1. Introduction
Agarwood or eaglewood (also known as
chen xiang in China,
agar in India,
oud in the Middle East,
gaharu in the South East Asia and
jinkoh in Japan) is the resinous wood of the
Aquilaria spp. trees. It is actually an angiosperm existing in the Thymelaeaceae family [
1]. This cherished fragrant type of wood has for long been used as an incense, especially among the Buddhist, Hindu as well as for Islamic ceremonies [
2]. Moreover, agarwood also plays a fundamental role in the traditional Chinese medicine due to of its medicinal value. It is considered to have sedative as well as carminative properties. Again, it has been used in relieving gastric problems, coughs, anti-emetic effects, rheumatism as well as high fever [
2,
3]. In China, the main source of agarwood is the Aquilaria sinensis, a large evergreen tree, distributed in the Hainan, Guangdong, Guangxi, and Fujian provinces.
Agarwood mainly forms in the wood tissues of wild or cultivated Aquilaria trees after wounding. This can mainly be caused by external factors like physical injury, insect gnawing, or microbial infection [
4]. Usually, the tree takes several years to form the agarwood around the tissues wound. A lot of factors have led to the depletion of wild
Aquilaria trees. These include agarwood’s immense value and rarity, indiscriminate cutting of trees, as well as over-harvesting. Our laboratory has therefore patented an effective method, which is the whole-tree agarwood inducing technique (Agar-Wit) [
2,
5,
