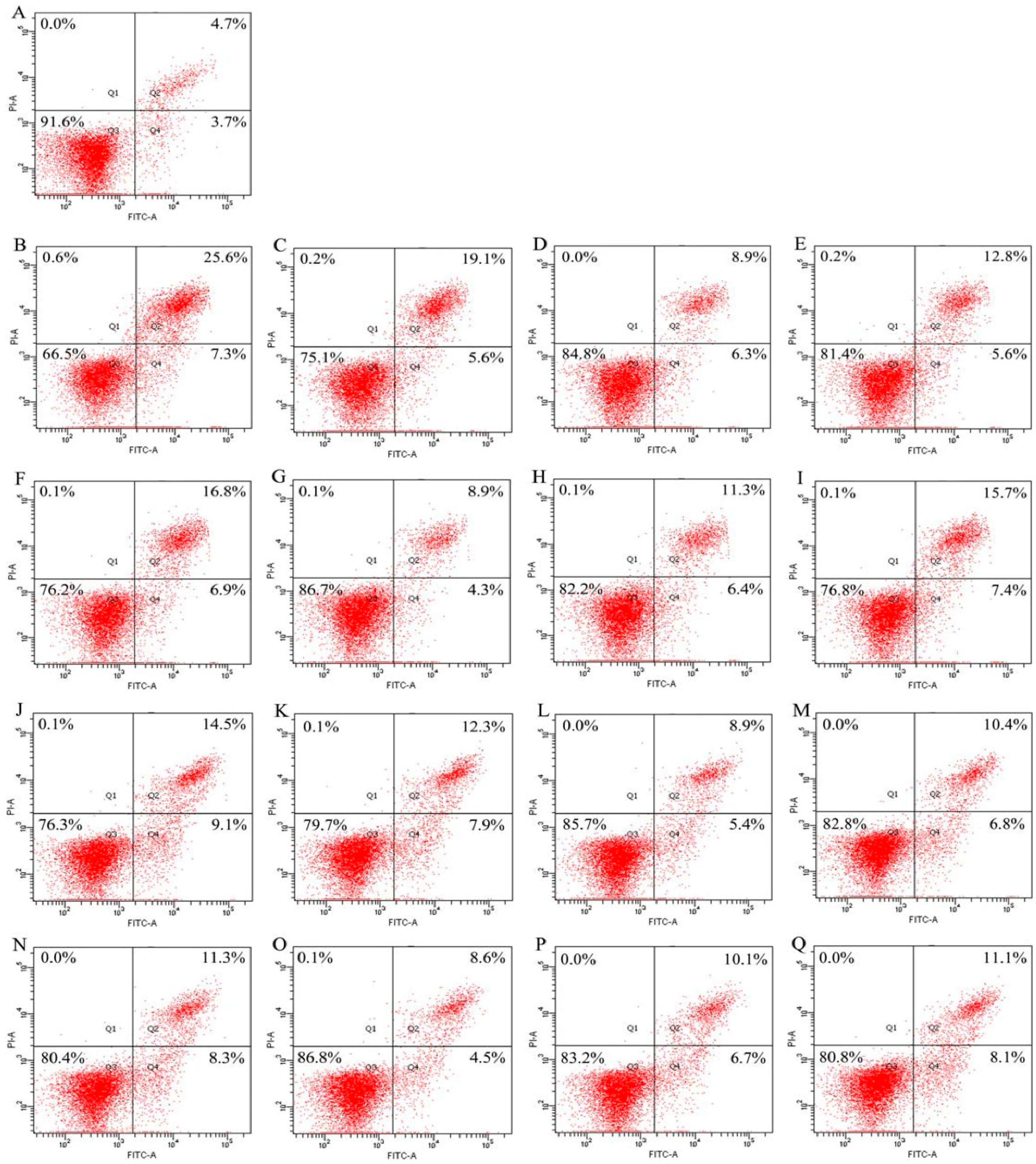
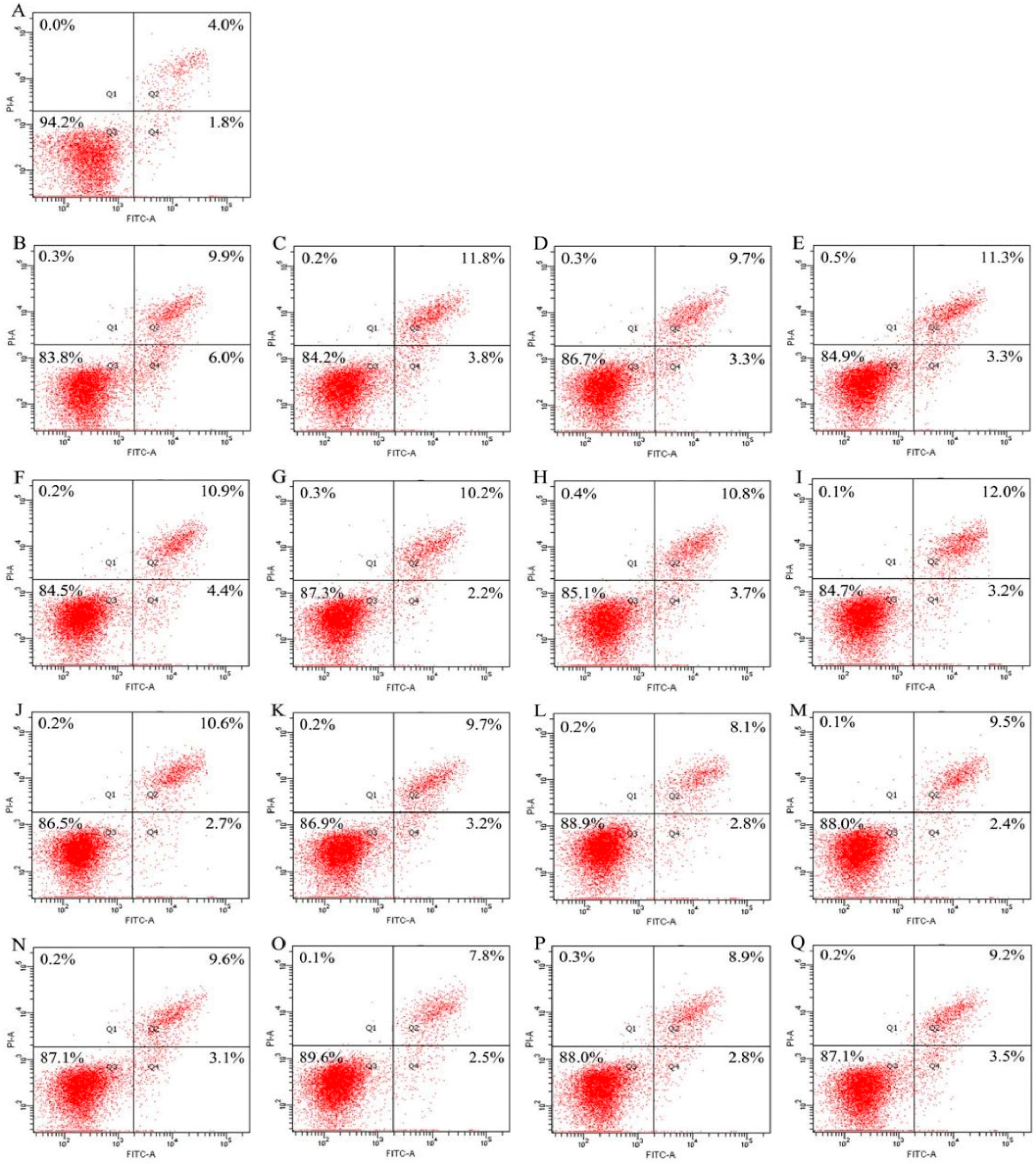
2.4. Cooperation between Genistein and the Hydrolysates in Osteoblast Anti-Apoptosis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of De-Isoflavoned Soy Protein and Protein Hydrolysates
4.3. Cell Line and Culture Conditions
4.4. In Vitro Effect of Genistein on the Osteoblasts
4.5. Assay of Cell Proliferation
4.6. Apoptosis Assay
4.7. Chemical Analyses
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Gen | genistein |
| CH | casein hydrolysate |
| GH | gelatin hydrolysate |
| SH | soy protein hydrolysate |
| DH | degree of hydrolysis |
| CCK-8 | cell counting kit-8 |
| DMSO | dimethyl sulfoxide |
| E | 17β-estradiol |
| WST-8 | 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt |
| EDTA | ethylenediamine tetra-acetic acid |
| EP | etoposide |
| FACS | fluorescence-activated cell sorting |
| FBS | fetal bovine serum |
| hFOB 1.19 cells | human fetal osteoblastic cells |
| OPA | o-phthaldialdehyde |
| PBS | phosphate-buffered saline |
References
- Anonymous. Consensus development conference: Diagnosis, prophylaxis and treatment of osteoporosis. Am. J. Med. 1993, 94, 646–650. [Google Scholar]
- Xu, W.X.; Liu, Y.; Liu, S.Z.; Zhang, Y.; Qiao, G.F.; Yan, J.L. Arsenic trioxide exerts a double effect on osteoblast growth in vitro. Environ. Toxicol. Pharmacol. 2014, 38, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Kruger, M.C.; Wolber, F.M. Osteoporosis: Modern paradigms for last century’s bones. Nutrients 2016, 8, 376. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R. Osteoporosis treatment: Focus on safety. Eur. J. Int. Med. 2013, 24, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.F.; Qin, L.Q.; Wang, P.Y.; Katoh, R. Soy isoflavone intake increases bone mineral density in the spine of menopausal women: Meta-analysis of randomized controlled trials. Clin. Nutr. 2007, 27, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Marini, H.; Minutoli, L.; Polito, F.; Bitto, A.; Altavilla, D.; Atteritano, M.; Gaudio, A.; Mazzaferro, S.; Frisina, A.; Frisina, N.; et al. Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: A randomized trial. Ann. Int. Med. 2007, 146, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.F.; Qin, L.Q.; Wang, P.Y.; Katoh, R. Soy isoflavone intake inhibits bone resorption and stimulates bone formation in menopausal women: Meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2008, 62, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.L.; Hu, Y.C.; Hsieh, B.S.; Cheng, H.L.; Hsu, H.W.; Huang, L.W.; Su, S.J. Combined effect of soy isoflavones and vitamin D3 on bone loss in ovariectomized rats. Nutrition 2013, 29, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Xue, H.G.; Chen, J.Y.; Chai, W.; Ni, M. Genistein induces adipogenic differentiation in human bone marrow mesenchymal stem cells and suppresses their osteogenic potential by upregulating PPARγ. Exp. Ther. Med. 2016, 11, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, R.; Meisel, H. Food-derived peptides with biological activity: From research to food applications. Curr. Opin. Biotechnol. 