Substituent Inductive Effects on the Electrochemical Oxidation of Flavonoids Studied by Square Wave Voltammetry and Ab Initio Calculations
Abstract
:1. Introduction
2. Results
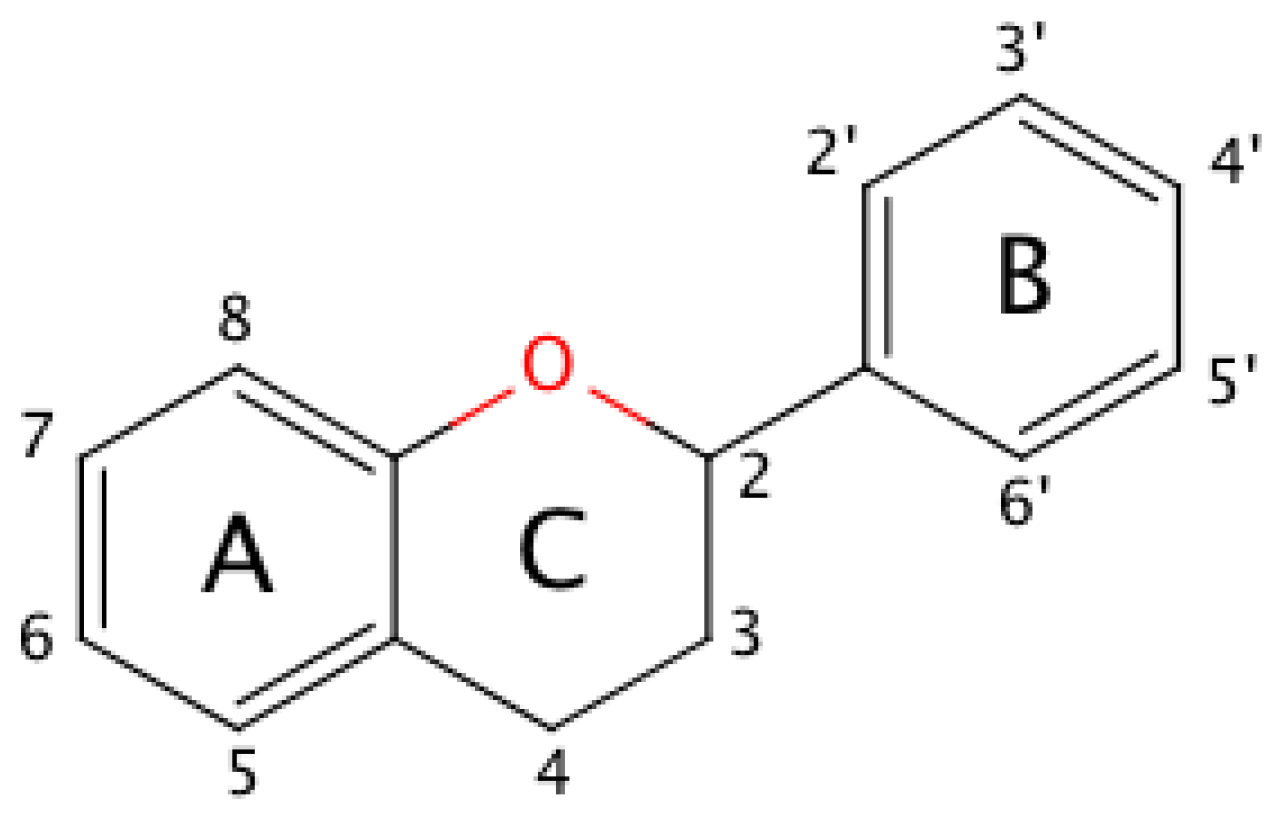
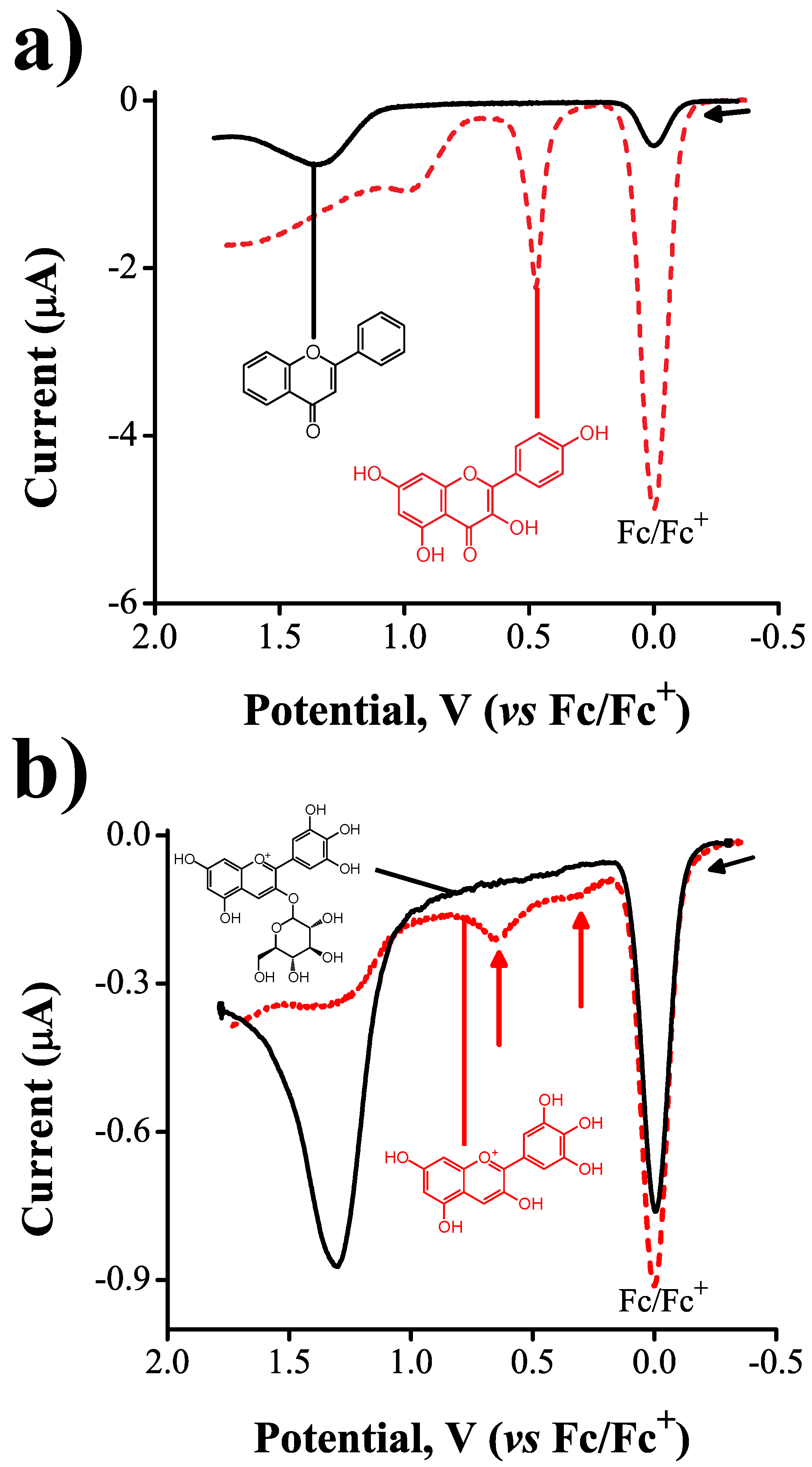
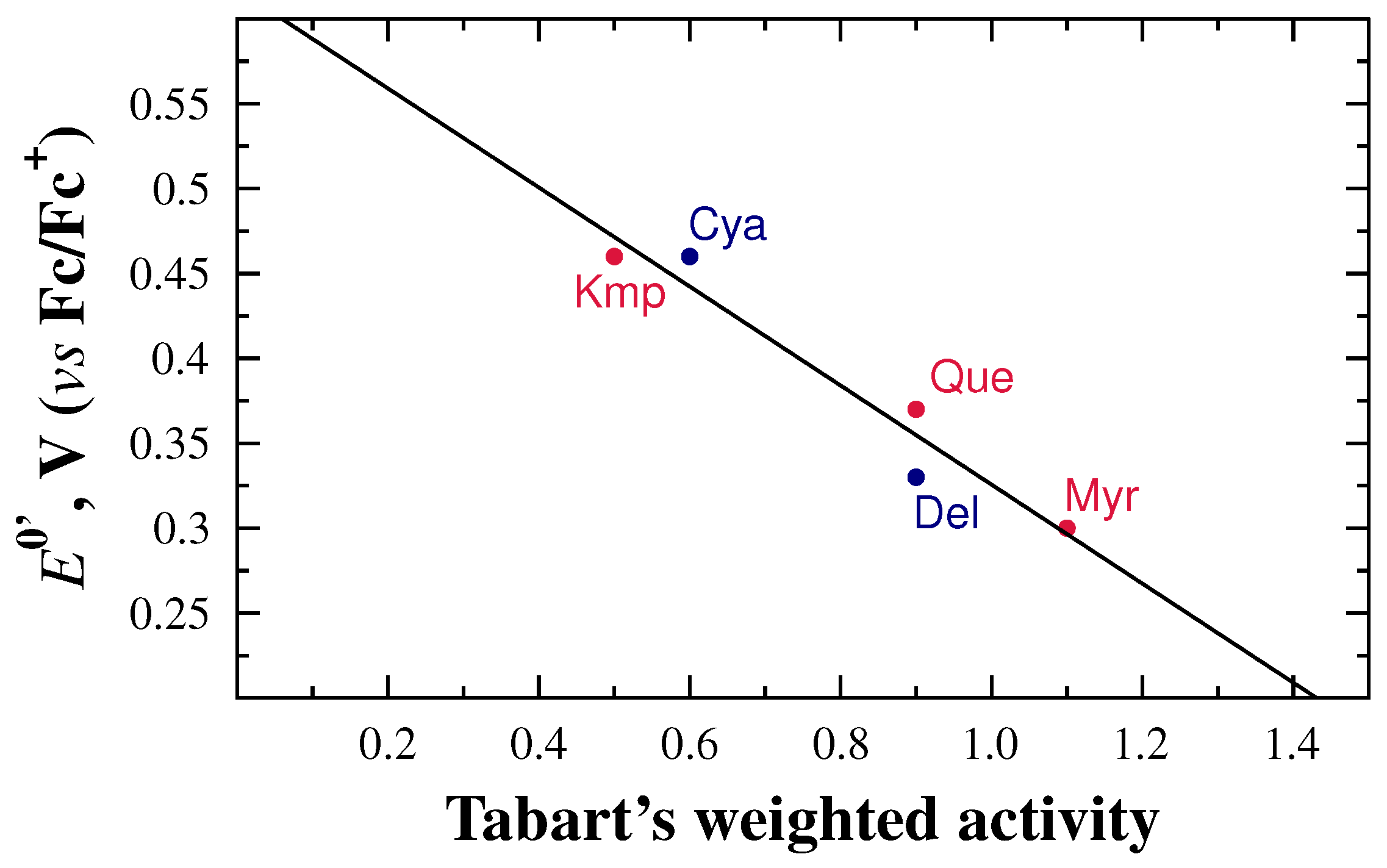
2.1. General Relationships between Structure and Oxidation Potential
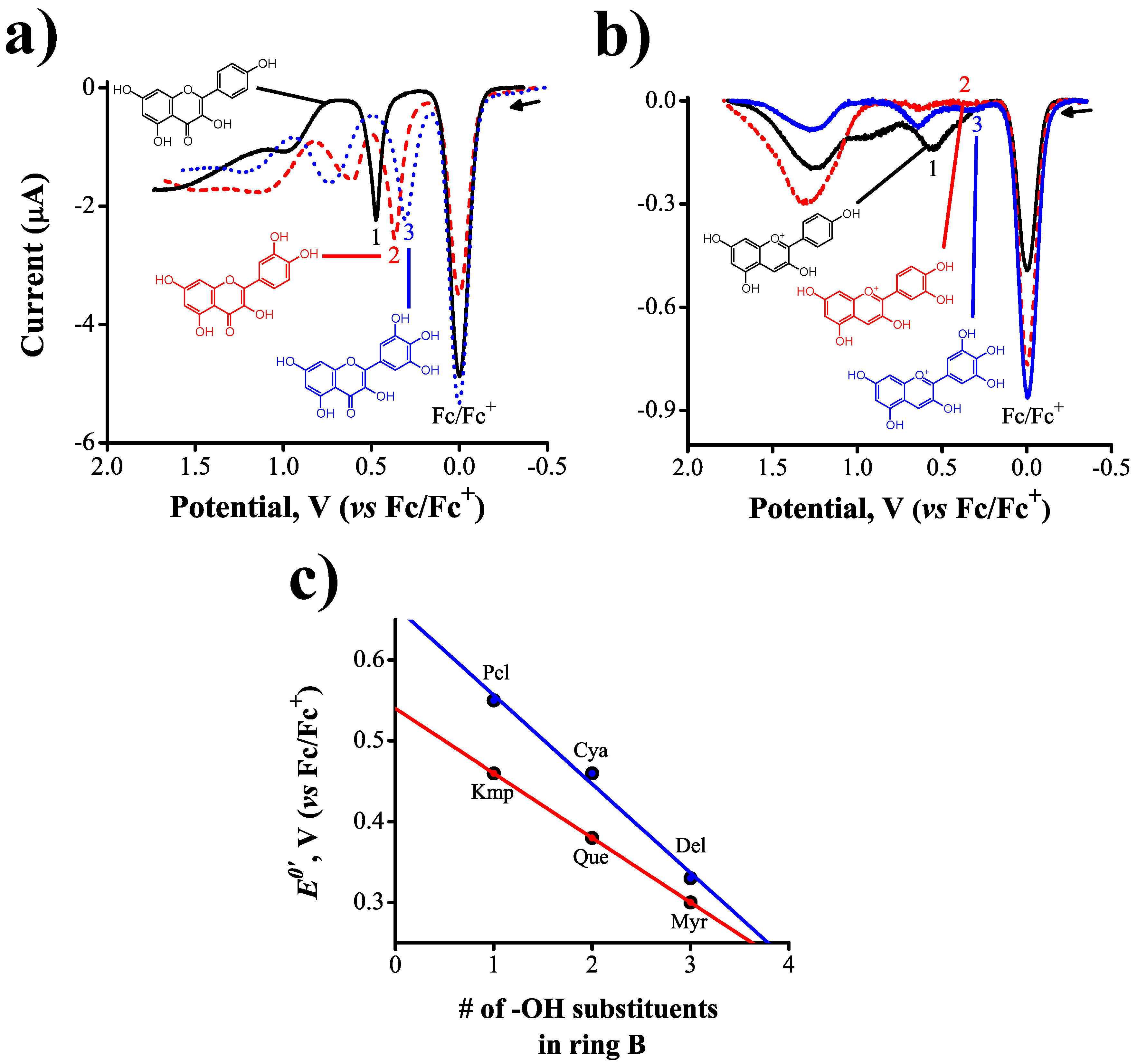
2.2. Inductive Effects on Oxidation Potentials
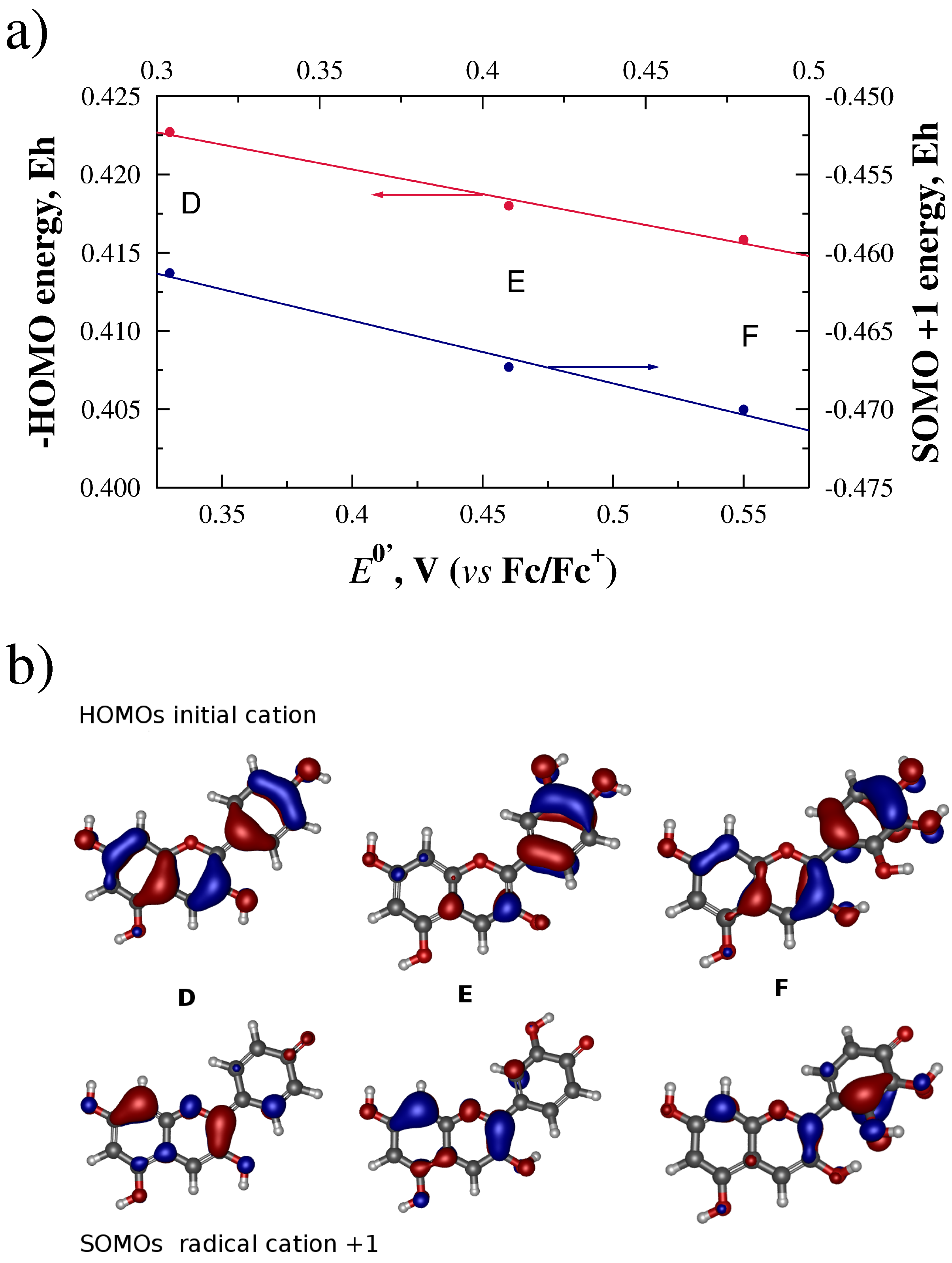
2.3. Orbital Energy and Electron Density Considerations
3. Discussion
4. Materials and Methods
4.1. Chemicals and Solutions
4.2. Instrumentation
4.3. Ab Initio Calculations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ESR | Electron Paramagnetic Resonance |
| Fc | ferrocene |
| FRAP | ferric reducing antioxidant potential |
| HOMO | highest occupied molecular orbital |
| HF | Hartree–Fock |
| ORAC | oxygen-radical absorbance capacity |
| SOMO | single occupied molecular orbital |
| SVP | Karlsruhe split valence polarization basis set |
| SWV | square wave voltammetry |
| TAC | Total antioxidant capacity |
| TEAC | Trolox equivalent antioxidant capacity |
| TEATFB | tetraethylammonium tetrafluoroborate |
| Cya | cyanidin |
| Del | delphinidin |
| Kmp | kaempferol |
| Myr | myricetin |
| Pel | pelargonidin |
| Que | quercetin |
References
