Preparation of Thermo-Responsive Poly(ionic liquid)s-Based Nanogels via One-Step Cross-Linking Copolymerization
Abstract
:1. Introduction
2. Results and Discussion
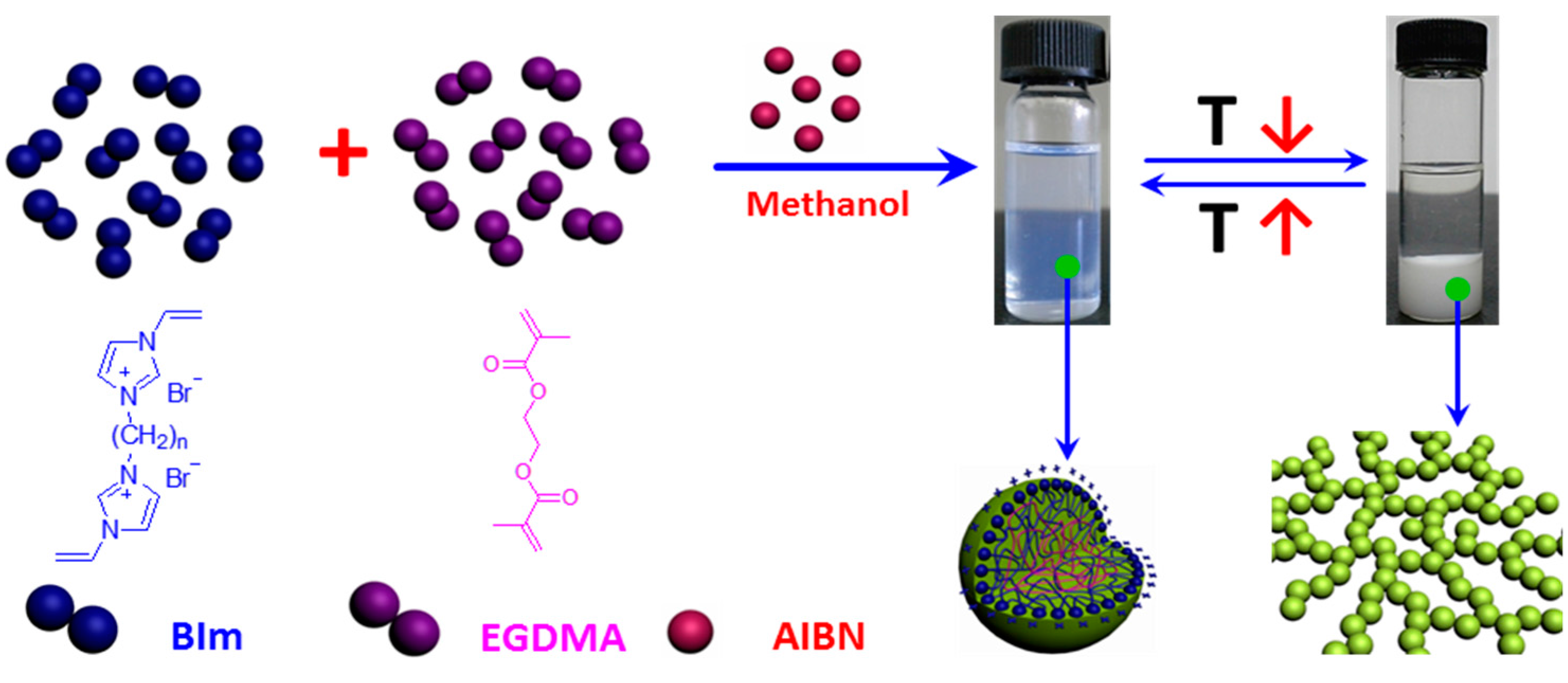
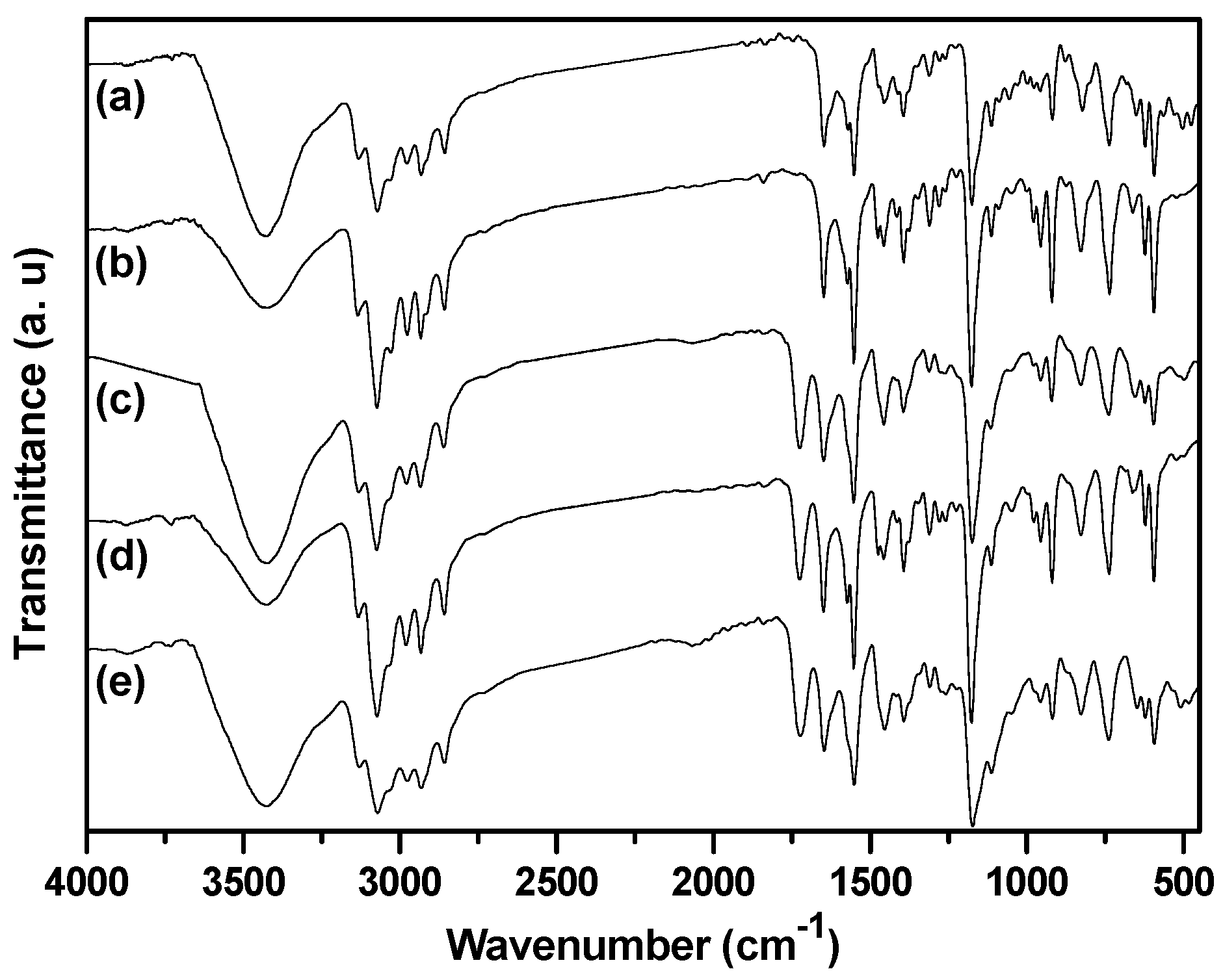
2.1. Synthesis of Biimidazolium Salt-Based Monomers
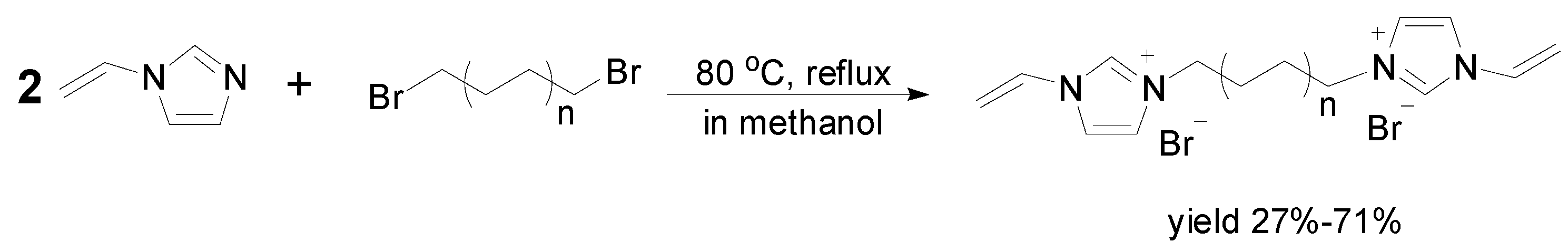
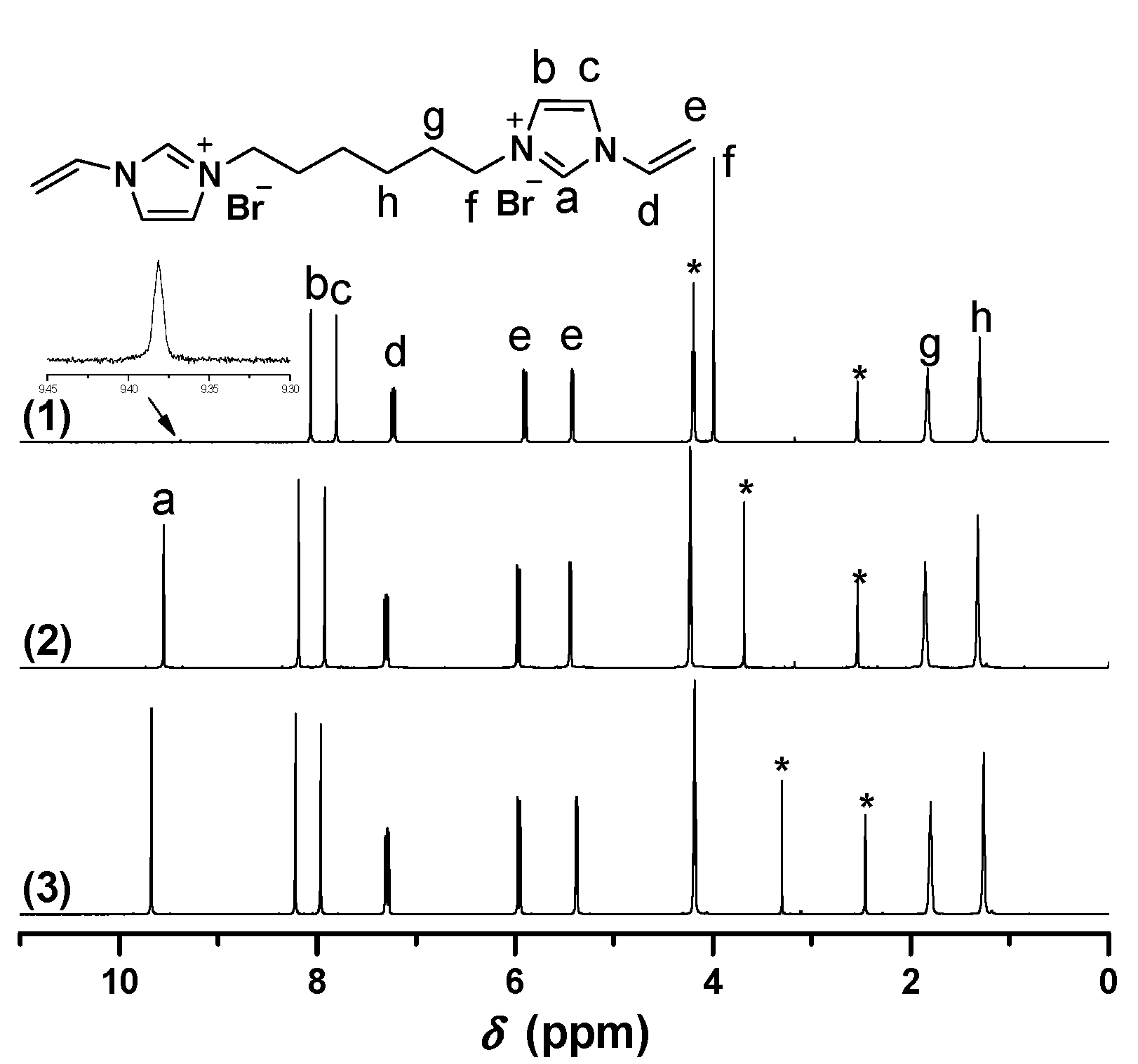
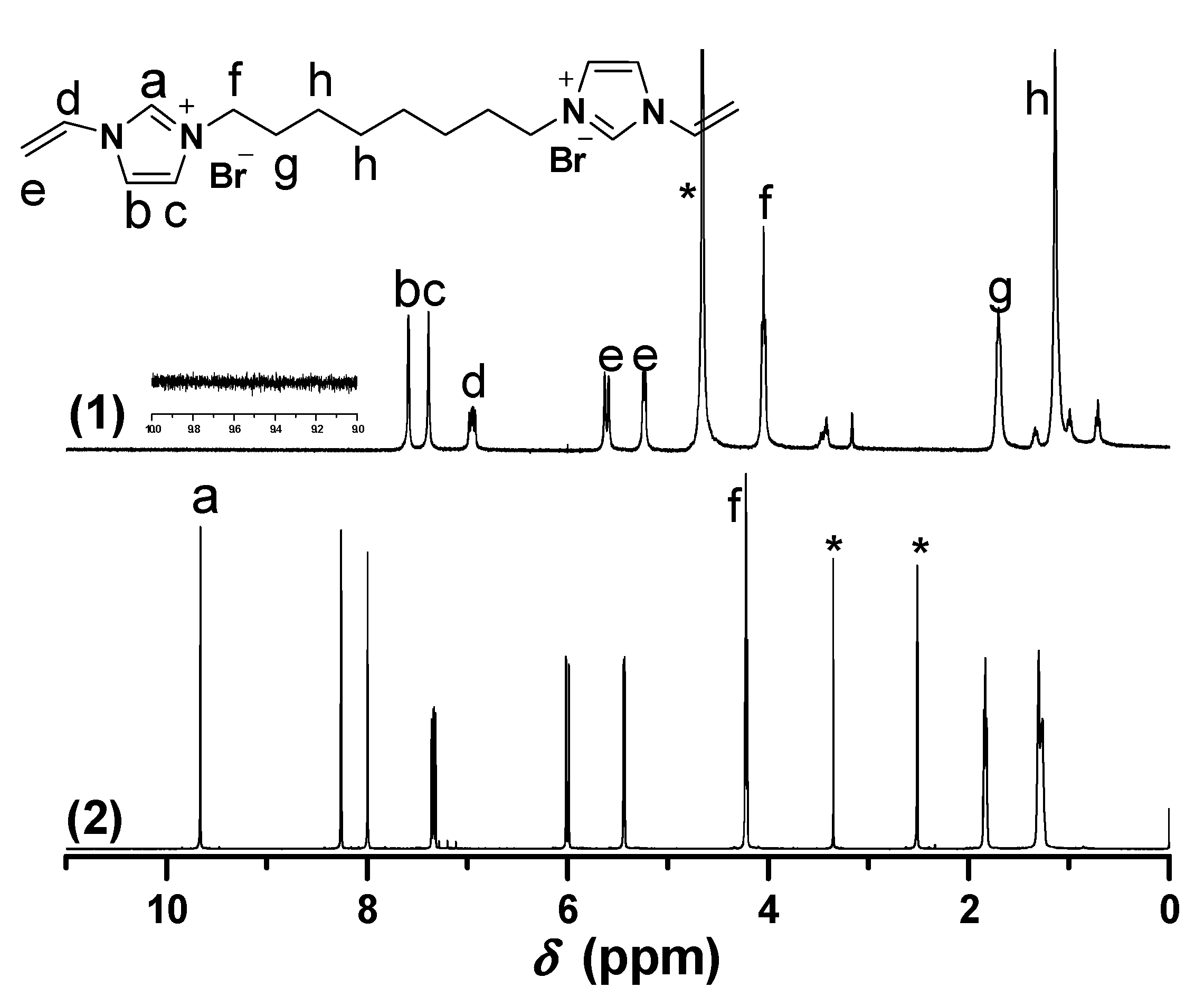
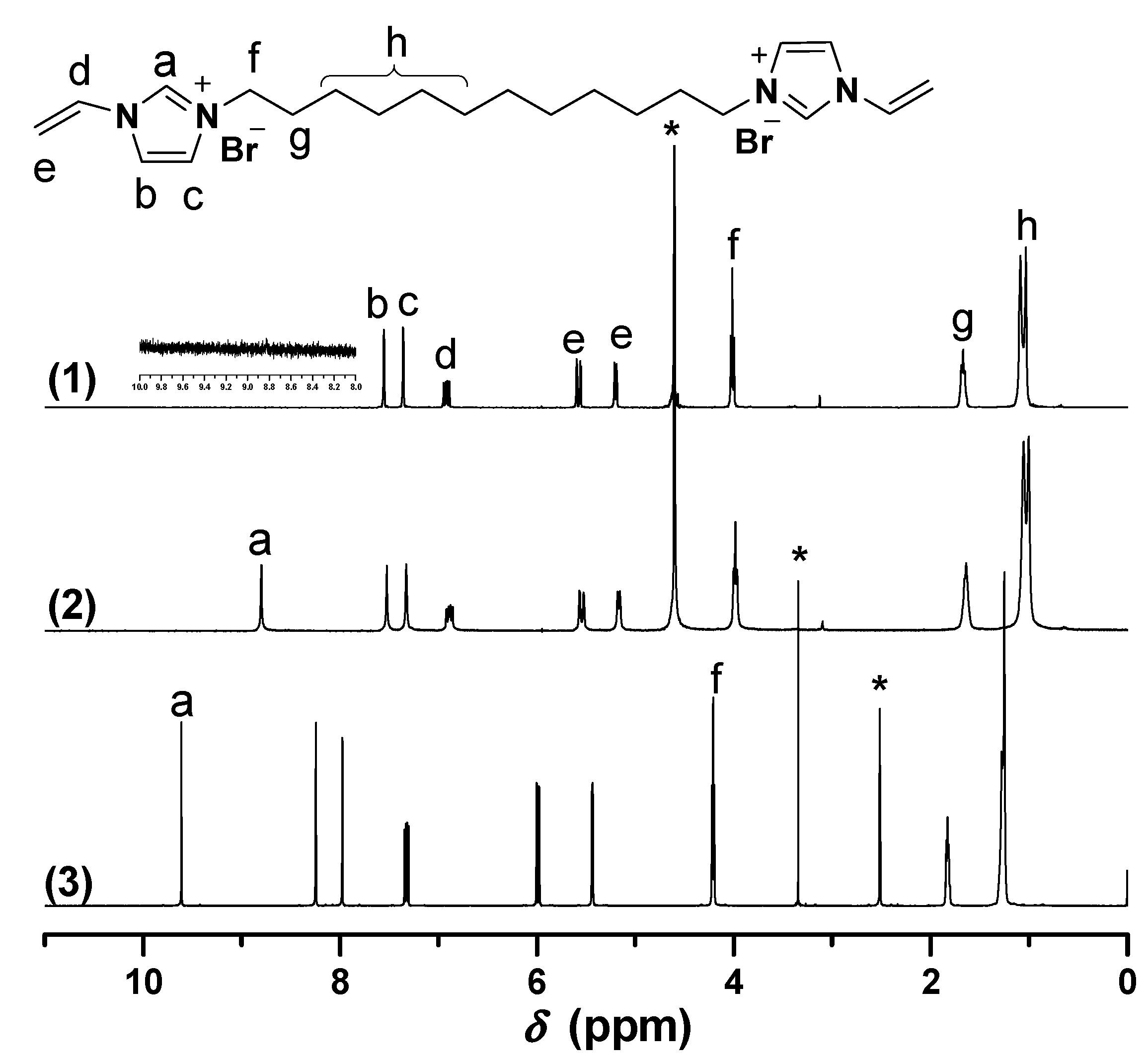
2.2. Characterization of BIm Salt-Based Nanogels and Their Thermo-Responsive Behavior
| Entry | Monomer and Cross-Linker | Feed Ratio (Molar Ratio) a | Dh (nm) | PDI | ξ-Potential (mV) |
|---|---|---|---|---|---|
| 1 | [C6VIm]Br + EGDMA | 1:1 | precipitated | - | - |
| 2 | [C6VIm]Br + EGDMA | 3:1 | 159 | 0.28 | 11.5 |
| 3 | [C6VIm]Br + EGDMA | 5:1 | 137 | 0.26 | 12.4 |
| 4 | [C6VIm]Br + EGDMA | 10:1 | 103 | 0.43 | 14.1 |
| 5 | [C6VIm]Br + EGDMA | 15:1 | 71 | 0.09 | 18.1 |
| 6 | [C8VIm]Br + EGDMA | 10:1 | 125 | 0.36 | 13.5 |
| 7 | [C12VIm]Br + EGDMA | 10:1 | 148 | 0.45 | 11.9 |
| 8 | [C6VIm]Br + DVB | 3:1 | 111 | 0.39 | 14.6 |
| 9 | [C6VIm]Br + DVB | 5:1 | 88 | 0.29 | 16.2 |
| 10 | [C6VIm]Br + DVB | 10:1 | 47 | 0.33 | 17.1 |
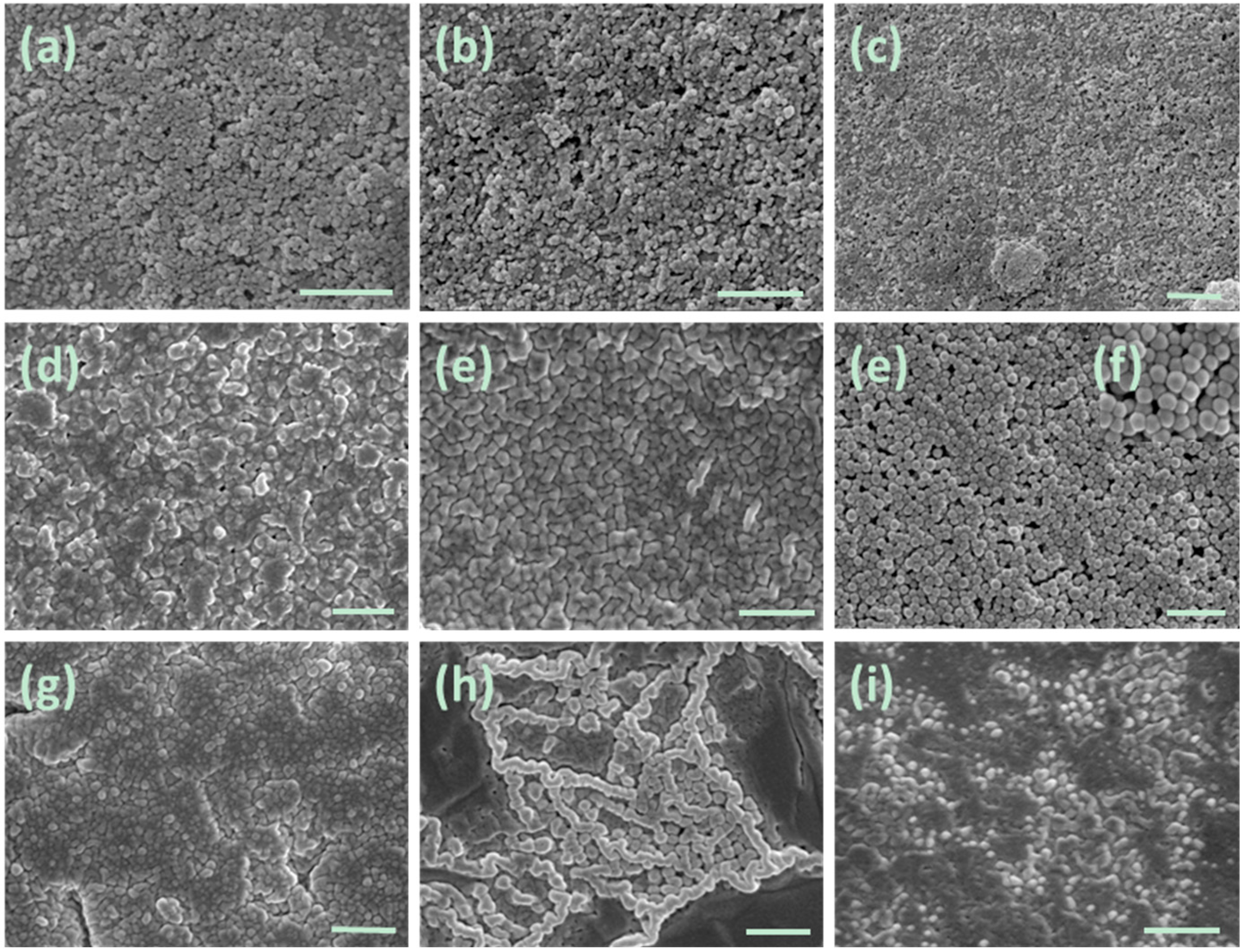
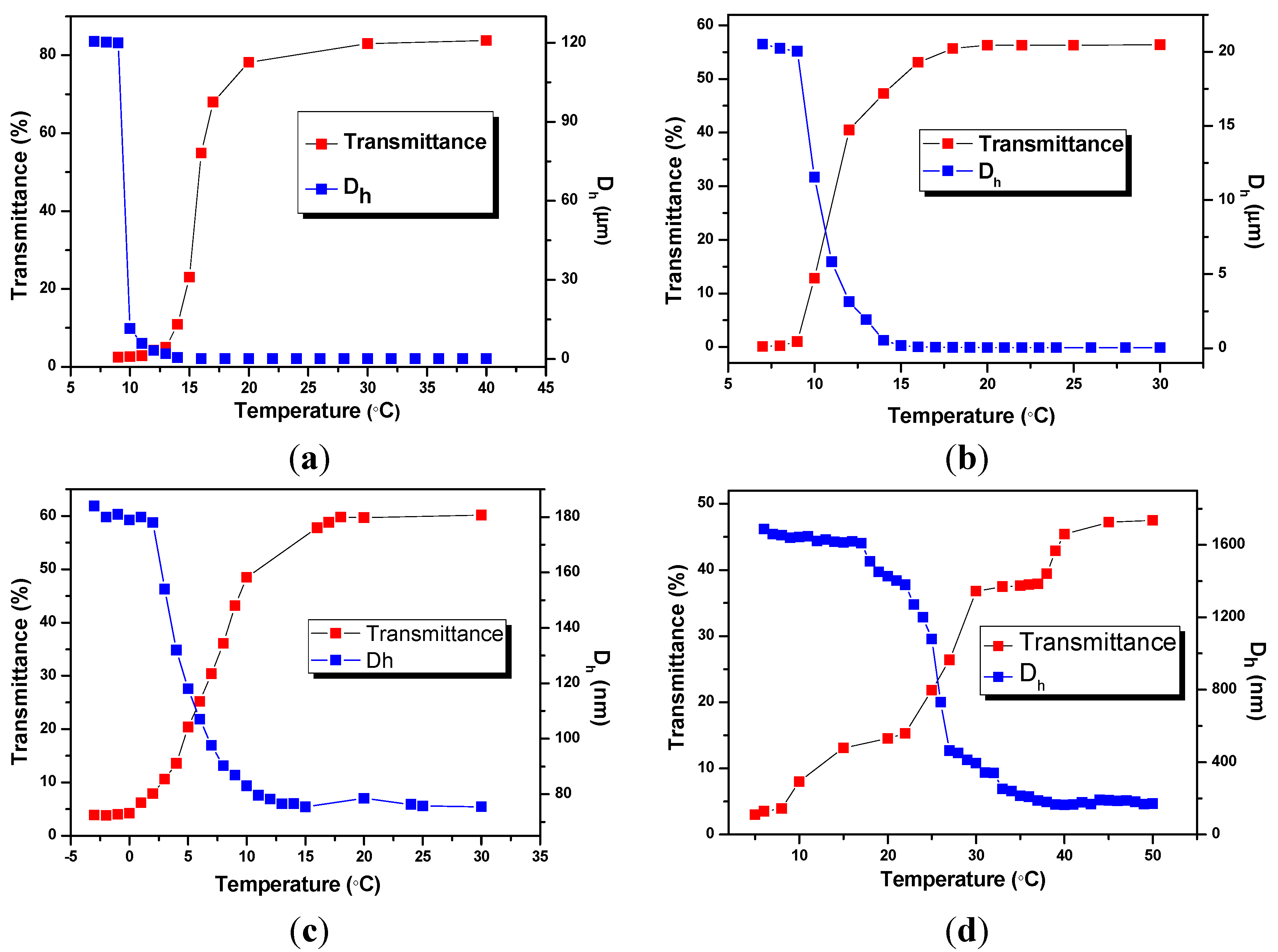