2007, 18, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, R.W. Role of collagen hydrolysate in bone and joint disease. Semin. Arthritis Rheum. 2000, 30, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Liu, H.; Ren, J.H.; Li, X.; Guo, S.T. The positive effect of soybean protein hydrolysates-calcium complexes on bone mass of rapidly growing rats. Food Funct. 2013, 4, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Tulipano, G.; Bulgari, O.; Chessa, S.; Nardone, A.; Cocchi, D.; Caroli, A. Direct effects of casein phosphopeptides on growth and differentiation of in vitro cultured osteoblastic cells (MC3T3-E1). Regul. Pept. 2010, 160, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Donida, B.M.; Mrak, E.; Gravaghi, C.; Villa, I.; Cosentino, S.; Zacchi, E.; Perego, S.; Rubinacci, A.; Fiorilli, A.; Tettamanti, G.; et al. Casein phosphopeptides promote calcium uptake and modulate the differentiation pathway in human primary osteoblast-like cells. Peptides 2009, 30, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Graef, G.L.; Nielsen, F.H.; Johnson, L.K.; Cao, J. Soy protein is beneficial but high-fat diet and voluntary running are detrimental to bone structure in mice. Nutr. Res. 2015, 35, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.R.; Zhang, J.; Lazarenko, O.P.; Cao, J.J.; Blackburn, M.L.; Badger, T.M.; Ronis, M.J.J. Soy protein isolates prevent loss of bone quantity associated with obesity in rats through regulation of insulin signaling in osteoblasts. FASEB J. 2013, 27, 3514–3523. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.M.; Racette, S.B.; Van Pelt, R.E.; Peterson, L.R.; Villareal, D.T. Effects of soy protein isolate and moderate exercise on bone turnover and bone mineral density in postmenopausal women. Menopause 2007, 14, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Guillerminet, F.; Beaupied, H.; Fabien-soule, V.; Tome, D.; Benhamou, C.L.; Roux, C.; Blais, A. Hydrolyzed collagen improves bone metabolism and biomechanical parameters in ovariectomized mice: An in vitro and in vivo study. Bone 2010, 46, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Watanabe-kamiyama, M.; Shimizu, M.; Kamiyama, S.; Taguchi, Y.; Sone, H.; Morimatsu, F.; Shirakawa, H.; Furukawa, Y.; Komai, M. Absorption and effectiveness of orally administered low molecular weight collagen hydrolysate in rats. J. Agric. Food Chem. 2010, 58, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Ham, J.S.; Kim, H.K.; Lee, C.H.; Kim, D.W.; Seol, K.H.; Oh, M.H.; Kim, D.H.; Jang, A. Effect of pig skin gelatin hydrolysates on the bone mineral density of ovariectomized rats. Korean J. Food Sci. Anim. Resour. 2012, 32, 234–240. [Google Scholar] [CrossRef]