6], in China. In fact, it is currently being filed for international patent. In this technique, there were small holes deep into the xylem drilled into the main trunk of
Aquilaira tree by use of an electric drill. The agarwood inducer was then slowly injected into the xylem tissues through a simple and cheap transfusion set to induce formation of high-quality agarwood in a shorter time compared to other conventional techniques.
Previous phytochemical investigations on the Chinese agarwood revealed that chromone derivatives are among the main chemical components [
7]. Some of these chemical components were also found to possess significant anti-inflammatory activity [
8]. Nonetheless, chemical constituents of the Chinese agarwood were induced by the Agar-Wit technique from
A. sinensis to contain just few reports [
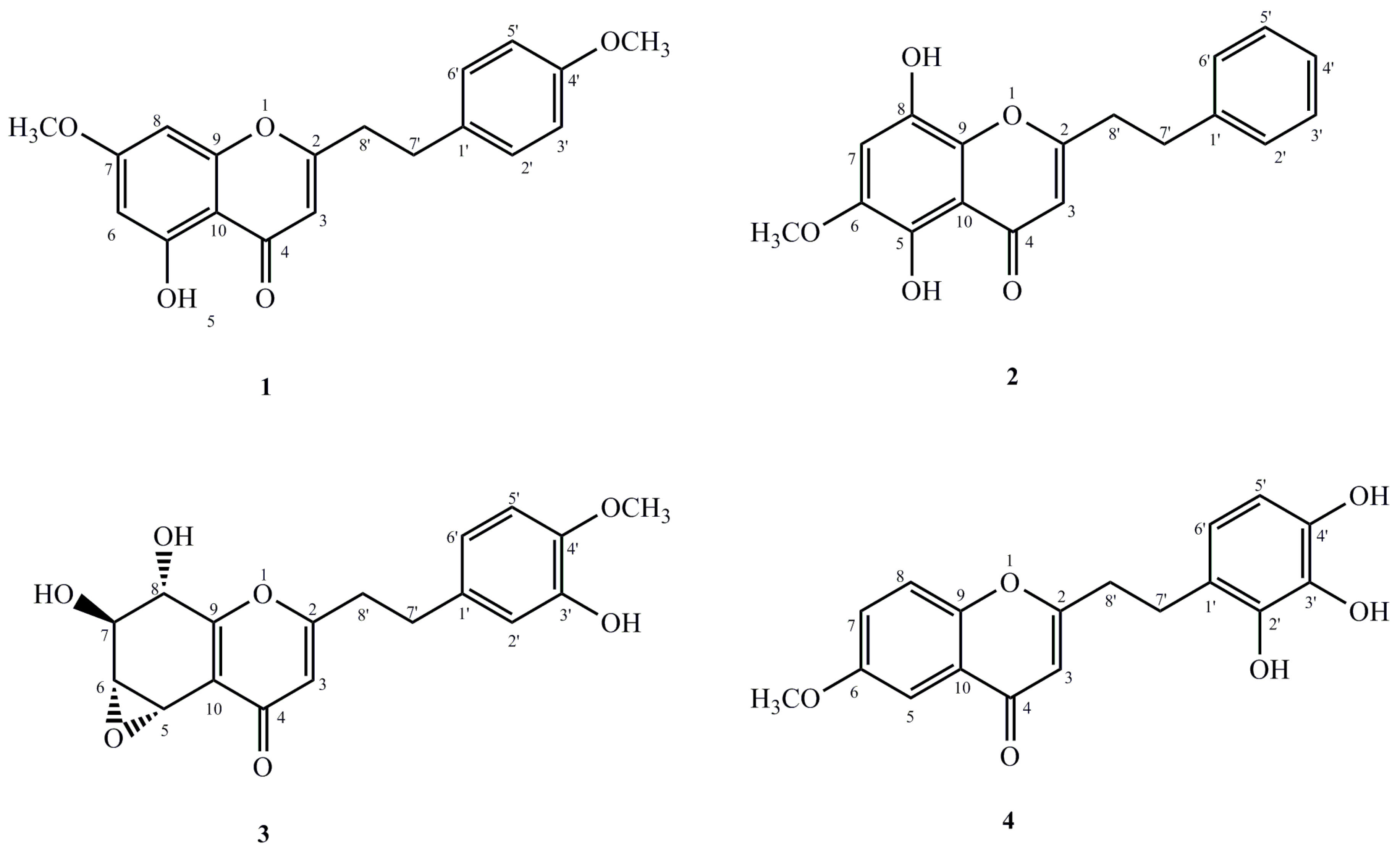
2]. As the inventor, it necessitated an investigation of the characteristic chromones of this agarwood. Our results gave out four new 2-(2-phenylethyl)chromone derivatives (
1–
4) (
Figure 1) from the ethanol extract. This was also shown to be a moderate anti-inflammation activity. In this paper, the isolation and structural elucidation of these new compounds are described, merged with their inhibitory activities against LPS-induced NO production in macrophages.
2. Results and Discussion
Compound
1 was isolated as pale yellow amorphous powder. Its molecular formula was determined to be C
19H
18O
5 from the molecular ion peak at
m/
z 349.1023 [M + Na]
+ (calcd for C
19H
18O
5Na. 349.1052) from the HR-ESI-MS. Again, the IR spectrum indicated the presence of hydroxyl group (3415 cm