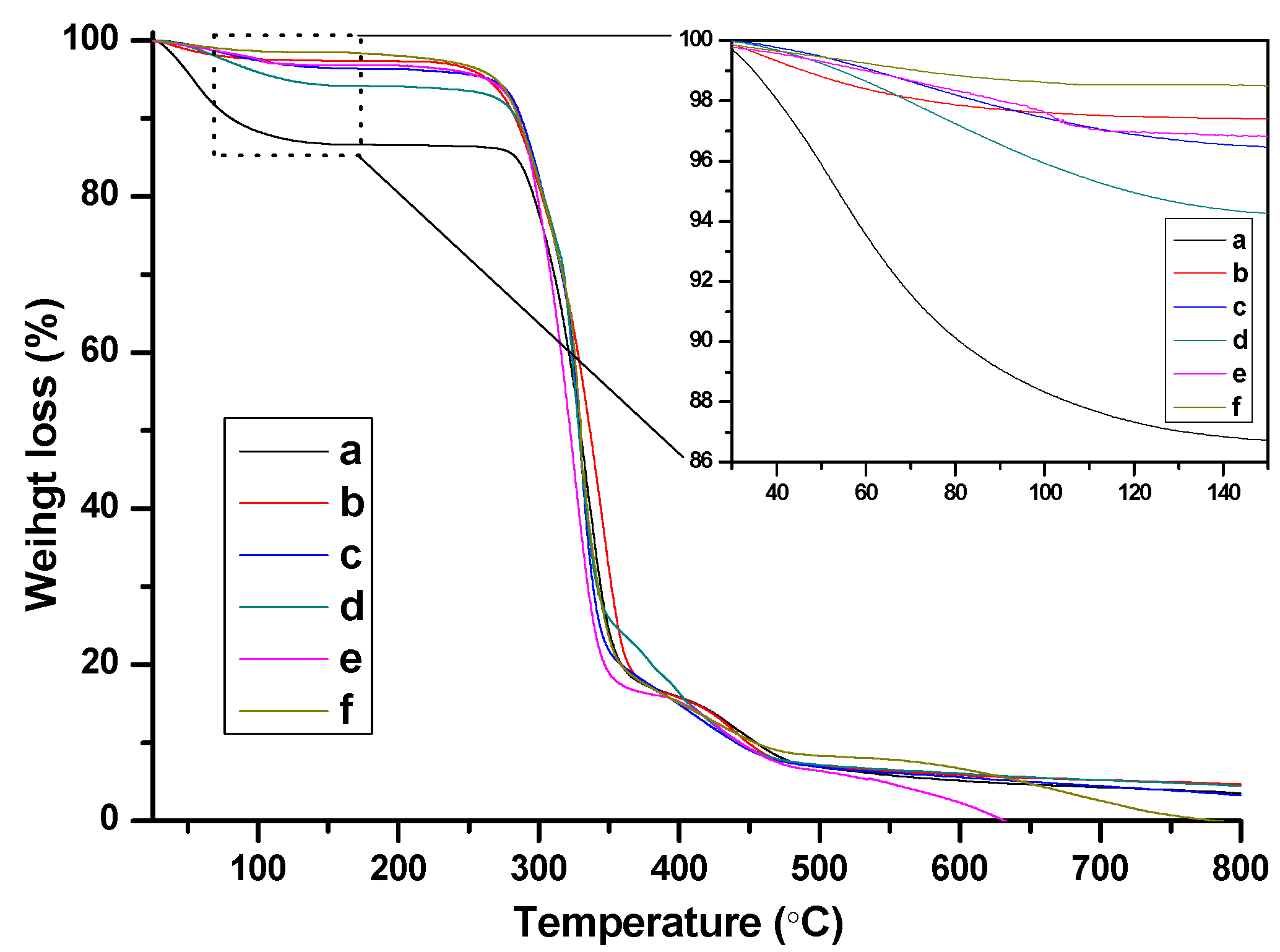
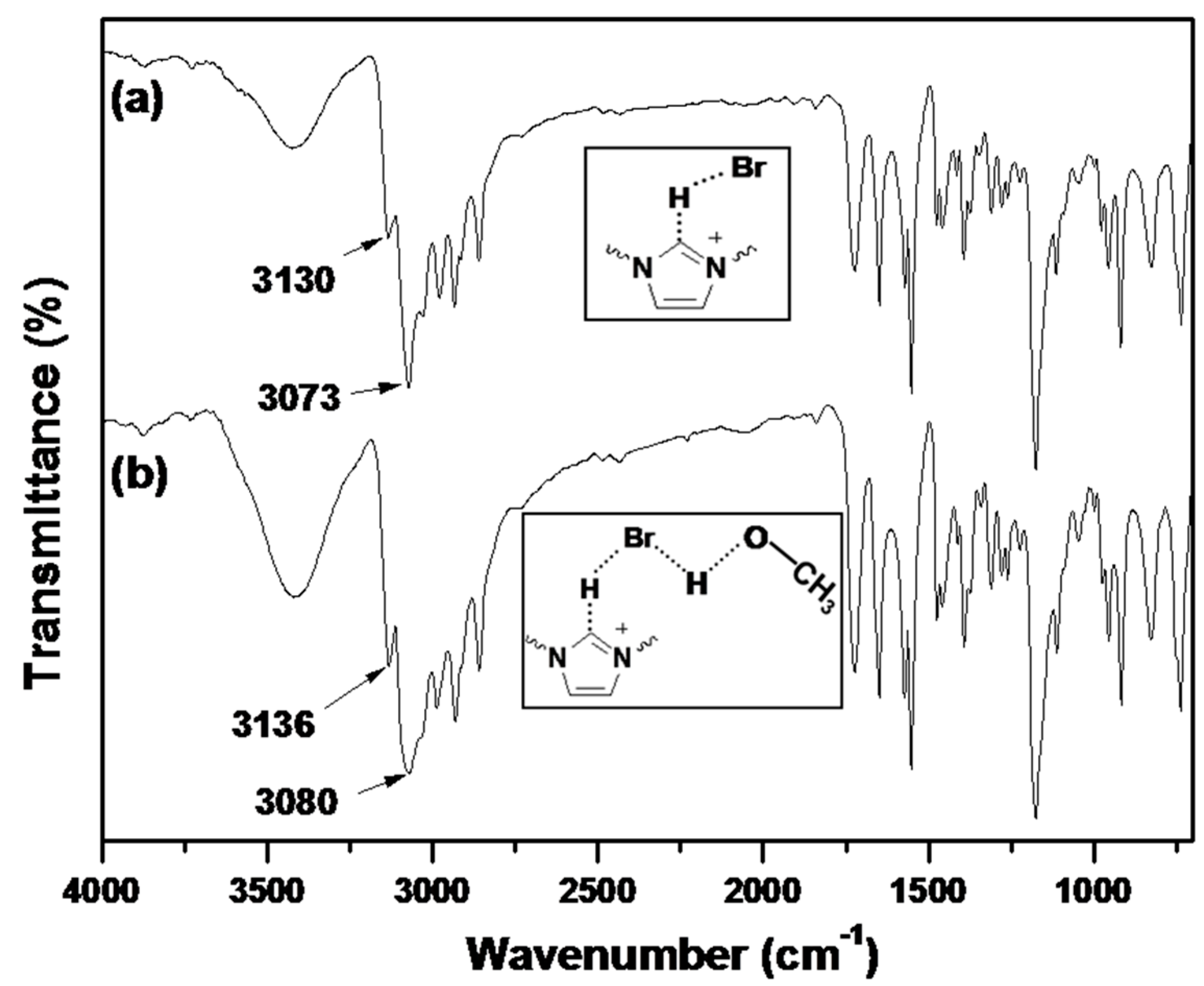
2.3. Catalysis Performance of BIm Salt-Based Nanogels for the Cycloaddition Reaction
| Entry | Catalyst b | Temperature (°C) | CO2 (MPa) | Yield (%) | Selectivity (%) |
|---|---|---|---|---|---|
| 1 | 3:1 | 160 | 3 | 99.7 | 89.0 |
| 2 | 5:1 | 160 | 3 | 99.8 | 93.0 |
| 3 | 10:1 | 160 | 3 | 100 | 100 |
| 4 | 15:1 | 160 | 3 | 100 | 96.4 |
| 5 | 5:1 | 120 | 3 | 87.7 | 99.3 |
| 6 | 5:1 | 140 | 3 | 96.1 | 96.1 |
| 7 | 5:1 | 150 | 3 | 99.5 | 99.2 |
| 8 | 5:1 | 160 | 2 | 95.7 | 97.3 |
| 9 | 5:1 | 160 | 5 | 99.7 | 100 |
3. Experimental Section
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Parvulescu, V.I.; Hardacre, C. Catalysis in ionic liquids. Chem. Rev. 2007, 107, 2615–2665. [Google Scholar] [CrossRef]
- Han, D.; Row, K.H. Recent applications of ionic liquids in separation technology. Molecules 2010, 15, 2405–2426. [Google Scholar] [CrossRef] [PubMed]
- Dominguez de Maria, P. “Nonsolvent” Applications of ionic liquids in biotransformations and organocatalysis. Angew. Chem. Int. Ed. 2008, 47, 6960–6968. [Google Scholar] [CrossRef] [PubMed]
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2083. [Google Scholar] [CrossRef] [PubMed]