- Jackix, E.D.; Cuneo, F.; Amaya-Farfan, J.; de Assuncao, J.V.; Quintaes, K.D. A food supplement of hydrolyzed collagen improves compositional and biodynamic characteristics of vertebrae in ovariectomized rats. J. Med. Food 2010, 13, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.K.; Kim, S.; Imm, J.Y.; Whang, K.Y. Whey protein concentrate hydrolysate prevents bone loss in ovariectomized rats. J. Med. Food 2015, 18, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhao, X.H. In vitro responses of hFOB 1.19 cells towards chum salmon (Oncorhynchus keta) skin gelatin hydrolysates in cell proliferation, cycle progression and apoptosis. J. Funct. Foods 2013, 5, 279–288. [Google Scholar] [CrossRef]
- Ohara, H.; Matsumoto, H.; Ito, K.; Iwai, K.; Sato, K. Comparison of quantity and structures of hydroxyproline-containing peptides in human blood after oral ingestion of gelatin hydrolysates from different sources. J. Agric. Food Chem. 2007, 55, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Fujioka, M.; Sugimoto, K.; Mu, G.; Ishimi, Y. Assessment of effectiveness of oral administration of collagen peptide on bone metabolism in growing and mature rats. J. Bone Miner. Metab. 2004, 22, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.W.; Zhao, X.H. In vitro proliferation and anti-apoptosis of the papain-generated casein and soy protein hydrolysates towards osteoblastic cells (hFOB 1.19). Int. J. Mol. Sci. 2015, 16, 13908–13920. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.A.; Enger, R.J.; Riggs, L.B.; Spelsberg, T.C. Development and characterization of a conditionally immortalized human fetal osteoblastic cell line. J. Bone Miner. Res. 1995, 10, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, M.; Jalal, S.M.; Rickard, D.J.; Harris, S.A.; Bolander, M.E.; Spelsberg, T.C. Further characterization of human fetal osteoblastic hFOB 1.19 and hFOB/ERα cells: Bone formation in vivo and karyotype analysis using multicolor fluorescent in situ hybridization. J. Cell. Biochem. 2002, 87, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Hock, J.M.; Krishnan, V.; Onyia, J.E.; Bidwell, J.P.; Milas, J.; Stanislaus, D. Osteoblast apoptosis and bone turnover. J. Bone Miner. Res. 2001, 16, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C. Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 2000, 21, 115–137. [Google Scholar] [CrossRef] [PubMed]
- Moriishi, T.; Maruyama, Z.; Fukuyama, R.; Ito, M.; Miyazaki, T.; Kitaura, H.; Ohnishi, H.; Furuichi, T.; Kawai, Y.; Masuyama, R.; et al. Overexpression of Bcl2 in osteoblasts inhibits osteoblast differentiation and induces osteocyte apoptosis. PLoS ONE 2011, 6, e27487. [Google Scholar] [CrossRef] [PubMed]