−1) and α,β-unsaturated carbonyl group (1620 cm
−1). The
1H-NMR spectroscopic data (
Table 1) of
1 depicted the presence of two methoxyl groups at δ
H 3.90 and 3.88 (each 3H, s), one hydroxyl signal at δ
H 12.80, as well as two aromatic protons at δ
H 6.37 and 6.49. These were assigned to H-6 and H-8 respectively. In addition, a set of typical A
2B
2 coupling systems at δ
H 7.84 (2H, d, 9.0 Hz); δ
C 128.9, and δ
H 7.02; δ
C 114.8 (2H, d, 9.0 Hz), as well as four methylene protons at δ
H 2.35 (2H, d, 7.2 Hz, H-7′) and δ
H 2.80 (2H, d, 7.2 Hz, H-8′) were also observed from HSQC spectrum. Analysis of the
13C-NMR spectroscopic data (
Table 2, see
Supplementary Materials) showed that
1 had two methylene groups at δ
C 33.0 and 36.5, an α,β-unsaturated ketone at δ
C 108.0, 169.8 and 182.7, two methoxyl groups at δ
C 55.7 and 55.9, as well as other two aromatic rings in an AB and A
2B
2 patterns. Considering these, which suggested that compound
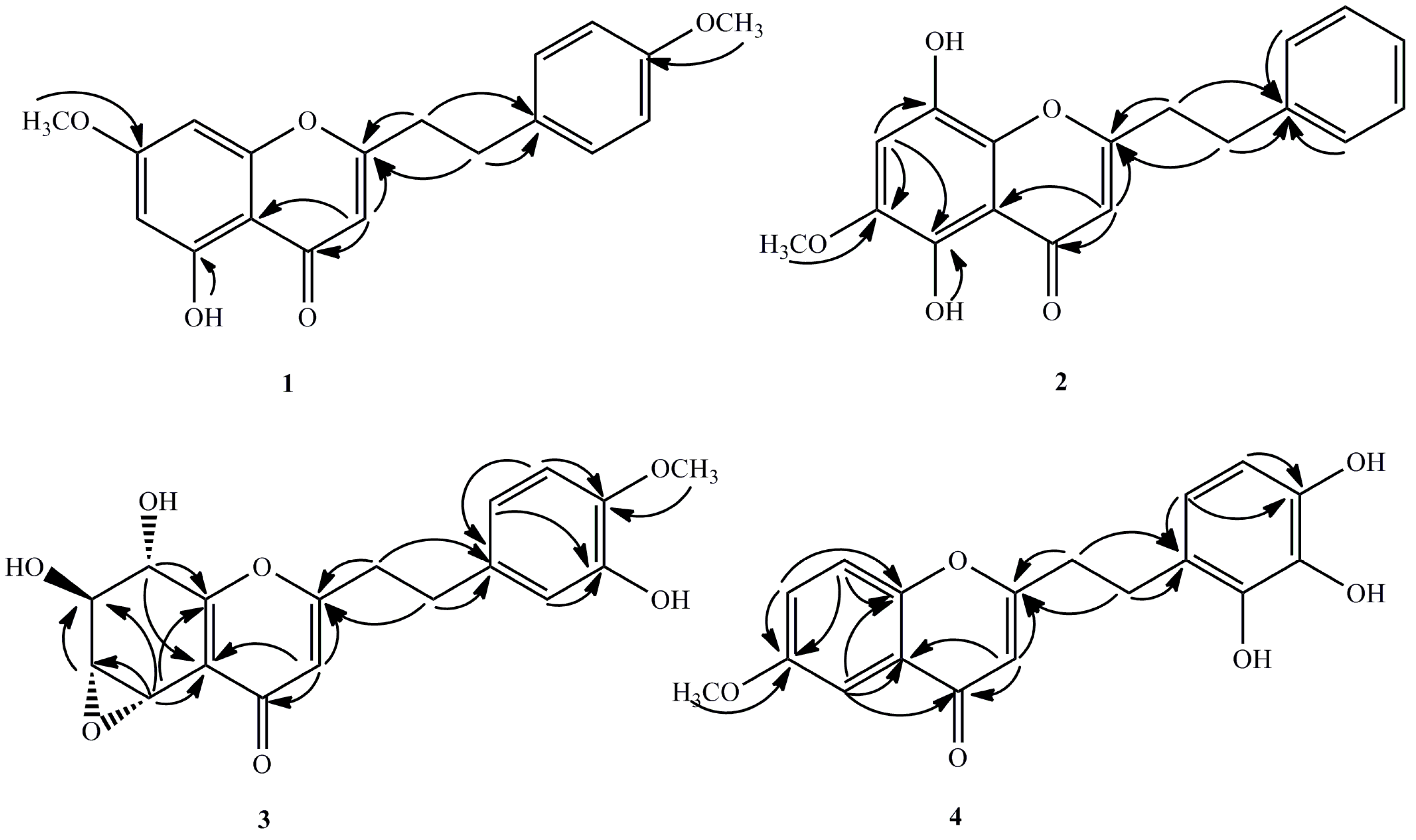
1 was a 2-(2-phenylethyl)chromone derivative with one hydroxyl as well as two methoxyl groups. Based on the HMBC spectroscopic experiment (
Figure 2), the hydroxyl group was located at C-5 since the hydroxyl proton (δ
H 12.80) correlated with the carbon at C-5 (δ
C 149.8), C-10 (δ
C 110.8) as well as C-6 (δ
C 98.3). The methoxyl groups were attached to C-7 and C-4′, respectively. This is also on the basis of correlations from δ
H 3.90 to the aromatic carbon at δ
C 165.9, the δ
H 3.88 to the carbon at δ
C 157.0. Therefore, compound
1 was realised to be 5-hydroxyl-7-methoxy-2-[2-(4′-methoxyphenyl)ethyl]chromone.
