Tetrandrine Induces Apoptosis of Human Nasopharyngeal Carcinoma NPC-TW 076 Cells through Reactive Oxygen Species Accompanied by an Endoplasmic Reticulum Stress Signaling Pathway
Abstract
:1. Introduction
2. Results
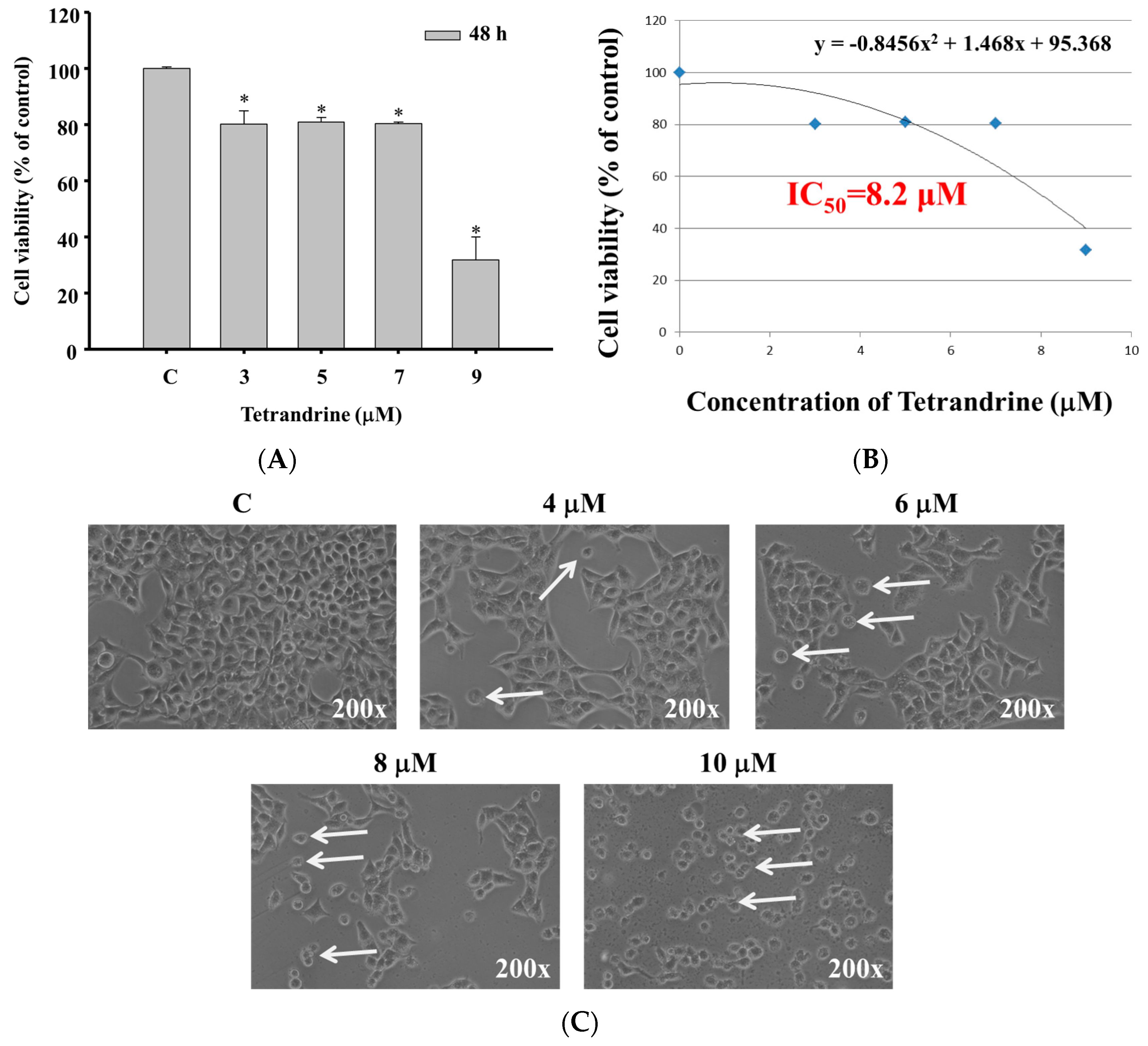
2.1. TET Induced Cell Morphological Changes and Decreased the Total Viable Cell Number in NPC-TW 076 Cells