- Ishimi, Y. Osteoporosis and lifestyle. J. Nutr. Sci. Vitaminol. 2015, 61, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, T.; Onouchi, T.; Takahashi, M.; Ito, H.; Orimo, H. Effect of soy protein on bone metabolism in postmenopausal Japanese women. Osteoporos. Int. 2000, 11, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.C.; Woo, J.; Lam, S.; Chen, Y.; Sham, A.; Lau, J. Soy protein consumption and bone mass in early postmenopausal Chinese women. Osteoporos. Int. 2003, 14, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Oka, J.; Tabata, I.; Higuchi, M.; Toda, T.; Fuku, N.; Ezaki, J.; Sugiyama, F.; Uchiyama, S.; Yamada, K.; et al. Effects of isoflavone and exercise on BMD and fat mass in postmenopausal Japanese women: A 1-year randomized placebo-controlled trial. J. Bone Miner. Res. 2006, 21, 780–789. [Google Scholar] [CrossRef] [PubMed]
- King, T.J.; Shandala, T.; Lee, A.M.; Foster, B.K.; Chen, K.M.; Howe, P.R.; Xian, C.J. Potential effects of phytoestrogen genistein in modulating acute methotrexate chemotherapy-induced osteoclastogenesis and bone damage in rats. Int. J. Mol. Sci. 2015, 16, 18293–18311. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Liu, Z.H.; Tong, Z.H.; Zhao, Z.N.; Liang, H.D. Soybean isoflavone treatment induces osteoblast differentiation and proliferation by regulating analysis of Wnt/β-catenin pathway. Gene 2015, 573, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.; Thorpe, J.; Ambrosio, L.; Santin, M. The soybean isoflavone genistein induces differentiation of MG63 human osteosarcoma osteoblasts. J. Nutr. 2006, 136, 1166–1170. [Google Scholar] [PubMed]
- Fu, Y.; Zhao, X.H. Utilization of chum salmon (Oncorhynchus keta) skin gelatin hydrolysates to attenuate hydrogen peroxide-induced oxidative injury in rat hepatocyte BRL cell model. J. Aquat. Food Prod. Technol. 2015, 24, 648–660. [Google Scholar] [CrossRef]
- Fu, Y.; Young, J.F.; Løkke, M.M.; Lametsch, R.; Aluko, R.E.; Therkildsen, M. Revalorisation of bovine collagen as a potential precursor of angiotensin I-converting enzyme (ACE) inhibitory peptides based on in silico and in vitro protein digestions. J. Funct. Foods 2016, 24, 196–206. [Google Scholar] [CrossRef]
- Liu, J.L.; Zhang, B.; Song, S.J.; Ma, M.; Si, S.Y.; Wang, Y.H.; Xu, B.X.; Feng, K.; Wu, J.G.; Guo, Y.C. Bovine collagen peptides compounds promote the proliferation and differentiation of MC3T3-E1 pre-osteoblasts. PLoS ONE 2014, 9, e99920. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Oohashi, K.; Watanabe, M.; Kasugai, S. Increase in bone mineral density through oral administration of shark gelatin to ovariectornized rats. Nutrition 2005, 21, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Budek, A.Z.; Bjornvad, C.R.; Molgaard, C.; Bugel, S.; Vestergaard, M.; Pulkkinen, P.; Michaelsen, K.; Sangild, P.T. Effects of casein, whey and soy proteins on volumetric bone density and bone strength in immunocompromised piglets. e-SPEN Eur. J. Clin. Nutr. Metab. 2007, 2, 57–62. [Google Scholar] [CrossRef]
