Palladium-Catalyzed C–H Arylation of 1,2,3-Triazoles
Abstract
:1. Introduction
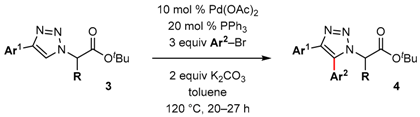
2. Results
3. Materials and Methods
3.1. General Methods
3.2. General Precedure for C–H Arylation and Compound Characterization
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Cy-JohnPhos | (2-Biphenyl)dicyclohexylphosphine |
| dba | dibenzylideneacetone |
| DMF | N,N-Dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| NMP | N-Methylpyrrolidine |
References
- Kolb, H.C.; Sharpless, K.B. The growing impact of click chemistry on drug discovery. Drug Discov. Today 2003, 8, 1128–1137. [Google Scholar] [CrossRef]
- Agalave, S.G.; Maujan, S.R.; Pore, V.S. Click chemistry: 1,2,3-triazoles as pharmacophores. Chem. Asian J. 2011, 6, 2696–2718. [Google Scholar] [CrossRef] [PubMed]
- Mosesa, J.E.; Moorhouse, A.D. The growing applications of click chemistry. Chem. Soc. Rev. 2007, 36, 1249–1262. [Google Scholar] [CrossRef] [PubMed]
- Lauria, A.; Mingoia, R.D.F.; Terenzi, A.; Martorana, A.; Barone, G.; Almerico, A.M. 1,2,3-triazole in heterocyclic compounds, endowed with biological activity, through 1,3-dipolar cycloadditions. Eur. J. Org. Chem. 2014, 3289–3306. [Google Scholar] [CrossRef]
- Angella, Y.L.; Burgess, K. Peptidomimetics via copper-catalyzed azide–alkyne cycloadditions. Chem. Soc. Rev. 2007, 36, 1674–1689. [Google Scholar] [CrossRef] [PubMed]
- Hawker, C.J.; Wooley, K.L. The convergence of synthetic organic and polymer chemistries. Science 2005, 309, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.-F. 1,3-dipolar cycloadditions of azides and alkynes: A universal ligation tool in polymer and materials science. Angew. Chem. Int. Ed. 2007, 46, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, X.; Xue, P.; Sun, H.H.Y.; Williams, I.D.; Sharpless, K.B.; Fokin, V.V.; Jia, G. Ruthenium-catalyzed cycloaddition of alkynes and organic azides. J. Am. Chem. Soc. 2005, 127, 15998–15999. [Google Scholar] [CrossRef] [PubMed]
- Bock, V.D.; Hiemstra, H.; van Maarseveen, J.H. CuI-catalyzed alkyne–azide “click” cycloadditions from a mechanistic and synthetic perspective. Eur. J. Org. Chem. 2006, 51–68. [Google Scholar] [CrossRef]
- Hein, J.E.; Fokin, V.V. Copper-catalyzed azide–alkyne cycloaddition (CuAAC) and beyond: New reactivity of copper(I) acetylides. Chem. Soc. Rev. 2010, 39, 1302–1315. [Google Scholar] [CrossRef] [PubMed]
- Totobenazara, J.; Burke, A.J. New click-chemistry methods for 1,2,3-triazoles synthesis: Recent advances and applications. Tetrahedron Lett. 2015, 56, 2853–2859. [Google Scholar] [CrossRef]
- Singh, M.S.; Chowdhury, S.; Koley, S. Advances of azide-alkyne cycloaddition-click chemistry over the recent decade. Tetrahedron 2016, 72, 5257–5283. [Google Scholar] [CrossRef]
- Krasiński, A.; Fokin, V.V.; Sharpless, K.B. Direct synthesis of 1,5-disubstituted-4-magnesio-1,2,3-triazoles, revisited. Org. Lett. 2004, 6, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- Majireck, M.M.; Weinreb, S.M. A study of the scope and regioselectivity of the ruthenium-catalyzed [3 + 2]-cycloaddition of azides with internal alkynes. J. Org. Chem. 2006, 71, 8680–8683. [Google Scholar] [CrossRef] [PubMed]
- Boren, B.C.; Narayan, S.; Rasmussen, L.K.; Zhang, L.; Zhao, H.; Lin, Z.; Jia, G.; Fokin, V.V. Ruthenium-catalyzed azide-alkyne cycloaddition: Scope and mechanism. J. Am. Chem. Soc. 2008, 130, 8923–8930. [Google Scholar] [CrossRef] [PubMed]
- Hein, J.E.; Tripp, J.C.; Krasnova, L.B.; Sharpless, K.B.; Fokin, V.V. Copper(I)-catalyzed cycloaddition of organic azides and 1-iodoalkynes. Angew. Chem. Int. Ed. 2009, 48, 8018–8021. [Google Scholar] [CrossRef] [PubMed]
- Meza-Aviña, M.E.; Patel, M.K.; Lee, C.B.; Dietz, T.J.; Croatt, M.P. Selective formation of 1,5-substituted sulfonyl triazoles using acetylides and sulfonyl azides. Org. Lett. 2011, 13, 2984–2987. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.D.; Greaney, M.F. Zinc mediated azide-alkyne ligation to 1,5- and 1,4,5-substituted 1,2,3-triazoles. Org. Lett. 2013, 15, 4826–4829. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Li, H.; Song, C.; Ma, Y.; Zhou, L.; Tung, C.-H.; Xu, Z. Cu/Pd-catalyzed, three-component click reaction of azide, alkyne, and aryl halide: One-pot strategy toward trisubstituted triazoles. Org. Lett. 2015, 17, 2860–2863. [Google Scholar] [CrossRef] [PubMed]