Compound
2 was obtained as yellow amorphous powder. The molecular formula established as C
18H
16O
5 by its HR-ESI-MS at
m/
z 335.0902 [M + Na]
+ (calcd for C
18H
16O
5Na, 335.0895). The IR spectrum also demonstrated absorption bands of hydroxyl group (3424 cm
−1) as well as aromatic ring (1610, 1512 and 1455 cm
−1). The UV spectrum further depicted the presence of an α,β-unsaturated carbonyl group out of the distinct absorption maximum at 242 and 322 nm. Moreover, the
1H-NMR spectrum (
Table 1) also outlaid the presence of one methoxyl group at δ
H 3.93 (3H, s), one hydroxyl signal at δ
H 12.66, as well as one singlet aromatic protons at δ
H 6.86, and an A
2B
2X coupling system at δ
H 7.21(2H, t, 7.2 Hz), δ
C 128.4, δ
H 7.29(2H, d, 7.2 Hz), δ
C 128.9, and δ
H 7.27 (m), δ
C 123.3. The
13C-NMR spectrum (
Table 2, see
Supplementary Materials) of
2 illustrated signals for two methylene groups at δ
C 33.3 and 36.4, a trisubstituted double bond at δ
C 108.1 and 170.3, one methoxyl groups at δ
C 57.3, and a carbonyl group at δ
C 184.2. Basing on these findings, we realized that compound
2 was a 2-(2-phenylethyl)chromone derivative with two hydroxyl and one methoxyl groups which was then affirmed by the HMBC spectrum (
Figure 2). In the HMBC spectrum, the correlations from methoxy signal (δ
H 3.93) to the carbon at δ
C 143.5 (C-6) and δ
H 6.86 (s, H-7) to δ
C 143.5 (C-6) indicated that the methoxy (δ
H 3.93) was located at C-6. Moreover, one hydroxyl group was linked to C-5 on the basis of the correlations between 5-OH (δ
H 12.66) and C-5 (δ
C 150.7). The other hydroxyl group was located at C-8, due to the downfield-shifted carbon at C-8 (δ
C 151.2) as well as the molecular formula above. Thus, the structure of compound
2 was assigned to be 5,8-dihydroxy-6-methoxy-2-(2-phenylethyl)chromone.
Compound
3 was obtained as a pale brown amorphous powder. The molecular formula established as C
18H
18O
7 by its HR-ESI-MS at
m/
z 371.1103 [M + Na]
+ (calcd for C
18H
18O
7Na, 371.1107) the IR spectrum showed absorption bands of hydroxyl groups (3410, 3010 cm
−1) and aromatic ring (1610, 1500 and 1425 cm
−1). The
1H-NMR spectrum (
Table 1) showed that the presence of one methoxyl group at δ
H 3.90 (3H, s), two methylene groups at δ
H 2.90, 2.98 (each 2H, t,
J = 7.2 Hz), four oxygenated methine protons at δ
H 4.08 (dd,
J = 10.2, 7.2 Hz), 4.32 (dd,
J = 10.2, 4.8 Hz), 4.94 (d,
J = 7.2 Hz), 5.02 (d,
J = 4.8 Hz), and four aromatic protons at 6.62 (dd,
J = 8.4, 1.8 Hz), 6.76 (d,
J = 8.4 Hz), 6.74 (d,
J = 1.8 Hz), 6.13 (s). The
13C-NMR spectrum (
Table 2, see
supporting information) displayed 18 carbon signals of two methylene groups (δ
C 32.4 and 35.6), a phenyl group (δ
C 119.8, 111.1, 146.0, 145.6, 114.5, and 132.3), a conjugated moiety (δ
C 114.1, 122.0, 157.0, 169.9, 179.8), four oxygenated methine carbons (δ
C 56.5, 61.9, 69.1, and 70.8), and one methoxy carbon (δ
C 56.2). From the
1H-NMR,
1H-
1H COSY, and HSQC spectra, these four carbons were concluded to form a series of consecutive methines (δ
C 56.5, δ
H 5.02; δ
C 61.9, δ
H 4.32; δ
C 69.1, δ
H 4.08; δ
C 70.8, δ
H 4.94). In the HMBC spectrum (
Figure 2), the methine proton at δ
H 5.02, which was located at one end of the consecutive methine, showed correlation peaks with the two olefinic carbons [δ
C 122.0 (C-10) and 157.0 (C-9)], whereas the methine proton at δ
H 4.94, which was located at the other end of the methine, correlated with the latter two olefinic carbons (δ
C 122.0 and 157.0). These correlations indicate that these methines form a part of a tetrasubstituted tetrahydrochromone moiety. The HMBC correlations from δ
H 3.90 and 6.76 to δ
C 146.0 indicated that the methoxy located at C-4′. The hydroxy linked at C-3′ (δ
C 145.6) because the δ
H 6.74 (H-2′) and δ
H 6.62 (H-6′) correlated with the carbon at C-3′. On the basis of
1H-NMR and
13C-NMR data, the structure of compound
3 is very similar to a reported compound named rel-(1a
R,2
R,3
R,7b
S)-1a,2,3,7b-Tetrahydro-2,3-dihydroxy-5-[2-(3-hydroxy-4-methoxy phenyl)ethyl]-7
H-oxireno[
f][1] benzopyran-7-one [
9], except for two strongly downfield-shifted signals at δ
C 61.9 (C-6) and 56.5 (C-5) compared with the known one at δ
C 54.8 (C-6) and 49.5 (C-5), due to the relative configuration of epoxy group in this region, which was further confirmed by the NOESY correlations. In the NOESY spectrum, the correlations between H-5, H-6 and H-8 indicated the epoxy group was α-oriented. The
J3-coupling constant (10.2 Hz) also supported an antiperiplanar relationship between H-6 and H-7. Therefore, the structure of compound
3 was assigned to be 5α,6α-epoxy-7β,8α,3′-trihydroxy-4′-methoxy-2-(2-phenylethyl)chromone.