- Clifford, M.; Brown, J. Flavonoids: Chemistry, Biochemistry and Applications; Taylor & Francis: Abingdon, UK, 2006. [Google Scholar]
- Kühnau, J. Die Flavonoide und ihre Rollen in der Menschlichen Ernährung: Ein Beitrag zur Kenntnis Semi-Essentieller Planzenstoffe. Qual. Plant. Plant Foods. Hum. Nutr. 1973, 23, 119–127. [Google Scholar] [CrossRef]
- Tapieiro, H.; Tew, K.; Ba, G.; Mathé, G. Polyphenols: Do they play a role in the prevention of human pathologies? Biomed. Pharmacother. 2002, 56, 200–207. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Reyes, A.G.; Arroyo-Curras, N.; Cano, B.G.; Lara-Diaz, V.J.; Guajardo-Salinas, G.E.; Islas, J.F.; Morales-Oyarvide, V.; Morales-Garza, L.A.; Galvez-Gastelum, F.J.; Grijalva, G.; et al. Black bean extract ameliorates liver fibrosis in rats with CCl4-induced injury. Ann. Hepatol. 2008, 7, 130. [Google Scholar] [PubMed]
- Kroon, P.; Williamson, G. Polyphenols: Dietary components with stablished benefits to health? J. Sci. Food Agric. 2005, 85, 1239–1240. [Google Scholar] [CrossRef]
- Silva, M.M.; Santos, M.R.; Caroco, G.; Rocha, R.; Justino, G.; Mira, L. Structure-antioxidant Activity Relationships of Flavonoids: A Re-examination. Free Radic. Res. 2002, 36, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.K.; Weng, M.S. Flavonoids as Nutraceuticals. In The Science of Flavonoids; Grotewold, E., Ed.; Springer: New York, NY, USA, 2006; Chapter 8; p. 213. [Google Scholar]