- Skoda-Földes, R. The use of supported acidic ionic liquids in organic synthesis. Molecules 2014, 19, 8840–8884. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 2009, 8, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Yan, F.; Texter, J. Advanced applications of ionic liquids in polymer science. Prog. Polymer Sci. 2009, 34, 431–448. [Google Scholar] [CrossRef]
- Nishimura, N.; Ohno, H. 15th anniversary of polymerised ionic liquids. Polymer 2014, 55, 3289–3297. [Google Scholar] [CrossRef]
- Mecerreyes, D. Polymeric ionic liquids: Broadening the properties and applications of polyelectrolytes. Prog. Polymer Sci. 2011, 36, 1629–1648. [Google Scholar] [CrossRef]
- Erdmenger, T.; Guerrero-Sanchez, C.; Vitz, J.; Hoogenboom, R.; Schubert, U.S. Recent developments in the utilization of green solvents in polymer chemistry. Chem. Soc. Rev. 2010, 39, 3317–3333. [Google Scholar] [CrossRef] [PubMed]
- Antonietti, M.; Kuang, D.B.; Smarsly, B.; Zhou, Y. Ionic liquids for the convenient synthesis of functional nanoparticles and other inorganic nanostructures. Angew. Chem. Int. Ed. 2004, 43, 4988–4992. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Kohno, Y.; Nakamura, N.; Ohno, H. Introduction of hydrophilic groups onto the ortho-position of benzoate anions induced phase separation of the corresponding ionic liquids with water. Chem. Commun. 