- Chuprakov, S.; Chernyak, N.; Dudnik, A.S.; Gevorgyan, V. Direct Pd-catalyzed arylation of 1,2,3-triazoles. Org. Lett. 2007, 9, 2333–2336. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, L.; Potukuchi, H.K.; Landsberg, D.; Vicente, R. Copper-catalyzed “click” reaction/direct arylation sequence: Modular syntheses of 1,2,3-triazoles. Org. Lett. 2008, 10, 3081–3084. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, L.; Vicente, R. Catalytic direct arylations in polyethylene glycol (PEG): Recyclable palladium(0) catalyst for C−H bond cleavages in the presence of air. Org. Lett. 2009, 11, 4922–4925. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, L.; Vicente, R.; Born, R. Palladium-catalyzed direct arylations of 1,2,3-triazoles with aryl chlorides using conventional heating. Adv. Synth. Catal. 2008, 350, 741–748. [Google Scholar] [CrossRef]
- Ackermann, L.; Althammer, A.; Fenner, S. Palladium-catalyzed direct arylations of heteroarenes with tosylates and mesylates. Angew. Chem. Int. Ed. 2009, 48, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Liégault, B.; Lapointe, D.; Caron, L.; Vlassova, A.; Fagnou, K. Establishment of broadly applicable reaction conditions for the palladium-catalyzed direct arylation of heteroatom-containing aromatic compounds. J. Org. Chem. 2009, 74, 1826–1834. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Tan, X.; Chen, C. Palladium-catalyzed direct functionalization of imidazolinone: Synthesis of dibromophakellstatin. J. Am. Chem. Soc. 2007, 129, 7768–7769. [Google Scholar] [CrossRef] [PubMed]
- Ryabov, A.D. Mechanisms of intramolecular activation of C–H bonds in transition-metal complexes. Chem. Rev. 1990, 90, 403–424. [Google Scholar] [CrossRef]
- Lapointe, D.; Fagnou, K. Overview of the mechanistic work on the concerted metallation–deprotonation pathway. Chem. Lett. 2010, 39, 1118–1126. [Google Scholar] [CrossRef]
- Gómez, M.; Granell, J.; Martinez, M. Variable-temperature and -pressure kinetics and mechanism of the cyclopalladation reaction of imines in aprotic solvent. Organometallics 1997, 16, 2539–2546. [Google Scholar] [CrossRef]
- Biswas, B.; Sugimoto, M.; Sakaki, S. C−H bond activation of benzene and methane by M(η2-O2CH)2 (M = Pd or Pt). A theoretical study. Organometallics 2000, 19, 3895–3908. [Google Scholar] [CrossRef]
- Kurzeev, S.A.; Kazankov, G.M.; Ryabov, A.D. Second- and inverse order pathways in the mechanism of orthopalladation of primary amines. Inorg. Chim. Acta 2002, 340, 192–196. [Google Scholar] [CrossRef]
- Davies, D.L.; Donald, S.M.A.; Macgregor, S.A. Computational study of the mechanism of cyclometalation by palladium acetate. J. Am. Chem. Soc. 2005, 127, 13754–13755. [Google Scholar] [CrossRef] [PubMed]
- García-Cuadrado, D.; Braga, A.A.C.; Maseras, F.; Echavarren, A.M. Proton abstraction mechanism for the palladium-catalyzed intramolecular arylation. J. Am. Chem. Soc. 2006, 128, 1066–1067. [Google Scholar] [CrossRef] [PubMed]
- Lafrance, M.; Rowley, C.N.; Woo, T.K.; Fagnou, K. Catalytic intermolecular direct arylation of perfluorobenzenes. J. Am. Chem. Soc. 2006, 128, 8754–8756. [Google Scholar] [CrossRef] [PubMed]
- Lafrance, M.; Fagnou, K. Palladium-catalyzed benzene arylation: Incorporation of catalytic pivalic acid as a proton shuttle and a key element in catalyst design. J. Am. Chem. Soc. 2006, 128, 16496–16497. [Google Scholar] [CrossRef] [PubMed]
- Moradi, W.A.; Buchwald, S.L. Palladium-catalyzed α-arylation of esters. J. Am. Chem. Soc. 2001, 123, 7996–8002. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, M.; Lee, S.; Liu, X.; Wolkowski, J.P.; Hartwig, J.F. Efficient synthesis of α-aryl esters by room-temperature palladium-catalyzed coupling of aryl halides with ester enolates. J. Am. Chem. Soc. 2002, 124, 12557–12565. [Google Scholar] [CrossRef] [PubMed]
- Bellina, F.; Rossi, R. Transition metal-catalyzed direct arylation of substrates with activated sp3-hybridized C–H bonds and some of their synthetic equivalents with aryl halides and pseudohalides. Chem. Rev. 2010, 110, 1082–1146. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 4 are available from the authors.