- Masek, A. Flavonoids as Natural Stabilizers and Color Indicators of Ageing for Polymeric Materials. Polymers 2015, 7, 1125–1144. [Google Scholar] [CrossRef]
- Chambers, J.Q. The Quinonoid Compounds; Chapter 12 Electrochemistry of Quinones; Wiley: Hoboken, NJ, USA, 1988. [Google Scholar]
- Frontana, C.; Gonzalez, I. Structural Factors Affecting the Reactivity of the Natural a-Hydroxy Benzoquinones. An Electrochemical and ESR Study. ECS Trans. 2007, 3, 13–23. [Google Scholar]
- Ghiselli, A.; Serafini, S.; Natella, F.; Scaccini, C. Total Antioxidant Capacity as a Tool to Assess Redox Status: Critical View and Experimental Data. Free Radic. Biol. Med. 2000, 29, 1106. [Google Scholar] [CrossRef]
- Aherne, S.; O’Brien, N. Dietary Flavonols: Chemistry, Food Content, and Metabolism. Nutrition 2002, 18, 75–81. [Google Scholar] [CrossRef]
- Butelli, E.; Titta, L.; Giorgio, M.; Mock, H.P.; Matros, A.; Peterek, S.; Schijlen, E.G.; Hall, R.D.; Bovy, A.G.; Luo, J.; et al. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat. Biotechnol. 2008, 26, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Huber, L.; Hoffmann-Ribani, R.; Rodríguez-Amaya, D. Quantitative variations in Brazilian vegetable sources of flavonols and flavones. Food Chem. 2009, 113, 1278–1282. [Google Scholar] [CrossRef]
- Fuhrman, B.; Aviram, M. Handbook of Antioxidants; Marcel Dekker: New York, NY, USA, 2002. [Google Scholar]