2013, 49, 10248–10250. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, K.; Ohno, H. LCST-Type Phase Changes of a Mixture of Water and Ionic Liquids Derived from Amino Acids. Angew. Chem. Int. Ed. 2007, 46, 1852–1855. [Google Scholar] [CrossRef] [PubMed]
- Ribot, J.C.; Guerrero-Sanchez, C.; Hoogenboom, R.; Schubert, U.S. Thermoreversible ionogels with tunable properties via aqueous gelation of an amphiphilic quaternary ammonium oligoether-based ionic liquid. J. Mater. Chem. 2010, 20, 8279–8284. [Google Scholar] [CrossRef]
- Ribot, J.; Guerrero-Sanchez, C.; Hoogenboom, R.; Schubert, U. Aqueous gelation of ionic liquids: Reverse thermoresponsive ion gels. Chem. Commun. 2010, 46, 6971–6973. [Google Scholar] [CrossRef] [PubMed]
- Kohno, Y.; Deguchi, Y.; Ohno, H. Ionic liquid-derived charged polymers to show highly thermoresponsive LCST-type transition with water at desired temperatures. Chem. Commun. 2012, 48, 11883–11885. [Google Scholar] [CrossRef] [PubMed]
- Ueki, T. Stimuli-responsive polymers in ionic liquids. Polymer J. 2014, 46, 646–655. [Google Scholar] [CrossRef]
- Yuan, J.; Mecerreyes, D.; Antonietti, M. Poly(ionic liquid)s: An update. Prog. Polymer Sci. 2013, 38, 1009–1036. [Google Scholar] [CrossRef]