2.2. TET Induced Nuclear Condensation in NPC-TW 076 Cells
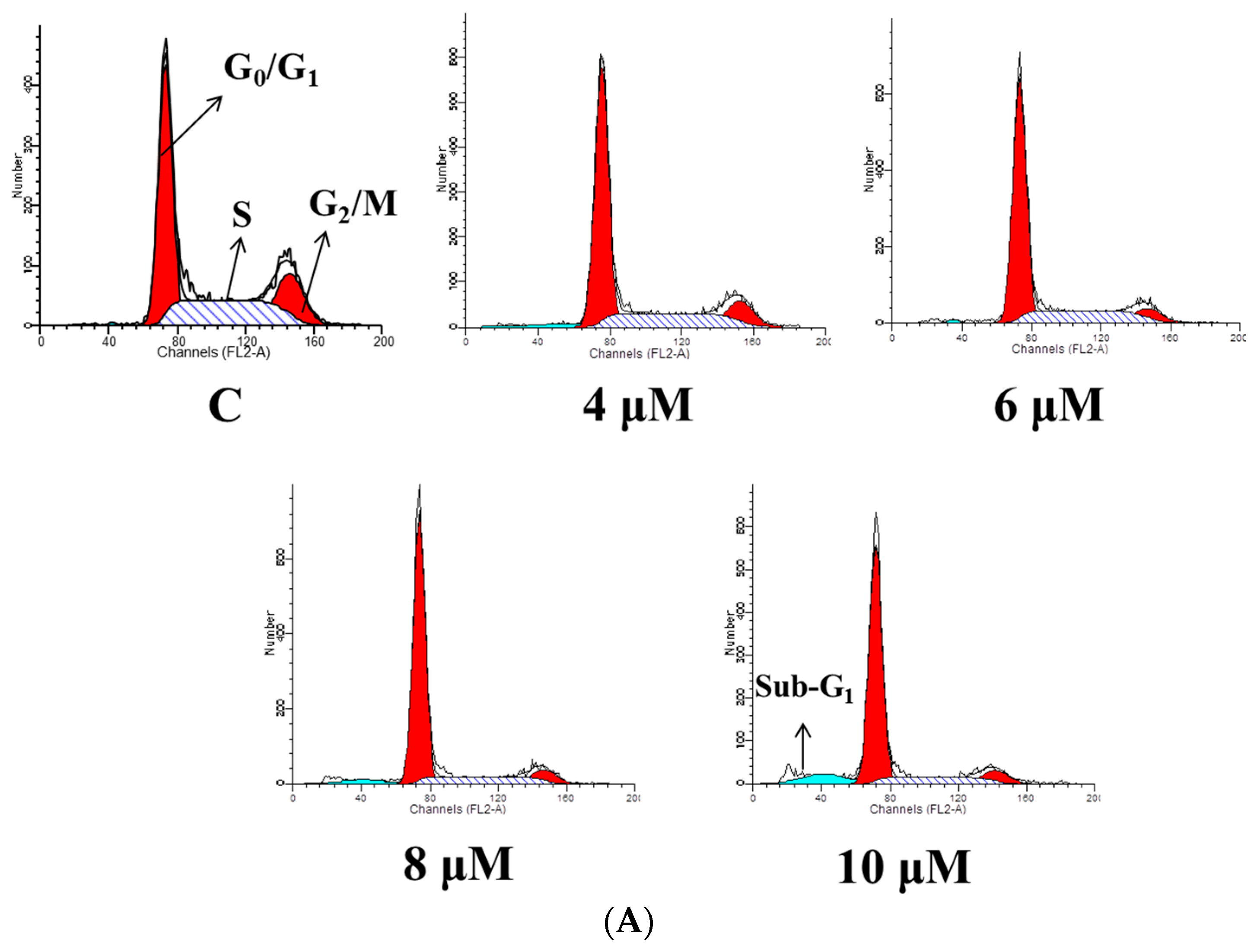
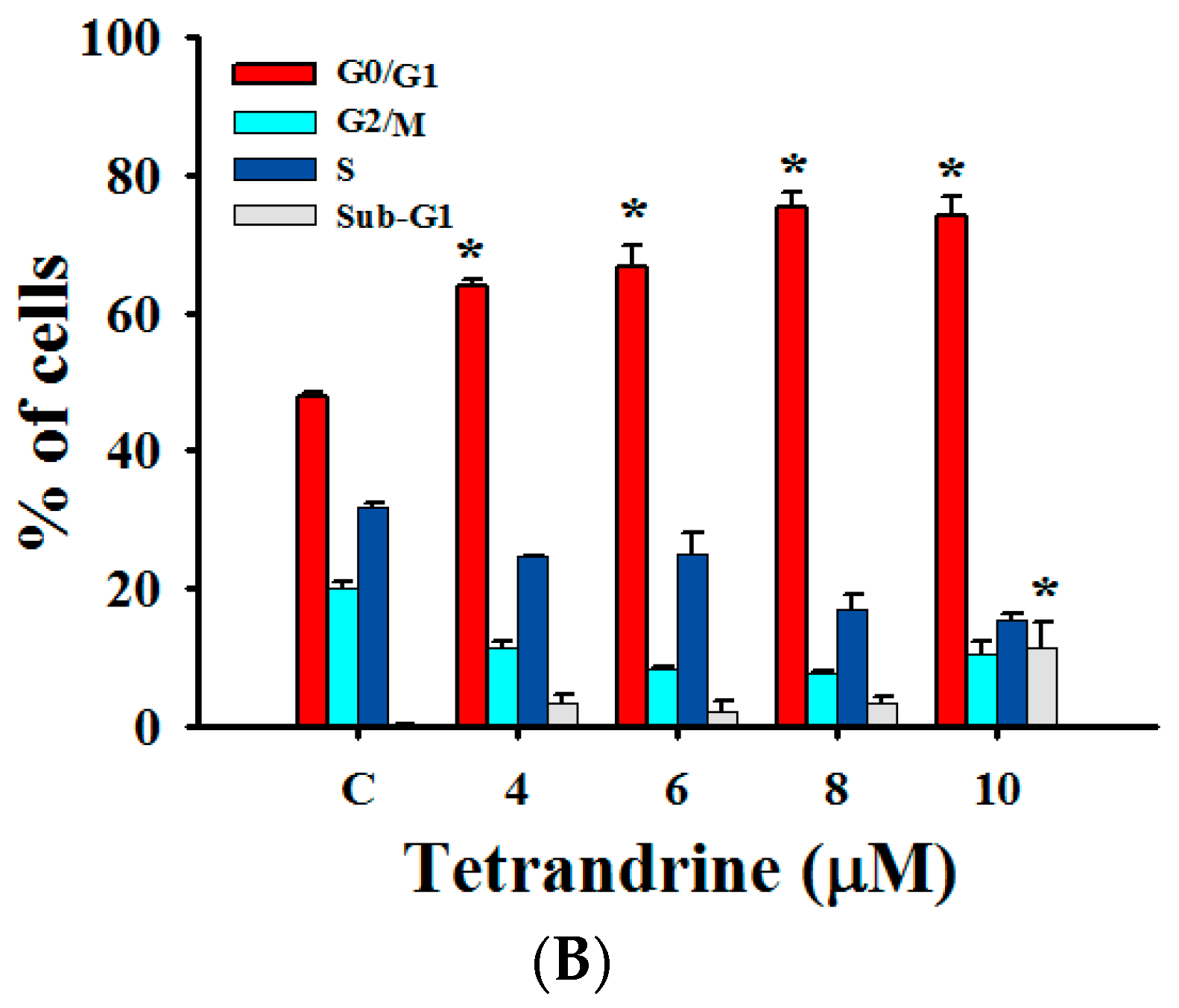
2.3. TET Induced G0/G1 Phase Arrest and Sub-G1 Phase in NPC-TW 076 Cells
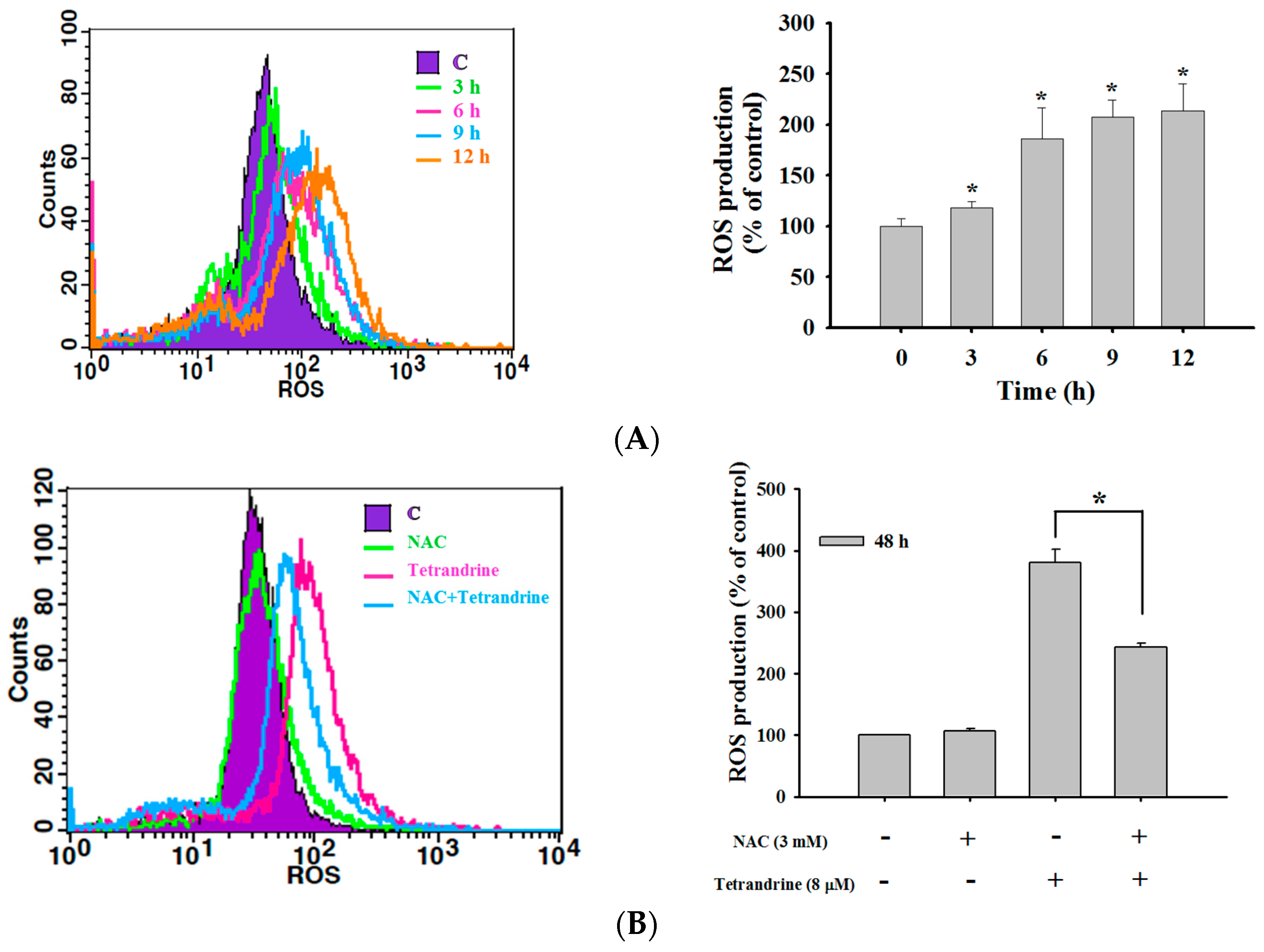
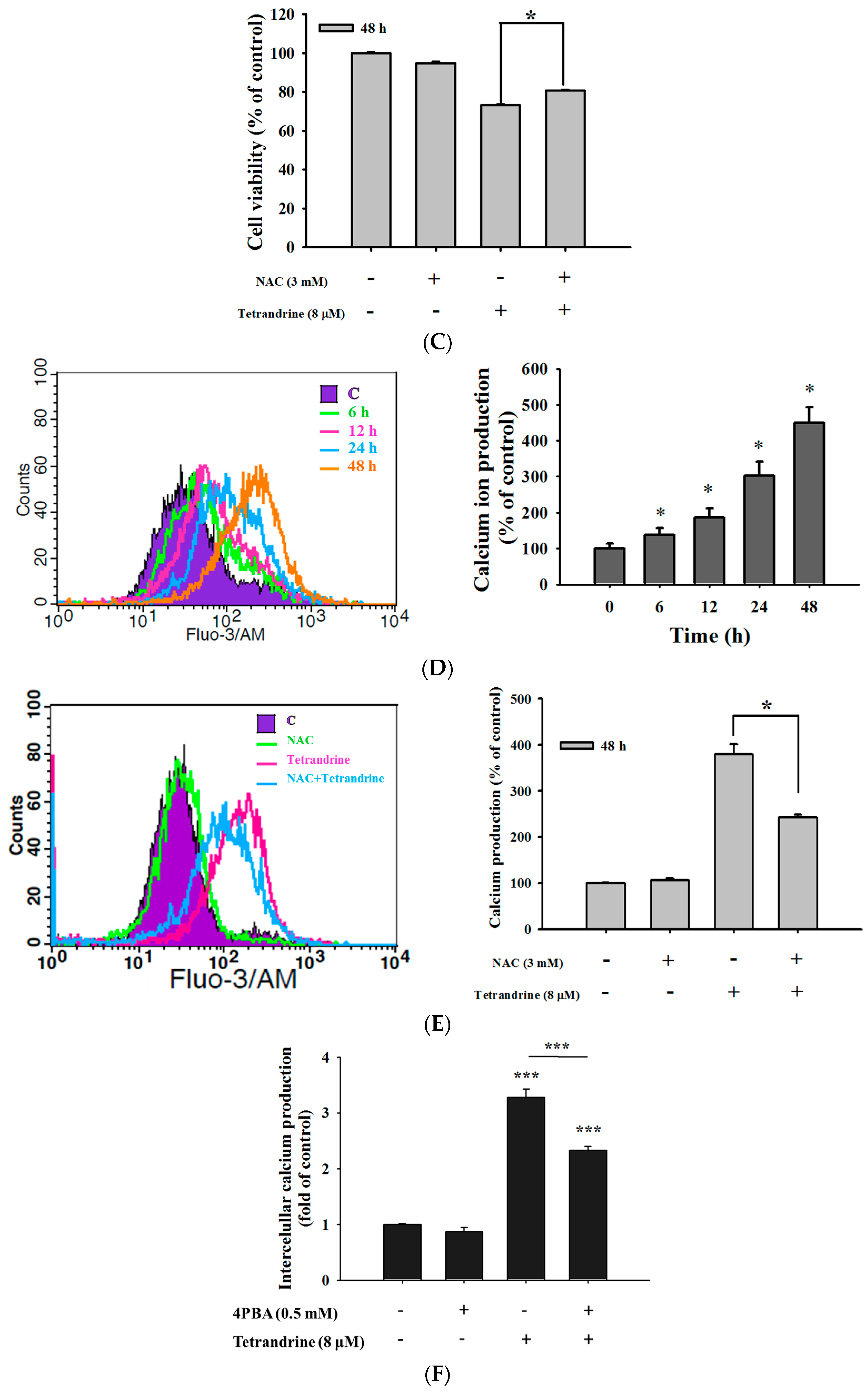
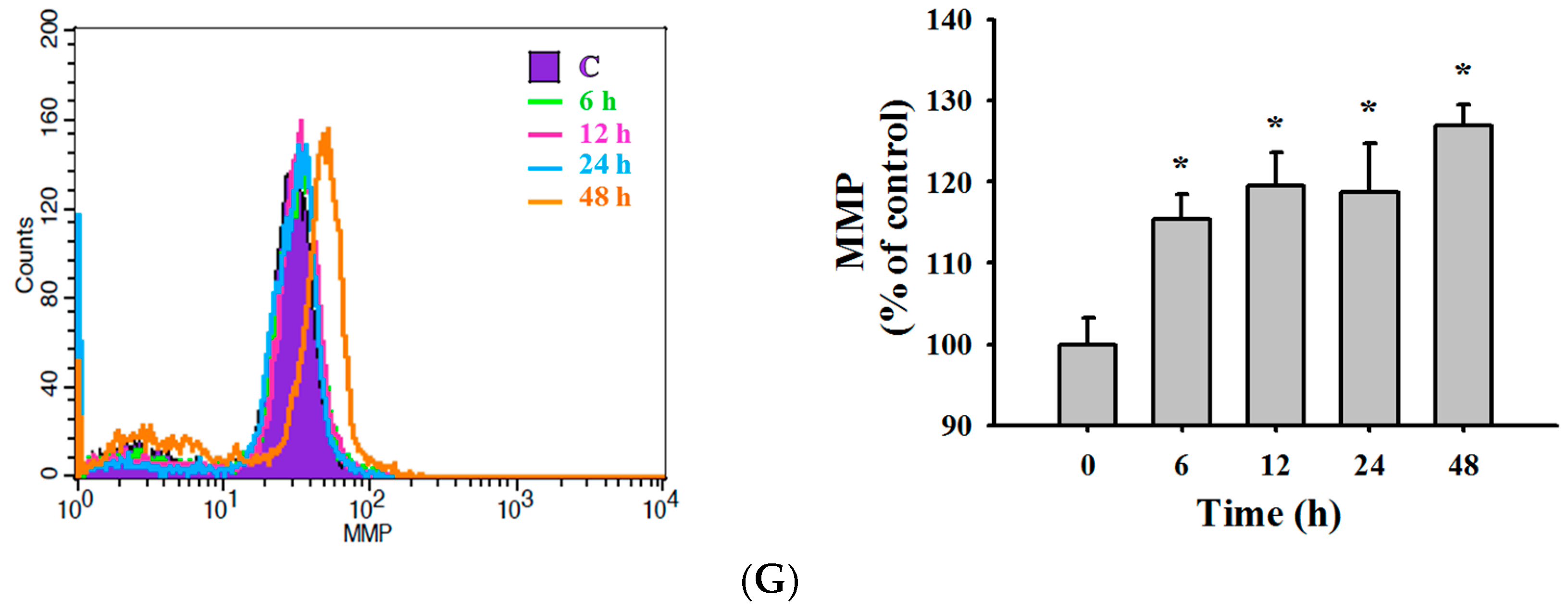