- Clifford, M. Anthocyanins-nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1063–1072. [Google Scholar] [CrossRef]
- Tabart, J.; Kevers, C.; Pincemail, J.; Defraigne, J.O.; Dommes, J. Comparative antioxidant capacities of phenolic compounds measured by varioust tests. Food Chem. 2009, 113, 1226–1233. [Google Scholar] [CrossRef]
- Csepregi, K.; Neugart, S.; Schreiner, M.; Hideg, E. Comparative Evaluation of Total Antioxidant Capacities of Plant Polyphenols. Molecules 2016, 21, 208. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Sochor, J.; Dobes, J.; Krystofova, O.; Ruttkay-Nedecky, B.; Babula, P.; Pohanka, M.; Jurikova, T.; Zitka, O.; Adam, V.; Klejdus, B.; et al. Electrochemistry as a Tool for Studying Antioxidant Properties. Int. J. Electrochem. Sci. 2013, 8, 8464–8489. [Google Scholar]
- Głód, B.; Kierstyn, I.; Piszcz, P. Total antioxidad potential assay with cyclic voltammetry and/or differential pulse voltammetry measurements. J. Electroanal. Chem. 2014, 719, 24–29. [Google Scholar] [CrossRef]
- Chevion, S.; Roberts, M.A.; Chevion, M. The use of cyclic voltammetry for the evaluation of antioxidant capacity. Free Radic. Biol. Med. 2000, 28, 860–870. [Google Scholar] [CrossRef]
- Masek, A.; Chrzescijanska, E. Effect of UV-A Irradiation and Temperature on the Antioxidant Activity of Quercetin Studied Using ABTS, DPPH and Electrochemistry Methods. Int. J. Electrochem. Sci. 2015, 10, 5276–5290. [Google Scholar]