- Terajima, M.; Perdivara, I.; Sricholpech, M.; Deguchi, Y.; Pleshko, N.; Tomer, K.B.; Yamauchi, M. Glycosylation and cross-linking in bone type I collagen. J. Biol. Chem. 2014, 289, 22636–22647. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.K.; Kuan, C.Y. Development of 4-hydroxyproline analysis kit and its application to collagen quantification. Food Chem. 2010, 119, 1271–1277. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Nanbu, P.N.; Kurokawa, M. Distribution of prolylhydroxyproline and its metabolites after oral administration in rats. Biol. Pharm. Bull. 2012, 35, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, S.; Mano, H.; Sampei, C.; Shimizu, J.; Wada, M. Chondroprotective effect of the bioactive peptide prolyl-hydroxyproline in mouse articular cartilage in vitro and in vivo. Osteoarthr. Cartil. 2009, 17, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Ohara, H.; Iida, H.; Ito, K.; Takeuchi, Y.; Nomura, Y. Effects of Pro-Hyp, a collagen hydrolysate-derived peptide, on hyaluronic acid synthesis using in vitro cultured synovium cells and oral ingestion of collagen hydrolysates in a guinea pig model of osteoarthritis. Biosci. Biotechnol. Biochem. 2010, 74, 2096–2099. [Google Scholar] [CrossRef] [PubMed]
- Tousen, Y.; Matsumoto, Y.; Matsumoto, C.; Nishide, Y.; Nagahata, Y.; Kobayashi, I.; Ishimi, Y. The combined effects of soya isoflavones and resistant starch on equol production and trabecular bone loss in ovariectomised mice. Br. J. Nutr. 2016, 116, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Morales, O.; Samuelsson, M.K.; Lindgren, U.; Haldosen, L.A. Effects of 1α, 25-dihydroxyvitamin D3 and growth hormone on apoptosis and proliferation in UMR 106 osteoblast-like cells. Endocrinology 2004, 145, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Tousen, Y.; Nishide, Y.; Tadaishi, M.; Kato, K.; Ishimi, Y. Combined effects of soy isoflavones and milk basic protein on bone mineral density in hind-limb unloaded mice. J. Clin. Biochem. Nutr. 2016, 58, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wu, N.N.; Peng, J.; Yang, X.Q.; Guo, J.; Yin, S.W.; Wang, J.M. Prevention of retinoic acid-induced osteoporosis in mice by isoflavone-enriched soy protein. J. Sci. Food Agric. 2016, 96, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Jilka, R.L.; Weinstein, R.S.; Bellido, T.; Parfitt, A.M.; Manolagas, S.C. Osteoblast programmed cell death (apoptosis): Modulation by growth factors and cytokines. J. Bone Miner. Res. 1998, 13, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Varshavsky, A. The N-end rule and regulation of apoptosis. Nat. Cell Biol. 2003, 5, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Varshavsky, A. The N-end rule pathway and regulation by proteolysis. Protein Sci. 2011, 20, 1298–1345. [Google Scholar] [CrossRef] [PubMed]