2.4. TET Induced Reactive Oxygen Species (ROS) and Ca2+ Productions but no Change in the Levels of Mitochondrial Membrane Potential (ΔΨm) in NPC-TW 076 Cells
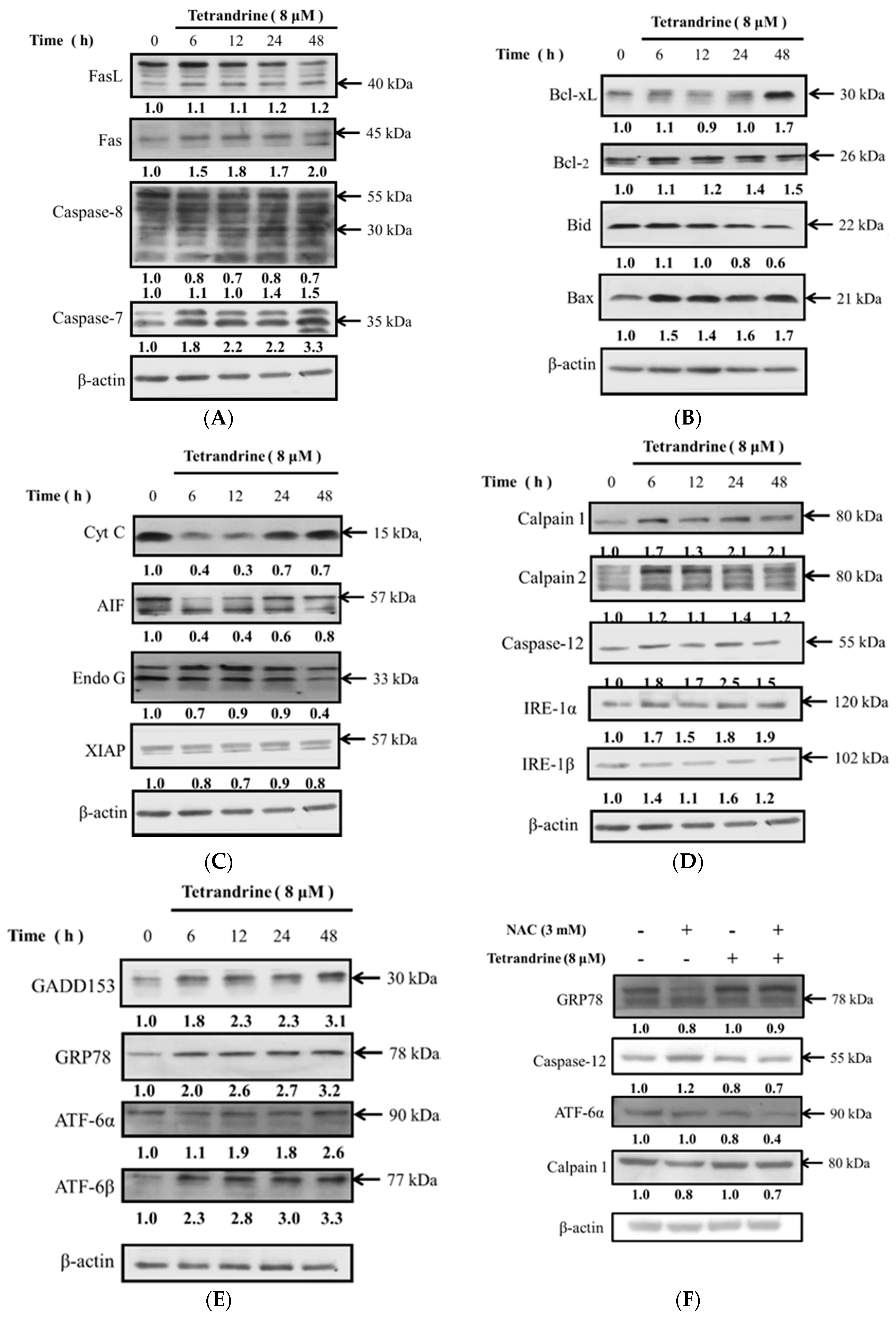
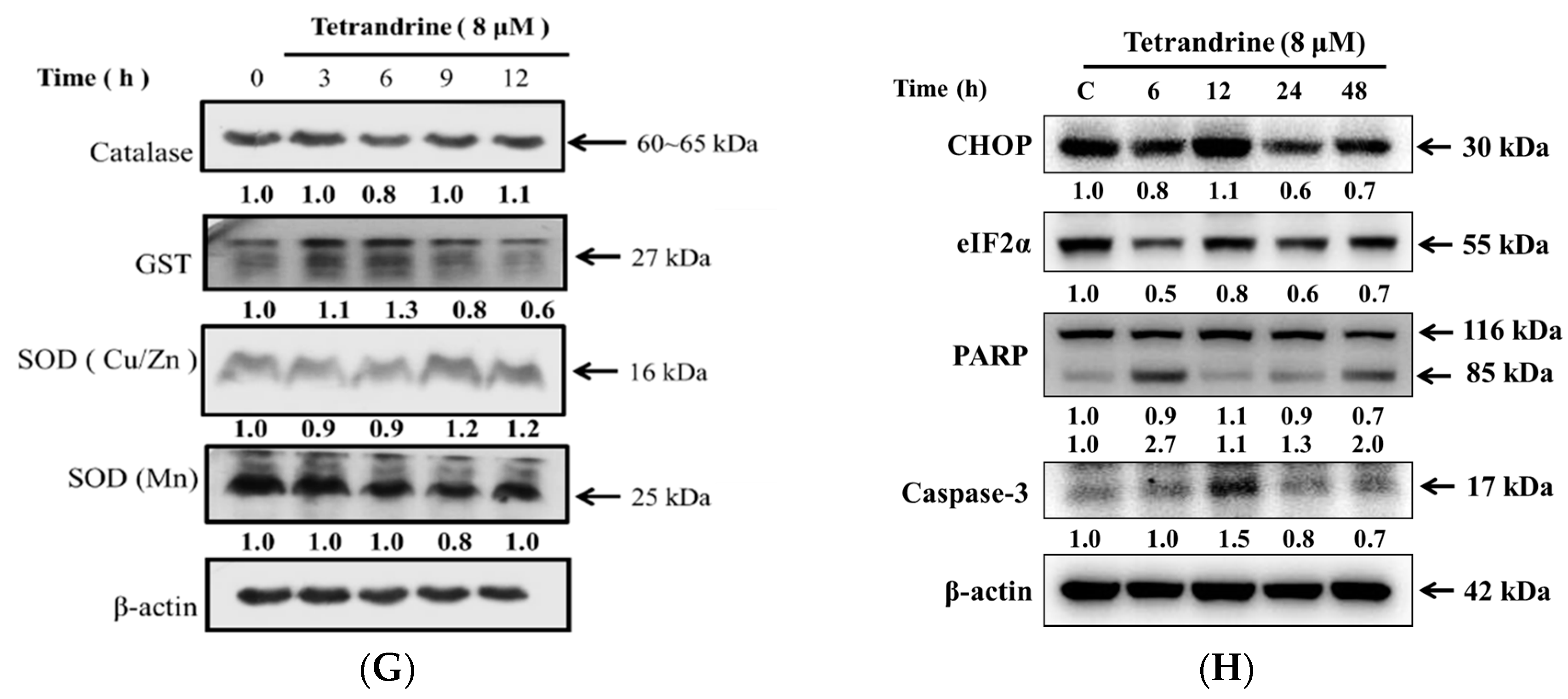
2.5. TET Altered Apoptosis Associated Protein Expression in NPC-TW 076 Cells
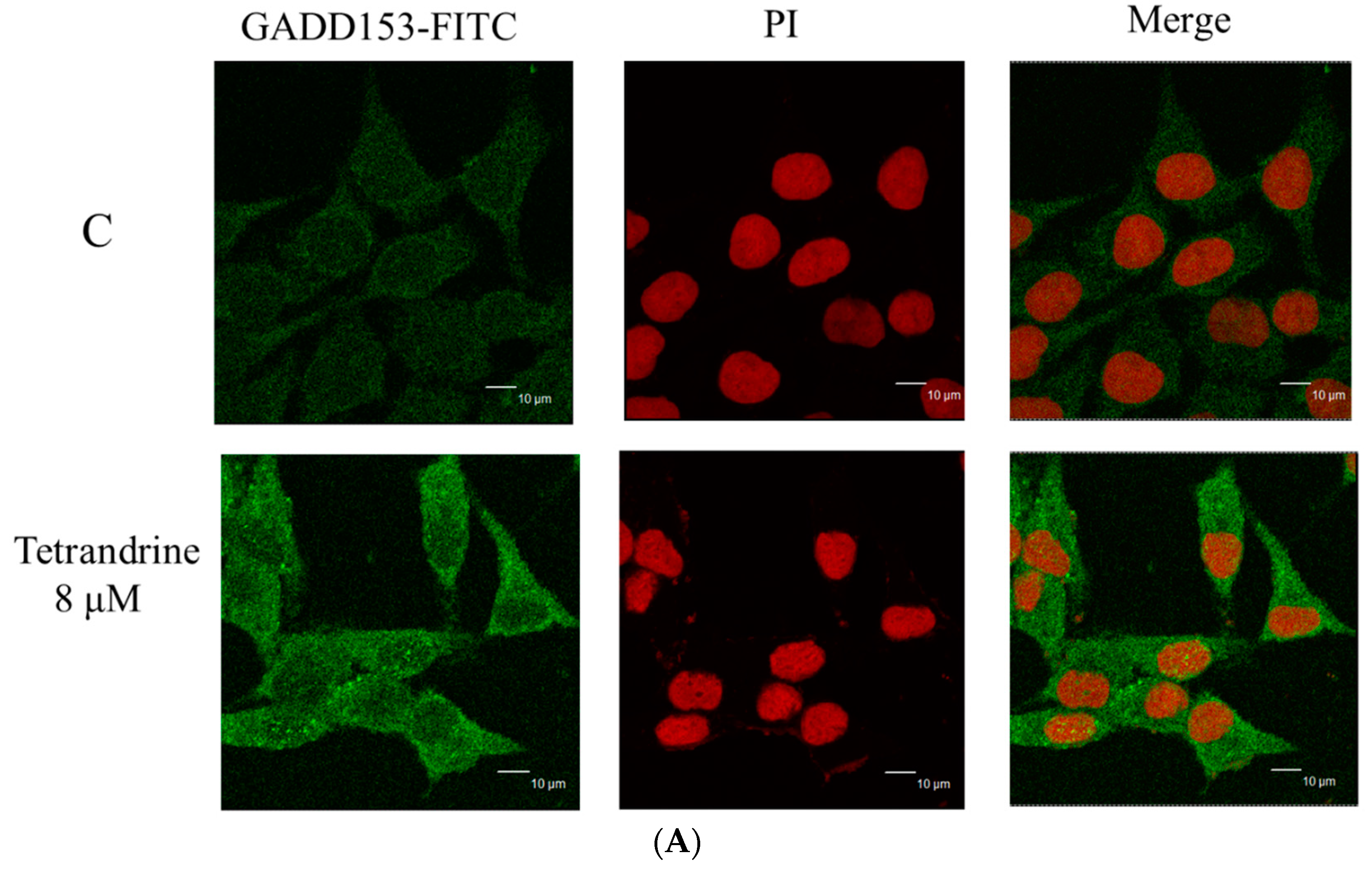