- Gulaboski, R.; Mirceski, V.; Mitrev, S. Development of a rapid and simple voltammetric method to determine total antioxidative capacity of edible oils. Food Chem. 2013, 138, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Piljac-Zegarac, J.; Valek, L.; Stipcevic, T.; Martinez, S. Electrochemical determination of antioxidant capacity of fruit tea infusions. Food Chem. 2010, 121, 820–825. [Google Scholar] [CrossRef]
- Arribas, A.S.; Martínez-Fernández, M.; Chicharro, M. The role of electroanalytical techniques in analysis of polyphenols in wine. TrAC Trends Anal. Chem. 2012, 34, 78–96. [Google Scholar] [CrossRef]
- Barros, L.; Cabrita, L.; Vilas Boas, M.; Carvalho, A.; Ferreira, C.F.R. Chemical, biochemical and electrochemical analysis to evaluate phytochemicals and antioxidant activity of wild plants. Food Chem. 2011, 127, 1600–1608. [Google Scholar] [CrossRef]
- Filipiak, M. Electrochemical Analysis of Polyphenolic Compounds. Anal. Sci. 2001, 17, i1667–i1670. [Google Scholar]
- Fraga, C. Plant Polyphenols: How to Translate their in vitro Antioxidant Actions to in vivo Conditions. IUMBM Life 2007, 59, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Ramaley, L.; Krause, M.S., Jr. Theory of square wave voltammetry. Anal. Chem. 1969, 41, 1362–1365. [Google Scholar] [CrossRef]