- Kohno, Y.; Saita, S.; Men, Y.; Yuan, J.; Ohno, H. Thermoresponsive polyelectrolytes derived from ionic liquids. Polymer Chem. 2015, 6, 2163–2178. [Google Scholar]
- Kohno, Y.; Arai, H.; Ohno, H. Ionic liquid/water mixtures: From hostility to conciliation. Chem. Commun. 2011, 47, 4772–4774. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Rodríguez, H.; Seddon, K.R. Phase behaviour of trihexyl (tetradecyl) phosphonium chloride, nonane and water. Green Chem. 2009, 11, 780–784. [Google Scholar] [CrossRef]
- Kagimoto, J.; Nakamura, N.; Kato, T.; Ohno, H. Novel thermotropic gels composed of only ions. Chem. Commun. 2009, 2405–2407. [Google Scholar] [CrossRef] [PubMed]
- Fukaya, Y.; Sekikawa, K.; Murata, K.; Nakamura, N.; Ohno, H. Miscibility and phase behavior of water-dicarboxylic acid type ionic liquid mixed systems. Chem. Commun. 2007, 3089–3091. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Soll, S.; Drechsler, M.; Muller, A.H.E.; Antomietti, M. Self-assembly of poly(ionic liquid)s: Polymerization, mesostructure formation, and directional alignment in one step. J. Am. Chem. Soc. 2011, 133, 17556–17559. [Google Scholar] [CrossRef] [PubMed]