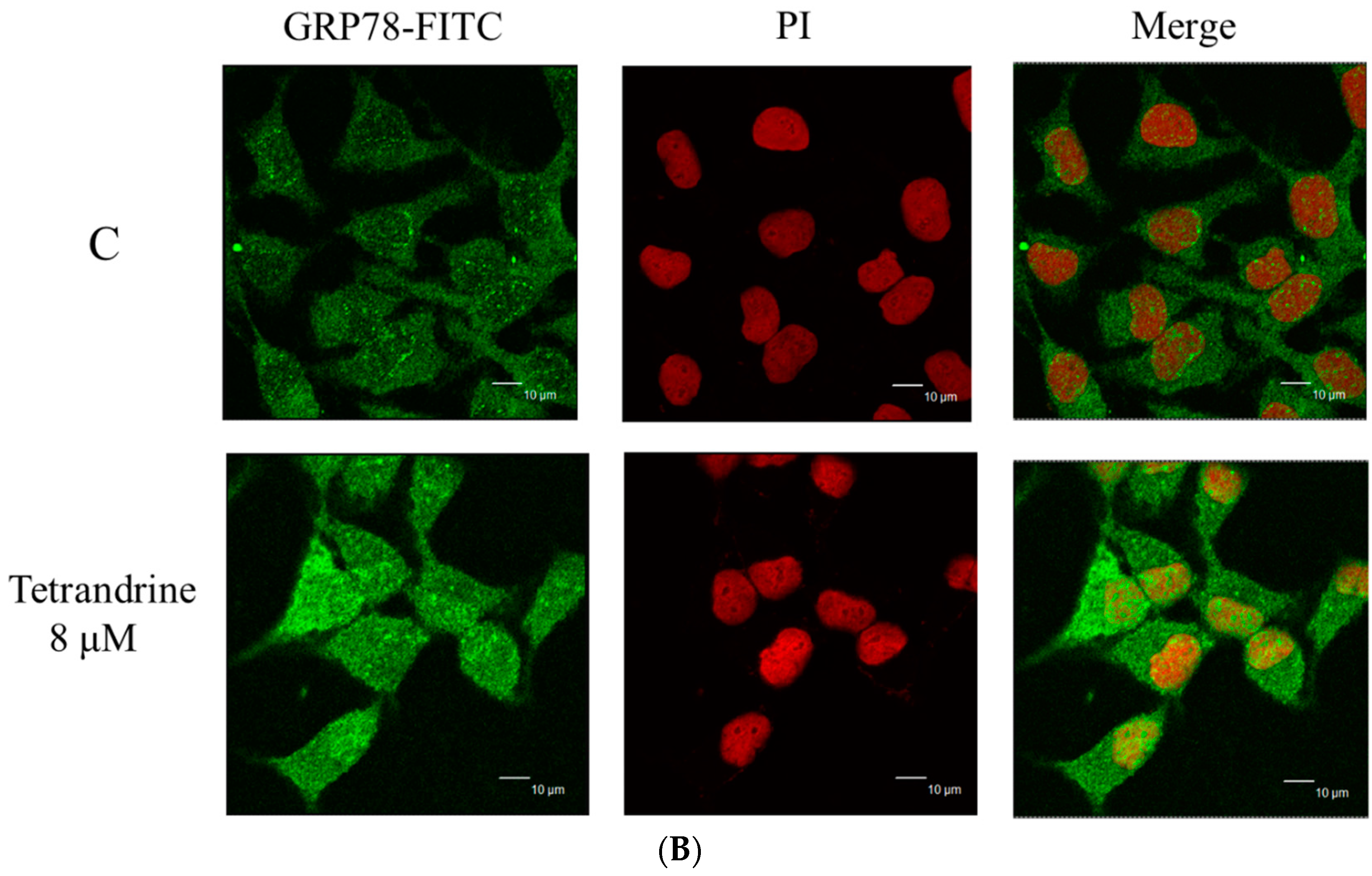
2.6. TET Altered the Translocation of Apoptotic Associated Proteins in NPC-TW 076 Cells
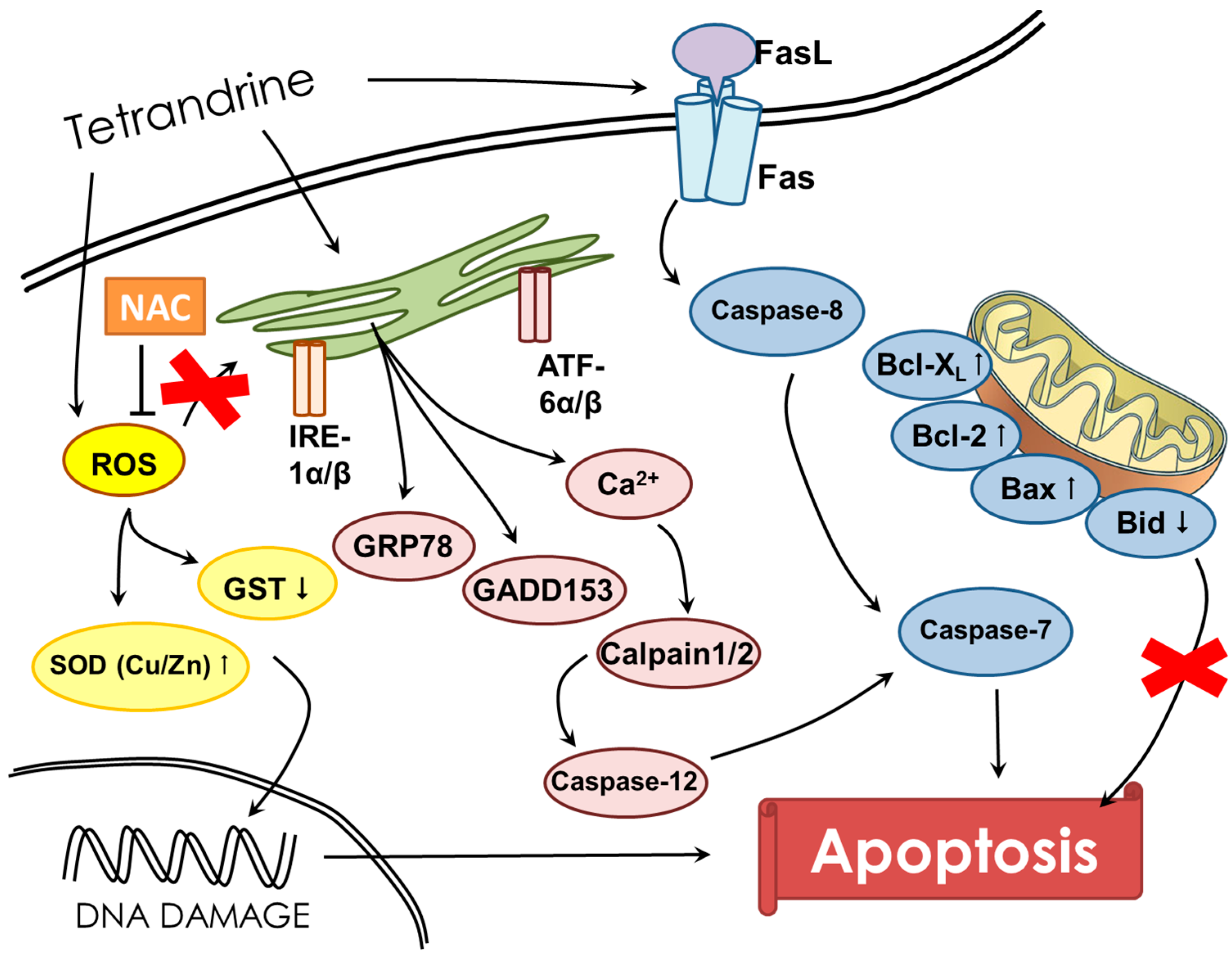
3. Discussion
4. Experimental Section
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. Cell Viability and Morphology Examinations
4.4. Chromatin Condensation Stained with DAPI
4.5. Cell Cycle and Sub-G1 Phase (Apoptosis) Assays
4.6. Measurements of Reactive Oxygen Species (ROS), Intracellular Ca2+ and Mitochondrial Membrane Potential (Ψm)
4.7. Western Blotting Analysis
4.8. Confocal Laser Scanning Microscopy Assay
4.9. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chang, E.T.; Adami, H.O. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1765–1777. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.C. Nasopharyngeal carcinoma: Molecular biomarker discovery and progress. Mol. Cancer 2007, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Mortality Report of the Department of Health; Ministry of Health and Welfare: Taipei, Taiwan, 2016.