- Osteryoung, J. Voltammetry for the future. Acc. Chem. Res. 1993, 26, 77–83. [Google Scholar] [CrossRef]
- Osteryoung, R.; Osteryoung, J. Pulse voltammetric methods of analysis. Philos. Trans. R. Soc. Lond. Ser. A 1981, 302, 315–326. [Google Scholar] [CrossRef]
- Osteryoung, J.; O’Dea, J.J. Square-Wave Voltammetry. In Electroanalytical Chemistry; Marcel Dekker, Inc.: New York, NY, USA, 1986; Volume 14, pp. 209–308. [Google Scholar]
- Jørgensen, L.; Cornett, C.; Justesen, U.; Skibsted, L.; Dragsted, L. Two-electron electrochemical oxidation of quercetin and kaempferol changes only the flavonoid C-ring. Free Radic. Res. 1998, 29, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Heijnen, C.; Haenen, G.; Vekemans, J.; Bast, A. Peroxynitrile scavenging of flavonoids: Structure activity relationship. Environ. Toxicol. Pharmacol. 2001, 10, 199–206. [Google Scholar] [CrossRef]
- Aparicio, S. A Systematic Computational Study of Flavonoids. Int. J. Mol. Sci. 2010, 11, 2017–2038. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.D. New Insights into the Oxidative Electrochemistry of Vitamin E. Acc. Chem. Res. 2007, 40, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Gritzner, G.; Kuta, J. Recommendations on Reporting Electrode Potentials in Non-aqueous Solvents. Pure Appl. Chem. 1984, 56, 461–466. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchinson, G.R. Avogadro: An advanced semantic chemical editor, visualization and analysis plataform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. The ORCA program system. Comput. Mol. Sci. 2012, 2, 73. [Google Scholar] [CrossRef]
- Schaefer, A.; Horn, H.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets for Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Allouche, A.R. Gabedit-a graphical user interface for computational chemistry softwares. J. Comput. Chem. 2011, 32, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Persistence of Vision (TM) Raytracer; Persistence of Vision Pty. Ltd.: Williamstown, Victoria, Australia, 2004; Available online: http://www.povray.org (accessed on 20 October 2016).
- Sample Availability: The compounds were purchased from commercial sources and are not currently available from the authors.


| Compounds | Structure |
|---|---|
| Flavonols | |
| kaempferol | 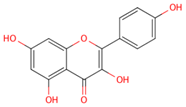 |
| quercetin | 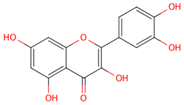 |
| myricetin | 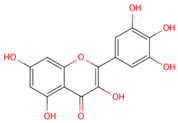 |
| Anthocyanidins | |
| pelargonidin | 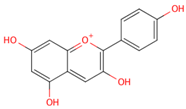 |
| cyanidin | 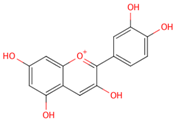 |
| delphinidin | 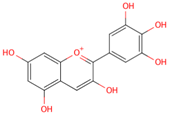 |
| Flavonols | OH | /V | HOMO | SOMO | |
| Anion | Cationic Radical | ||||
| kaempferol | 1 | 0.46 | |||
| quercetin | 2 | 0.37 | |||