| Entry | Catalyst | Ligand | Base | Temperature | Time | Solvent | Yield |
|---|---|---|---|---|---|---|---|
| 1 | CuI | – | t-BuLi | 120 °C | 24 h | DMF | 10% a |
| 2 | Pd(OAc)2 | – | n-Bu4NOAc | 120 °C | 24 h | NMP | 15% a |
| 3 | Pd(OAc)2 | – | n-Bu4NOAc | 120 °C | 20 h | DMF | 21% a |
| 4 | Pd(OAc)2 | – | Cs2CO3 | 120 °C | 20 h | DMF | 6% a |
| 5 | Pd(OAc)2 | – | K2CO3 | 120 °C | 20 h | DMF | 31% a |
| 6 | Pd(OAc)2 | PPh3 | K2CO3 | 120 °C | 20 h | DMF | 75% a 68% b |
| 7 | Pd(OAc)2 | P(o-Tol)3 | K2CO3 | 120 °C | 20 h | DMF | 70% a |
| 8 | Pd(OAc)2 | PPh3 | K2CO3 | 100 °C | 24 h | DMF | 77% a |
| 9 | Pd(OAc)2 | P(n-Bu)3 | K2CO3 | 100 °C | 24 h | DMF | <5% a |
| 10 | Pd(OAc)2 | PCy3 | K2CO3 | 100 °C | 24 h | DMF | 20% a |
| 11 | Pd(OAc)2 | P(2-furyl)3 | K2CO3 | 100 °C | 24 h | DMF | 29% a |
| 12 | Pd(OAc)2 | Cy-JohnPhos | K2CO3 | 100 °C | 24 h | DMF | 19% a |
| 13 | Pd2(dba)3 c | K2CO3 | 100 °C | 24 h | DMF | 7% a | |
| 14 | Pd(OAc)2 | PPh3 | K2CO3 | 120 °C | 20 h | toluene | 95% a 89% b |
| Entry | Ar1 | Ar2 | R | Yield |
|---|---|---|---|---|
| 1 | 4-pyridyl | phenyl | H | 89% |
| 2 | 4-pyridyl | 4-MeO-phenyl | H | 85% |
| 3 | 4-pyridyl | 4-EtO2C-phenyl | H | 92% |
| 4 | 4-pyridyl | 4-F3C-phenyl | H | 83% |
| 5 | 4-pyridyl | 4-NC-phenyl | H | 79% |
| 6 | 4-pyridyl | 4-F-phenyl | H | 51% |
| 7 | 4-pyridyl | 3-Me-phenyl | H | 86% |
| 8 | 4-pyridyl | 3-OHC-phenyl | H | 32% |
| 9 | 4-pyridyl | 2-MeO-phenyl | H | 82% |
| 10 | 4-pyridyl | 2-Me-phenyl | H | 49% |
| 11 | 4-pyridyl | 1-naphthyl | H | 78% |
| 12 | 4-pyridyl | phenyl | H | 80% |
| 13 | 4-pyridyl | phenyl | H | 84% |
| 14 | phenyl | phenyl | H | 80% |
| 15 | 4-MeO-phenyll | phenyl | H | 64% |
| 16 | 2-F3C-phenyl | phenyl | H | 50% |
| 17 | 4-pyridyl | phenyl | Me | 20% a |
| 18 | 4-pyridyl | phenyl | Et | 8% a |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; You, L.; Chen, C. Palladium-Catalyzed C–H Arylation of 1,2,3-Triazoles. Molecules 2016, 21, 1268. https://doi.org/10.3390/molecules21101268
Zhang C, You L, Chen C. Palladium-Catalyzed C–H Arylation of 1,2,3-Triazoles. Molecules. 2016; 21(10):1268. https://doi.org/10.3390/molecules21101268
Chicago/Turabian StyleZhang, Chengwei, Lin You, and Chuo Chen. 2016. "Palladium-Catalyzed C–H Arylation of 1,2,3-Triazoles" Molecules 21, no. 10: 1268. https://doi.org/10.3390/molecules21101268
APA StyleZhang, C., You, L., & Chen, C. (2016). Palladium-Catalyzed C–H Arylation of 1,2,3-Triazoles. Molecules, 21(10), 1268. https://doi.org/10.3390/molecules21101268