Compound
4 was obtained as a pale brown amorphous powder. The molecular formula of compound
4 was determined to be C
18H
16O
6 by HR-ESI-MS (
m/
z 351.0821 [M + Na]
+, calc. for C
18H
16O
6Na, 351.0845). The IR spectrum exhibited the presence of hydroxy group(s) at 3340 cm
−1. The
1H-NMR spectrum (
Table 1) showed the presence of one methoxyl group at δ
H 3.88 (3H, s), two methylene groups at δ
H 2.99, 2.89 (each 2H, t,
J = 7.2 Hz), six aromatic protons at δ
H 6.13 (s), 7.55 (d,
J = 3.0 Hz), 7.24, (dd,
J = 9.0, 3.0 Hz), 7.37 (d,
J = 9.0 Hz), 6.75 (d,
J = 8.4 Hz) and 7.05 (d,
J = 8.4 Hz). The
13C-NMR spectrum (
Table 2, see
supporting information) of
4 showed 18 carbon signals including one methoxy at δ
C 56.1, two methylene groups at δ
C 32.4 and 36.6, six methine at δ
C 109.7, 105.0, 123.8, 119.6, 115.7 and 129.6, and nine quaternary carbons at δ
C 168.6, 178.5, 157.0, 151.5, 124.4, 131.9, 151.2, 140.1 and 154.5. Based on the combined analyses of the IR,
1H-NMR, and
13C-NMR spectroscopic data, compound
4 was another 2-(2-phenylethyl)chromone derivative with one methoxyl group and three hydroxyl groups, which was further confirmed by HMBC correlations (
Figure 2). In the HMBC spectrum, the correlations from the methoxy (δ
H 3.88) and H-7 (δ
H 7.24) to the carbon at δ
C 157.0, indicated that the methoxy (δ
H 3.88) was located on C-6 (δ
C 157.0), in which the integration value of H-7 was enhanced when the methoxy protons at δ
H 3.88 was irradiated. The positions of the hydroxyl groups were attached to C-2′/3′/4′, respectively on the basis of the downfield carbons at δ
C 151.2 (C-2′), 140.1 (C-3′), 154.5 (C-4′), together with the molecular formula above. Thus, the structure of
4 was identified as 6-methoxy-2-[2-(2′,3′,4′-trihydroxy)phenyl)ethyl]chromone.
Considering this medicinal herb as a therapeutical agent of analgesics and asthmatic, the isolated compounds
1–
4 were studied for their anti-inflammatory activities on lipopolysaccharide (LPS)-induced nitric oxide (NO) production in RAW 264.7. The results showed that compound
1 showed significant inhibitory activities with IC
50 value of 4.6 μM and compound
3 displayed moderate activity with IC
50 value of 84 μM comparing of the positive drug control group aminoguanidine with IC
50 value of 1.8 μM, while compounds
2 and
4 were inactive (
Table 3).