- Piatkov, K.I.; Brower, C.S.; Varshavsky, A. The N-end rule pathway counteracts cell death by destroying proapoptotic protein fragments. Proc. Natl. Acad. Sci. USA 2012, 109, 1839–1847. [Google Scholar] [CrossRef] [PubMed]
- Eldeeb, M.A.; Fahlman, R.P. The anti-apoptotic form of tyrosine kinase Lyn that is generated by proteolysis is degraded by the N-end rule pathway. Oncotarget 2014, 5, 2714–2722. [Google Scholar] [CrossRef] [PubMed]
- Eldeeb, M.A.; Fahlman, R.P. Phosphorylation impacts N-end rule degradation of the proteolytically activated form of BMX kinase. J. Biol. Chem. 2016, 291, 22757–22768. [Google Scholar] [CrossRef] [PubMed]
- Eldeeb, M.; Fahlman, R.P. The-N-End Rule: The beginning determines the end. Protein Pept. Lett. 2016, 23, 343–348. [Google Scholar] [CrossRef]
- Jiang, S.J.; Zhao, X.H. Transglutaminase-induced cross-linking and glucosamine conjugation in soy protein isolates and its impacts on some functional properties of the products. Eur. Food Res. Technol. 2010, 231, 679–689. [Google Scholar] [CrossRef]
- Eckert, R.L.; Katzenellenbogen, B.S. Effect of estrogens and antiestrogen receptor dynamics and the induction of progesterone receptor in MCF-7 human breast cancer cells. Cancer Res. 1982, 42, 139–144. [Google Scholar] [PubMed]
- Zhang, J.; Liu, L.; Mu, X.M.; Jiang, Z.Z.; Zhang, L.Y. Effect of triptolide on estradiol release from cultured rat granulose cells. Endocr. J. 2012, 59, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, C.; Cnicoletti, I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat. Protoc. 2006, 1, 1458–1461. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of Association of Official Analytical Chemists International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Church, F.C.; Swaisgood, H.E.; Porter, D.H.; Catignani, G.L. Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk protein. J. Dairy Sci. 1983, 66, 1219–1227. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Bergman, I.; Loxley, R. Two improved and simplified methods for the spectrophotometric determination of hydroxyproline. Anal. Chem. 1963, 35, 1961–1965. [Google Scholar] [CrossRef]
- Basha, S.M.M.; Roberts, R.M. A simple colorimetric method for the determination of tryptophan. Anal. Biochem. 1977, 77, 378–386. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds genistein and protein hydrolysates are available from the authors.