- Lo, K.W.; Huang, D.P. Genetic and epigenetic changes in nasopharyngeal carcinoma. Semin. Cancer Biol. 2002, 12, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Chan, A.T. Nasopharyngeal carcinoma: Molecular pathogenesis and therapeutic developments. Expert Rev. Mol. Med. 2007, 9, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Young, L.S.; Murray, P.G. Epstein-Barr virus and oncogenesis: From latent genes to tumours. Oncogene 2003, 22, 5108–5121. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.B.; Hui, E.P.; Chan, A.T. Systemic approach to improving treatment outcome in nasopharyngeal carcinoma: Current and future directions. Cancer Sci. 2008, 99, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Ruan, L.; Wang, G.L.; Yi, H.; Chen, Y.; Tang, C.E.; Zhang, P.F.; Li, M.Y.; Li, C.; Peng, F.; Li, J.L.; et al. Raf kinase inhibitor protein correlates with sensitivity of nasopharyngeal carcinoma to radiotherapy. J. Cell. Biochem. 2010, 110, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Galluzzi, L.; Kroemer, G. Targeting mitochondria for cancer therapy. Nat. Rev. Drug Discov. 2010, 9, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Z. Effects and mechanisms of emodin on cell death in human lung squamous cell carcinoma. Br. J. Pharmacol. 2001, 134, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Boya, P.; Cohen, I.; Zamzami, N.; Vieira, H.L.; Kroemer, G. Endoplasmic reticulum stress-induced cell death requires mitochondrial membrane permeabilization. Cell. Death Differ. 2002, 9, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, D.A.; Simmons, T.D.; Slater, K.J.; Crouch, S.P. Measurement of the ADP:ATP ratio in human leukaemic cell lines can be used as an indicator of cell viability, necrosis and apoptosis. J. Immunol. Methods 2000, 240, 79–92. [Google Scholar] [CrossRef]
- Ishitsuka, K.; Hideshima, T.; Hamasaki, M.; Raje, N.; Kumar, S.; Podar, K.; Le Gouill, S.; Shiraishi, N.; Yasui, H.; Roccaro, A.M.; et al. Novel inosine monophosphate dehydrogenase inhibitor VX-944 induces apoptosis in multiple myeloma cells primarily via caspase-independent AIF/Endo G pathway. Oncogene 2005, 24, 5888–5896. [Google Scholar] [CrossRef] [PubMed]
- Ip, S.W.; Chu, Y.L.; Yu, C.S.; Chen, P.Y.; Ho, H.C.; Yang, J.S.; Huang, H.Y.; Chueh, F.S.; Lai, T.Y.; Chung, J.G. Bee venom induces apoptosis through intracellular Ca2+ -modulated intrinsic death pathway in human bladder cancer cells. Int. J. Urol. 2012, 19, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Lemos, J.R.; Iadecola, C. Herbal alkaloid tetrandrine: Fron an ion channel blocker to inhibitor of tumor proliferation. Trends Pharmacol. Sci. 2004, 25, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.J.; Bao, J.L.; Chen, X.P.; Huang, M.; Wang, Y.T. Alkaloids isolated from natural herbs as the anticancer agents. Evid. Based Complement. Altern. Med. 2012, 2012, 485042. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Lee, B.H. Induction of apoptosis in human hepatoblastoma cells by tetrandrine via caspase-dependent Bid cleavage and cytochrome c release. Biochem. Pharmacol. 2003, 66, 725–731. [Google Scholar] [CrossRef]
- Kuo, P.L.; Lin, C.C. Tetrandrine-induced cell cycle arrest and apoptosis in Hep G2 cells. Life Sci. 2003, 73, 243–252. [Google Scholar] [CrossRef]
- Yu, V.W.; Ho, W.S. Tetrandrine inhibits hepatocellular carcinoma cell growth through the caspase pathway and G2/M phase. Oncol. Rep. 2013, 29, 2205–2210. [Google Scholar] [PubMed]
- Chen, T.; Ji, B.; Chen, Y. Tetrandrine triggers apoptosis and cell cycle arrest in human renal cell carcinoma cells. J. Nat. Med. 2014, 68, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.S.; Chang, S.H.; Chung, Y.S.; Shin, J.Y.; Park, S.J.; Lee, E.S.; Hwang, S.K.; Kwon, J.T.; Tehrani, A.M.; Woo, M.; et al. Synergistic effect of ERK inhibition on tetrandrine-induced apoptosis in A549 human lung carcinoma cells. J. Vet. Sci. 2009, 10, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Su, B.; Liu, R.; Wu, D.; He, D. Tetrandrine induces apoptosis and triggers caspase cascade in human bladder cancer cells. J. Surg. Res. 2011, 166, e45–e51. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.; Chen, Y.; Chen, J.C.; Lin, T.Y.; Tseng, S.H. Tetrandrine induces apoptosis and growth suppression of colon cancer cells in mice. Cancer Lett. 2010, 287, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.T.; Chiang, L.C.; Lin, Y.T.; Lin, C.C. Antiproliferative and apoptotic effects of tetrandrine on different human hepatoma cell lines. Am. J. Chin. Med. 2006, 34, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.H.; Wan, J.Y.; Gong, X.; Li, H.Z.; Cheng, Y. Tetrandrine enhances cytotoxicity of cisplatin in human drug-resistant esophageal squamous carcinoma cells by inhibition of multidrug resistance-associated protein 1. Oncol. Rep. 2012, 28, 1681–1686. [Google Scholar] [PubMed]
- Gao, J.L.; Ji, X.; He, T.C.; Zhang, Q.; He, K.; Zhao, Y.; Chen, S.H.; Lv, G.Y. Tetrandrine Suppresses Cancer Angiogenesis and Metastasis in 4T1 Tumor Bearing Mice. Evid. Based Complement. Altern. Med. 2013, 2013, 265061. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Kou, B.; Ma, Z.K.; Tang, X.S.; Lv, C.; Ye, M.; Chen, J.Q.; Li, L.; Wang, X.Y.; He, D.L. Tetrandrine suppresses proliferation, induces apoptosis, and inhibits migration and invasion in human prostate cancer cells. Asian J. Androl. 2015, 17, 850–853. [Google Scholar] [PubMed]