- Saita, S.; Kohno, Y.; Nakamura, N.; Ohno, H. Ionic liquids showing phase separation with water prepared by mixing hydrophilic and polar amino acid ionic liquids. Chem. Commun. 2013, 49, 8988–8990. [Google Scholar]
- Xiong, Y.B.; Wang, H.; Wang, R.M.; Yan, Y.F.; Zheng, B.; Wang, Y.P. A facile one-step synthesis to cross-linked polymeric nanoparticles as highly active and selective catalysts for cycloaddition of CO2 to epoxides. Chem. Commun. 2010, 46, 3399–3401. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.B.; Wang, Y.J.; Wang, H.; Wang, R.M. A facile one-step synthesis to ionic liquid-based cross-linked polymeric nanoparticles and their application for CO2 fixation. Polymer Chem. 2011, 2, 2306–2315. [Google Scholar] [CrossRef]
- Xiong, Y.B.; Liu, J.J.; Wang, Y.J.; Wang, H.; Wang, R.M. One-step synthesis of thermosensitive nanogels based on highly cross-linked poly(ionic liquid)s. Angew. Chem. Int. Ed. 2012, 51, 9114–9118. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.B.; Wang, Y.J.; Wang, H.; Wang, R.M.; Cui, Z. Novel one-step synthesis to cross-linked polymeric nanoparticles as highly active and selective catalysts for cycloaddition of CO2 to epoxides. J. Appl. Polymer Sci. 2012, 123, 1486–1493. [Google Scholar] [CrossRef]