| Amino Acids | Hydrolysates | Amino Acids | Hydrolysates | ||||
|---|---|---|---|---|---|---|---|
| GH2 | SH2 | CH2 | GH2 | SH2 | CH2 | ||
| Ala | 100.1 | 39.0 | 31.2 | Lys | 37.7 | 73.7 | 80.2 |
| Arg | 90.0 | 87.6 | 34.8 | Met | 8.1 | 14.1 | 32.0 |
| Asp | 54.1 | 128.1 | 77.2 | Phe | 22.6 | 52.1 | 51.2 |
| Cys | ND | 14.2 | ND | Pro | 146.4 | 61.2 | 109.2 |
| Glu | 112.9 | 245.5 | 229.5 | Ser | 34.7 | 59.8 | 59.8 |
| Gly | 266.1 | 47.1 | 21.8 | Thr | 19.2 | 39.4 | 46.9 |
| His | 6.2 | 25.5 | 27.3 | Trp | ND | 12.5 | 14.6 |
| 4-Hyp | 110.7 | ND | ND | Tyr | 0.5 | 34.8 | 56.7 |
| Ile | 16.2 | 46.8 | 55.1 | Val | 31.5 | 44.5 | 67.3 |
| Groups | Dose Levels | Treatment Times | |
|---|---|---|---|
| 48 h | 72 h | ||
| Gen | 2.5 μg/L | 109.4 ± 4.1 | 112.3 ± 6.0 |
| 17β-Estradiol | 10−8 mol/L | 134.2 ± 2.9 | 126.3 ± 3.5 |
| GH1 | 0.02 g/L | 108.4 ± 2.6 | 109.4 ± 1.6 |
| GH1 | 0.05 g/L | 121.8 ± 2.5 | 117.9 ± 2.2 |
| GH1 | 0.1 g/L | 114.6 ± 1.9 | 113.2 ± 1.4 |
| Gen-GH1 | 2.5 μg/L+ 0.02 g/L | 113.8 ± 1.9 | 115.1 ± 2.2 |
| Gen-GH1 | 2.5 μg/L + 0.05 g/L | 127.2 ± 2.6 | 123.1 ± 2.8 |
| Gen-GH1 | 2.5 μg/L + 0.1 g/L | 119.2 ± 1.3 | 117.9 ± 2.9 |
| GH2 | 0.02 g/L | 122.7 ± 2.8 | 110.0 ± 1.4 |
| GH2 | 0.05 g/L | 131.1 ± 4.3 | 125.3 ± 2.1 |
| GH2 | 0.1 g/L | 124.0 ± 2.0 | 116.7 ± 1.4 |
| Gen-GH2 | 2.5 μg/L + 0.02 g/L | 125.3 ± 3.4 | 115.8 ± 2.1 |
| Gen-GH2 | 2.5 μg/L + 0.05 g/L | 140.9 ± 1.3 | 131.2 ± 4.1 |
| Gen-GH2 | 2.5 μg/L + 0.1 g/L | 130.2 ± 2.3 | 122.2 ± 3.6 |
| SH1 | 0.02 g/L | 113.2 ± 5.7 | 115.8 ± 6.2 |
| SH1 | 0.05 g/L | 110.4 ± 4.1 | 110.9 ± 2.6 |
| SH1 | 0.1 g/L | 104.6 ± 6.0 | 104.6 ± 0.8 |
| Gen-SH1 | 2.5 μg/L + 0.02 g/L | 114.6 ± 4.5 | 118.7 ± 4.4 |
| Gen-SH1 | 2.5 μg/L + 0.05 g/L | 111.1 ± 4.4 | 114.8 ± 4.7 |
| Gen-SH1 | 2.5 μg/L + 0.1 g/L | 104.8 ± 3.2 | 108.7 ± 2.2 |
| SH2 | 0.02 g/L | 119.8 ± 5.7 | 107.7 ± 3.3 |
| SH2 | 0.05 g/L | 121.3 ± 2.4 | 110.6 ± 5.6 |