| myricetin | 3 | 0.30 | |||
| Anthocyanidins | OH | /V | HOMO | SOMO+1 | SOMO+2 |
| Cation | Cationic Radical | Cationic Radical | |||
| pelargonidin | 1 | 0.55 | |||
| cyanidin | 2 | 0.46 | |||
| delphinidin | 3 | 0.33 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arroyo-Currás, N.; Rosas-García, V.M.; Videa, M. Substituent Inductive Effects on the Electrochemical Oxidation of Flavonoids Studied by Square Wave Voltammetry and Ab Initio Calculations. Molecules 2016, 21, 1422. https://doi.org/10.3390/molecules21111422
Arroyo-Currás N, Rosas-García VM, Videa M. Substituent Inductive Effects on the Electrochemical Oxidation of Flavonoids Studied by Square Wave Voltammetry and Ab Initio Calculations. Molecules. 2016; 21(11):1422. https://doi.org/10.3390/molecules21111422
Chicago/Turabian StyleArroyo-Currás, Netzahualcóyotl, Víctor M. Rosas-García, and Marcelo Videa. 2016. "Substituent Inductive Effects on the Electrochemical Oxidation of Flavonoids Studied by Square Wave Voltammetry and Ab Initio Calculations" Molecules 21, no. 11: 1422. https://doi.org/10.3390/molecules21111422
APA StyleArroyo-Currás, N., Rosas-García, V. M., & Videa, M. (2016). Substituent Inductive Effects on the Electrochemical Oxidation of Flavonoids Studied by Square Wave Voltammetry and Ab Initio Calculations. Molecules, 21(11), 1422. https://doi.org/10.3390/molecules21111422