- Wulf, A.; Fumino, K.; Ludwig, R. Spectroscopic evidence for an enhanced anion-cation interaction from hydrogen bonding in pure imidazolium ionic liquids. Angew. Chem. Int. Ed. 2010, 49, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Peppel, T.; Roth, C.; Fumino, K.; Paschek, D.; Kockerling, M.; Ludwig, R. The influence of hydrogen-bongding defects on the properties of ionic liquids. Angew. Chem. Int. Ed. 2011, 50, 6661–6665. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Jin, Q.; Tan, L.; Wu, P.; Yan, F. Trace of the interesting “V”-shape dynamic mechanism of interaction between water and ionic liquids. J. Phys. Chem. B 2008, 112, 14251–14259. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, G.C.; McClellan, A.L. The Hydrogen Bond; Freeman: San Francisco, CA, USA, 1960; pp. 348–363. [Google Scholar]
- Xie, Y.; Zhang, Z.F.; Jiang, T.; He, J.L.; Han, B.X.; Wu, T.B.; Ding, K.L. CO2 cycloaddition reactions catalyzed by an ionic liquid grafted onto a highly cross-linked polymer matrix. Angew. Chem. Int. Ed. 2007, 119, 7393–7396. [Google Scholar] [CrossRef]
- Peng, J.J.; Deng, Y.Q. Cycloaddition of carbon dioxide to propylene oxide catalyzed by ionic liquids. New J. Chem. 2001, 25, 639–641. [Google Scholar]