| SH2 | 0.1 g/L | 118.0 ± 1.2 | 104.4 ± 2.2 |
| Gen-SH2 | 2.5 μg/L + 0.02 g/L | 121.1 ± 1.2 | 110.9 ± 3.3 |
| Gen-SH2 | 2.5 μg/L + 0.05 g/L | 123.1 ± 0.9 | 112.3 ± 3.4 |
| Gen-SH2 | 2.5 μg/L + 0.1 g/L | 119.6 ± 1.8 | 107.1 ± 3.9 |
| CH1 | 0.02 g/L | 107.6 ± 4.9 | 106.6 ± 5.1 |
| CH1 | 0.05 g/L | 108.6 ± 4.4 | 105.9 ± 5.8 |
| CH1 | 0.1 g/L | 102.9 ± 6.6 | 105.1 ± 5.6 |
| Gen-CH1 | 2.5 μg/L + 0.02 g/L | 108.6 ± 3.3 | 108.1 ± 4.4 |
| Gen-CH1 | 2.5 μg/L + 0.05 g/L | 110.5 ± 2.9 | 107.4 ± 1.3 |
| Gen-CH1 | 2.5 μg/L + 0.1 g/L | 101.9 ± 4.9 | 104.4 ± 4.6 |
| CH2 | 0.02 g/L | 107.9 ± 3.9 | 108.0 ± 3.3 |
| CH2 | 0.05 g/L | 112.4 ± 5.1 | 114.6 ± 4.6 |
| CH2 | 0.1 g/L | 105.6 ± 1.9 | 113.1 ± 6.7 |
| Gen-CH2 | 2.5 μg/L + 0.02 g/L | 107.9 ± 7.0 | 107.3 ± 5.8 |
| Gen-CH2 | 2.5 μg/L + 0.05 g/L | 114.6 ± 5.1 | 116.8 ± 5.5 |
| Gen-CH2 | 2.5 μg/L + 0.1 g/L | 106.7 ± 3.4 | 113.9 ± 3.8 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Fu, Y.; Zhao, X.-H. The Cooperative Effect of Genistein and Protein Hydrolysates on the Proliferation and Survival of Osteoblastic Cells (hFOB 1.19). Molecules 2016, 21, 1489. https://doi.org/10.3390/molecules21111489
Wang S, Fu Y, Zhao X-H. The Cooperative Effect of Genistein and Protein Hydrolysates on the Proliferation and Survival of Osteoblastic Cells (hFOB 1.19). Molecules. 2016; 21(11):1489. https://doi.org/10.3390/molecules21111489
Chicago/Turabian StyleWang, Shuo, Yu Fu, and Xin-Huai Zhao. 2016. "The Cooperative Effect of Genistein and Protein Hydrolysates on the Proliferation and Survival of Osteoblastic Cells (hFOB 1.19)" Molecules 21, no. 11: 1489. https://doi.org/10.3390/molecules21111489
APA StyleWang, S., Fu, Y., & Zhao, X.-H. (2016). The Cooperative Effect of Genistein and Protein Hydrolysates on the Proliferation and Survival of Osteoblastic Cells (hFOB 1.19). Molecules, 21(11), 1489. https://doi.org/10.3390/molecules21111489