3. Materials and Methods
3.1. General Experimental Procedures
1D and 2D NMR spectra were obtained with a Bruker AV III 600NMR spectrometer (Bruker, Billerica, German) using TMS as the internal standard. HRESIMS spectra were performed on a LTQ-Obitrap XL spectrometer (Thermo Fisher Scientific, Boston, MA, USA). Optical rotations were obtained on a Perkin-Elmer 341 digital polarimeter (PerkinElmer, Norwalk, CT, USA). UV and IR spectra were recorded on Shimadzu UV2550 and FTIR-8400S spectrometers (Shimadzu, Kyoto, Japan), respectively. Semi-preparative LC was performed on a Lumtech K-1001 analytic LC (Beijing, China) which is equipped with two pumps of K-501, a UV detector of K-2600, as well as an YMC Pack C18 column (250 mm × 10 mm, i.d., 5 μM, YMC Co. Ltd., Kyoto, Japan) eluted with CH3CN–H2O (or MeOH–H2O) at a flow rate of 2 mL/min. ODS (12 nm–50 μm, YMC Co. Ltd., Kyoto, Japan), Sephadex LH-20 (Pharmacia, Uppsala, Sweden), as well as silica gel (100–200 and 300–400 mesh, Qingdao Marine Chemical plant, Qingdao, China) were utilized for column chromatography. Moreover, pre-coated silica gel GF254 plates (Zhi Fu Huang Wu Pilot Plant of Silica Gel Development, Yantai, China) were utilized for TLC (CH2Cl2:MeOH 100:1), the spots on TLC were detected by spraying with 5% H2SO4 in EtOH. All solvents utilized were of analytical grade (Beijing Chemical Works).
3.2. Plant Material
Agarwood which was induced by Agar-Wit from 7 years old A. sinensis tree was harvested about 18 months later, which was collected from Pingding Town, Huazhou City, Guangdong Province, China, in September 2014. The sample was identified by Prof. Jian-he Wei, Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences and Peking Union Medical College, where a voucher specimen (No. 20140907) was deposited.
3.3. Extraction and Isolation
Dried and powdered Agarwood (10 kg) was also refluxed with 95% EtOH (50.0 L × 3) within conditions of reflux in yielding a semi-solid residue (850 g) which was made of the crude extract. The crude extracts were then dissolved successively with water (2 L), in the order, petroleum ether (MSO), dichloromethane (CH2Cl2), ethyl acetate (EtOAc) as well as n-butanol (nBuOH), in re-extracting water solution of the crude extract as well as obtaining different fractions.
The CH2Cl2 extract (86.5 g) was applied to silica gel (100–200 mesh) chromatographic column then it successively eluted with MSO–CH2Cl2 (v/v, 100:0–1:20, 3.0 L of each), CH2Cl2–MeOH (v/v, 100:0–0:100, 3.0 L of each) in providing twenty fractions (Fr. 1–Fr. 20). Fr. 1–9 mix with Fr. 10 (Fr. A) (10.5 g) was subjected to silica gel (200–300 mesh) chromatographic column as well as eluted with MSO–CH2Cl2 (v/v, 10:0–1:1, 0.5 L of each), CH2Cl2–MeOH (v/v, 100:0–0:100, 0.5 L of each) to get nine sub-fractions (Fr.A-1–Fr. A-9). Fr.A-3 (3.0 g) was subjected to further purified by semi-preparative liquid chromatography (LC) with CH3CN–H2O (v/v, 6:4) isolated to yield compound 1 (21.7 mg, tR = 35.9 min).
Accordingly, the EtOAc extract (35.1 g) was also isolated to silica gel (100–200 mesh) chromatographic column and successively eluted with CH2Cl2–MeOH (v/v,100:0–0:100, 3.0 L of each) to give 7 fractions (Fr.A–Fr.G). Fr. A (8.1 g) was applied to silica gel (100–200 mesh) chromatographic column by eluted with MSO–CH2Cl2 (v/v, 1:1, 1:3, 1 L of each), CH2Cl2–MeOH (v/v, 100:0–0:100, 1.0 L of each) to provide six sub-fractions(Fr.A-1–Fr.A-6). Fr.A-3 (2.5 g) was chromatographed by ODS gel (3 × 40 cm) eluted with MeOH–H2O (v/v, 3:7, 1.0 L of each) to increase polarity to give eight sub-fractions (Fr.A-3-1–Fr.A-3-8). Compound 2 (7.3 mg, tR = 22.2 min) and 4 (8.2 mg, tR = 18.1 min) were separated out from the mixture of Fr.A-3-4-18~29 by semi-preparative LC with CH3CN–H2O (v/v, 50:50). Fr.A-3-2 (0.4 g) which was then purified by semi-preparative LC with MeOH–H2O (v/v, 44:56) isocratic to produce compound 3 (7.3 mg, tR = 32.7 min).