- Sun, X.; Xu, R.; Deng, Y.; Cheng, H.; Ma, J.; Ji, J.; Zhou, Y. Effects of Tetrandrine on Apoptosis and Radiosensitivity of Nasopharyngeal Carcinoma Cell Line CNE. Acta Biochim. Biophys. Sin. (Shanghai) 2007, 39, 869–678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.; Huang, P.Y.; Xu, F.; Peng, P.J.; Guan, Z.Z. Phase II clinical study of gemcitabine in the treatment of patients with advanced nasopharyngeal carcinoma after the failure of platinum-based chemotherapy. Cancer Chemother. Pharmacol. 2008, 61, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.B.; Hui, E.P.; Wong, S.C.; Tung, S.Y.; Yuen, K.K.; King, A.; Chan, S.L.; Leung, S.F.; Kam, M.K.; Yu, B.K.; et al. Multicenter phase II study of gemcitabine and oxaliplatin in advanced nasopharyngeal carcinoma-correlation with excision repair cross-complementing-1 polymorphisms. Ann. Oncol. 2009, 20, 1854–1859. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Chang, J.Y.; Liu, T.W.; Lin, C.Y.; Yu, Y.C.; Hong, R.L. Phase II study of gemcitabine plus vinorelbine in the treatment of cisplatin-resistant nasopharyngeal carcinoma. Head Neck 2006, 28, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, X.; Li, W. Tetrandrine, a Chinese plant-derived alkaloid, is a potential candidate for cancer chemotherapy. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Sarsour, E.H.; Kumar, M.G.; Chaudhuri, L.; Kalen, A.L.; Goswami, P.C. Redox control of the cell cycle in health and disease. Antioxid. Redox Signal. 2009, 11, 2985–3011. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Sagara, Y.; Liu, Y.; Maher, P.; Schubert, D. The regulation of reactive oxygen species production during programmed cell death. J. Cell. Biol. 1998, 141, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I.O.; Beydemir, S.; Kufrevioglu, O.I. Evaluation of the antioxidant and antimicrobial activities of clary sage (Salvia sclarea L.). Turk. J. Agric. For. 2004, 28, 9. [Google Scholar]
- Wu, Y.; Zhang, H.; Dong, Y.; Park, Y.M.; Ip, C. Endoplasmic reticulum stress signal mediators are targets of selenium action. Cancer Res. 2005, 65, 9073–9079. [Google Scholar] [CrossRef] [PubMed]
- Mori, K. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell 2000, 101, 451–454. [Google Scholar] [CrossRef]
- Banerjee, A.; Ahmed, H.; Yang, P.; Czinn, S.J.; Blanchard, T.G. Endoplasmic reticulum stress and IRE-1 signaling cause apoptosis in colon cancer cells in response to andrographolide treatment. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Piao, M.J.; Hyun, J.W. Fisetin induces apoptosis in human nonsmall lung cancer cells via a mitochondria-mediated pathway. In Vitro Cell. Dev. Biol. Anim. 2015, 51, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, X.A.; Hu, J.; Jiang, J.K.; Li, Y.; Chan-Salis, K.Y.; Gu, Y.; Chen, G.; Thomas, C.; Pugh, B.F.; et al. ATF4 Gene Network Mediates Cellular Response to the Anticancer PAD Inhibitor YW3–56 in Triple-Negative Breast Cancer Cells. Mol. Cancer Ther. 2015, 14, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell. Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Hendershot, L.M. The ER function BiP is a master regulator of ER function. Mt. Sinai J. Med. 2004, 71, 289–297. [Google Scholar] [PubMed]
- Ma, Y.; Hendershot, L.M. ER chaperone functions during normal and stress conditions. J. Chem. Neuroanat. 2004, 28, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, Y.; Liu, X.; Yan, J.; Su, L.; Liu, X. A novel derivative of tetrandrine (H1) induces endoplasmic reticulum stress-mediated apoptosis and prosurvival autophagy in human non-small cell lung cancer cells. Tumour Biol. 2016, 37, 10403–10413. [Google Scholar] [CrossRef] [PubMed]
- Oyadomari, S.; Mori, M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell. Death Differ. 2004, 11, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-C.; Tsai, S.-Y.; Wang, F.-Y.; Liu, F.-H.; Syu, J.-N.; Tang, F.-Y. Leptin induces cell invasion and the upregulation of matrilysin in human colon cancer cells. Biomedicine 2013, 3, 174–180. [Google Scholar] [CrossRef]
- Lin, M.L.; Chen, S.S.; Ng, S.H. CHM-1 Suppresses Formation of Cell Surface-associated GRP78-p85alpha Complexes, Inhibiting PI3K-AKT Signaling and Inducing Apoptosis of Human Nasopharyngeal Carcinoma Cells. Anticancer Res. 2015, 35, 5359–5368. [Google Scholar] [PubMed]
- Yu, F.S.; Huang, A.C.; Yang, J.S.; Yu, C.S.; Lu, C.C.; Chiang, J.H.; Chiu, C.F.; Chung, J.G. Safrole induces cell death in human tongue squamous cancer SCC-4 cells through mitochondria-dependent caspase activation cascade apoptotic signaling pathways. Environ. Toxicol. 2012, 27, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-Y.; Fan, C.-W.; Maa, M.-C.; Leu, T.-H. Lipopolysaccharide-promoted proliferation of Caco-2 cells is mediated by c-Src induction and ERK activation. Biomedicine 2015, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hsia, T.C.; Lin, J.H.; Hsu, S.C.; Tang, N.Y.; Lu, H.F.; Wu, S.H.; Lin, J.G.; Chung, J.G. Cantharidin induces DNA damage and inhibits DNA repair-associated protein levels in NCI-H460 human lung cancer cells. Environ. Toxicol. 2015, 30, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-J.; Peng, S.-F.; Lin, M.-L.; Kuo, C.-L.; Lu, K.-W.; Liao, C.-L.; Ma, Y.-S.; Chueh, F.-S.; Liu, K.-C.; Yu, F.-S.; et al. Tetrandrine Induces Apoptosis of Human Nasopharyngeal Carcinoma NPC-TW 076 Cells through Reactive Oxygen Species Accompanied by an Endoplasmic Reticulum Stress Signaling Pathway. Molecules 2016, 21, 1353. https://doi.org/10.3390/molecules21101353
Lin Y-J, Peng S-F, Lin M-L, Kuo C-L, Lu K-W, Liao C-L, Ma Y-S, Chueh F-S, Liu K-C, Yu F-S, et al. Tetrandrine Induces Apoptosis of Human Nasopharyngeal Carcinoma NPC-TW 076 Cells through Reactive Oxygen Species Accompanied by an Endoplasmic Reticulum Stress Signaling Pathway. Molecules. 2016; 21(10):1353. https://doi.org/10.3390/molecules21101353
Chicago/Turabian StyleLin, Ya-Jing, Shu-Fen Peng, Meng-Liang Lin, Chao-Lin Kuo, Kung-Wen Lu, Ching-Lung Liao, Yi-Shih Ma, Fu-Shin Chueh, Kuo-Ching Liu, Fu-Shun Yu, and et al. 2016. "Tetrandrine Induces Apoptosis of Human Nasopharyngeal Carcinoma NPC-TW 076 Cells through Reactive Oxygen Species Accompanied by an Endoplasmic Reticulum Stress Signaling Pathway" Molecules 21, no. 10: 1353. https://doi.org/10.3390/molecules21101353
APA StyleLin, Y.-J., Peng, S.-F., Lin, M.-L., Kuo, C.-L., Lu, K.-W., Liao, C.-L., Ma, Y.-S., Chueh, F.-S., Liu, K.-C., Yu, F.-S., & Chung, J.-G. (2016). Tetrandrine Induces Apoptosis of Human Nasopharyngeal Carcinoma NPC-TW 076 Cells through Reactive Oxygen Species Accompanied by an Endoplasmic Reticulum Stress Signaling Pathway. Molecules, 21(10), 1353. https://doi.org/10.3390/molecules21101353