- Sun, J.; Cheng, W.G.; Fan, W.; Wang, Y.H.; Meng, Z.Y.; Zhang, S.J. Reusable and efficient polymer-supported task-specific ionic liquid catalyst for cycloaddition of epoxide with CO2. Catal. Today 2009, 148, 361–367. [Google Scholar] [CrossRef]
- Sample Availability: Samples of [C6VIm]Br and [C8VIm]Br are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Liu, J.; Zuo, Y.; Wang, R.; Xiong, Y. Preparation of Thermo-Responsive Poly(ionic liquid)s-Based Nanogels via One-Step Cross-Linking Copolymerization. Molecules 2015, 20, 17378-17392. https://doi.org/10.3390/molecules200917378
Zhang J, Liu J, Zuo Y, Wang R, Xiong Y. Preparation of Thermo-Responsive Poly(ionic liquid)s-Based Nanogels via One-Step Cross-Linking Copolymerization. Molecules. 2015; 20(9):17378-17392. https://doi.org/10.3390/molecules200917378
Chicago/Turabian StyleZhang, Jing, Jingjiang Liu, Yong Zuo, Rongmin Wang, and Yubing Xiong. 2015. "Preparation of Thermo-Responsive Poly(ionic liquid)s-Based Nanogels via One-Step Cross-Linking Copolymerization" Molecules 20, no. 9: 17378-17392. https://doi.org/10.3390/molecules200917378
APA StyleZhang, J., Liu, J., Zuo, Y., Wang, R., & Xiong, Y. (2015). Preparation of Thermo-Responsive Poly(ionic liquid)s-Based Nanogels via One-Step Cross-Linking Copolymerization. Molecules, 20(9), 17378-17392. https://doi.org/10.3390/molecules200917378