The structures of compounds 1–4 were determined by UV, IR, 1H-NMR, 13C-NMR, 1H-1H COSY, HSQC, HMBC, NOESY and HR-ESI-MS.
5-Hydroxy-7-methoxy-2-[2-(4′-methoxyphenyl)ethyl]chromone (
1). C
19H
18O
5, pale yellow amorphous powder; m.p. 185–187 °C; UV λ
max (CHCl
3) nm (log ε): 225 (4.14), 318 (3.66); IR (KBr) ν
max cm
−1: 1030, 1220, 1275, 1365, 1480, 1620, 3415; HR-ESI-MS
m/
z 349.1023 [M + Na]
+ (calcd. 349.1052);
1H-NMR spectra data, see
Table 1;
13C-NMR spectrum data, see
Table 2.
5,8-Dihydroxy-6-methoxy-2-(2-phenylethyl)chromone (
2). C
18H
16O
5, pale yellow amorphous powder; m.p. 124–127 °C; UV λ
max (CHCl
3) nm (log ε): 242 (4.6), 322 (4.15); IR (KBr) ν
max cm
−1 1512, 1455, 1610, 3424; HR-ESI-MS
m/
z 335.0902 [M + Na]
+ (calcd for C
18H
16O
5Na, 335.0895);
1H-NMR spectra data, see
Table 1;
13C-NMR spectrum data, see
Table 2.
5α,6α-Epoxy-7β,8α,3′-trihydroxy-4′-methoxy-2-(2-phenylethyl)chromone (
3). C
18H
18O
7, pale brown amorphous powder,
−12.5 (
c 0.10, MeOH); UV λ
max (MeOH) nm (log ε): 254 (4.02), 205 (4.45); IR (KBr) ν
max cm
−1 3410, 3010, 1660, 1610, 1500, 1425, 1265, 1240, 1120, 1100, 1015; HR-ESI-MS at
m/
z 371.1103 [M + Na]
+ (calcd for C
18H
18O
7Na, 371.1107);
1H-NMR spectra data, see
Table 1;
13C-NMR spectrum data, see
Table 2.
6-Methoxy-2-[2-(2′,3′,4′-trihydroxy)phenyl)ethyl]chromone (
4). C
18H
16O
6, pale brown amorphous powder; UV λ
max (MeOH) nm (log ε): 242 (4.56), 340 (3.75); IR (KBr) ν
max cm
−1 3340, 2545, 1610, 1512, 1405, 1310, 1284, 1140, 1020; HR-ESI-MS (
m/
z 351.0821 [M + Na]
+, calc. for C
18H
16O
6Na, 351.0845);
1H-NMR spectra data, see
Table 1;
13C-NMR spectrum data, see
Table 2.
3.4. Assay for Inhibitory Ability Against LPS-Induced NO Production in RAW 264.7 Macrophages
The in vitro anti-inflammatory activity was assessed through determining the nitrite concentration in the medium as well as the proliferation of RAW264.7 cells as illustrated in a previous study with some modify [
10,
11]. Shortly, the cells (10
5 cells/well) were co-incubated with drugs (Compounds
1–
4 and Aminoguanidine) as well as LPS (1 μg/mL) for 24 h at 37 °C. The tested samples were dissolved in DMSO, and then diluted with DMEM to make the final DMSO concentration of 0.1%. After that, the cells were coincubated with fresh medium (150 μL/well) and then treated with LPS (200 ng/mL), and the tested compounds at various concentrations (0.2–50.0 μM) for 24 h. Griess reagent was used to determine the NO production by detecting the nitrite in the culture supernatant. In short, 100 μL of the culture supernatant was reacted with an equal volume of Griess reagent and vibrated for 10 min at room temperature. The amount of NO was assessed by finding the nitrite concentration in the cultured RAW 264.7 macrophage supernatants with Griess reagent. Aliquots of supernatants (100 μL) were also incubated, in-sequence, with 50 μL of 1% sulfanilamide and 50 μL of 0.1% naphthylethylenediamine in 2.5% phosphoric acid solution. From this, the absorbance was recorded on a micro-plate reader at a wavelength of 570 nm. The results were expressed as IC
50 values which were calculated using the CalcuSyn program and expressed as the means with SD of three independent experiments.