Beneficial Effects of Ethanolic and Hexanic Rice Bran Extract on Mitochondrial Function in PC12 Cells and the Search for Bioactive Components
Abstract
:1. Introduction
2. Results and Discussion
2.1. Vitamin E Concentrations
| Vitamin E Congener (µg/g) | Ethanolic RBE | Hexanic RBE |
|---|---|---|
| α-Tocopherol | 86 | 112 |
| β-Tocopherol | 71 | 163 |
| γ-Tocopherol | 288 | 163 |
| δ-Tocopherol | 93 | 209 |
| α-Tocotrienol | 55 | 59 |
| β-Tocotrienol | not detected | not detected |
| γ-Tocotrienol | 2226 | 1791 |
| δ-Tocotrienol | 266 | 591 |
| Total vitamin E | 3084 | 3088 |
2.2. Mitochondrial Function
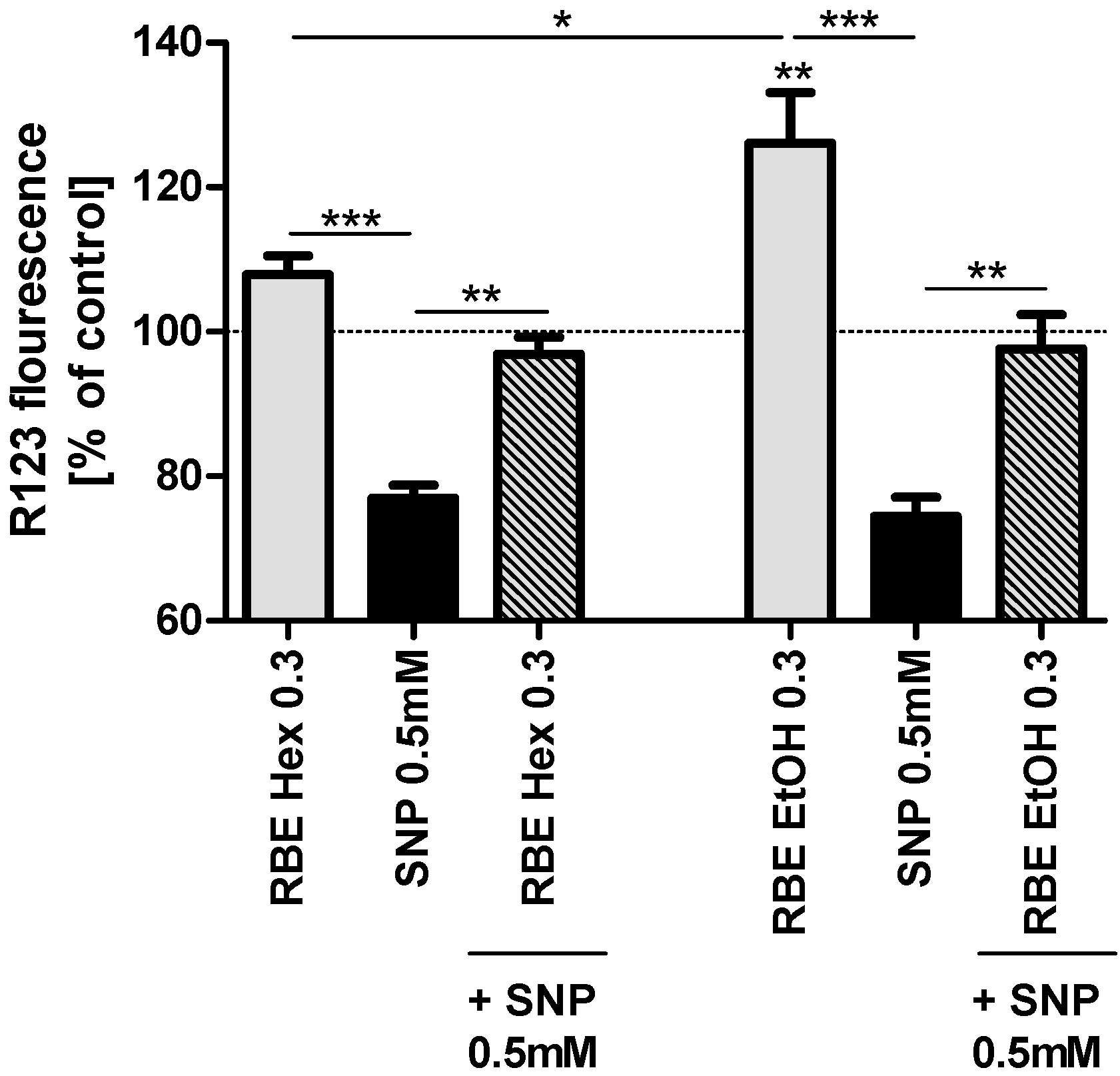
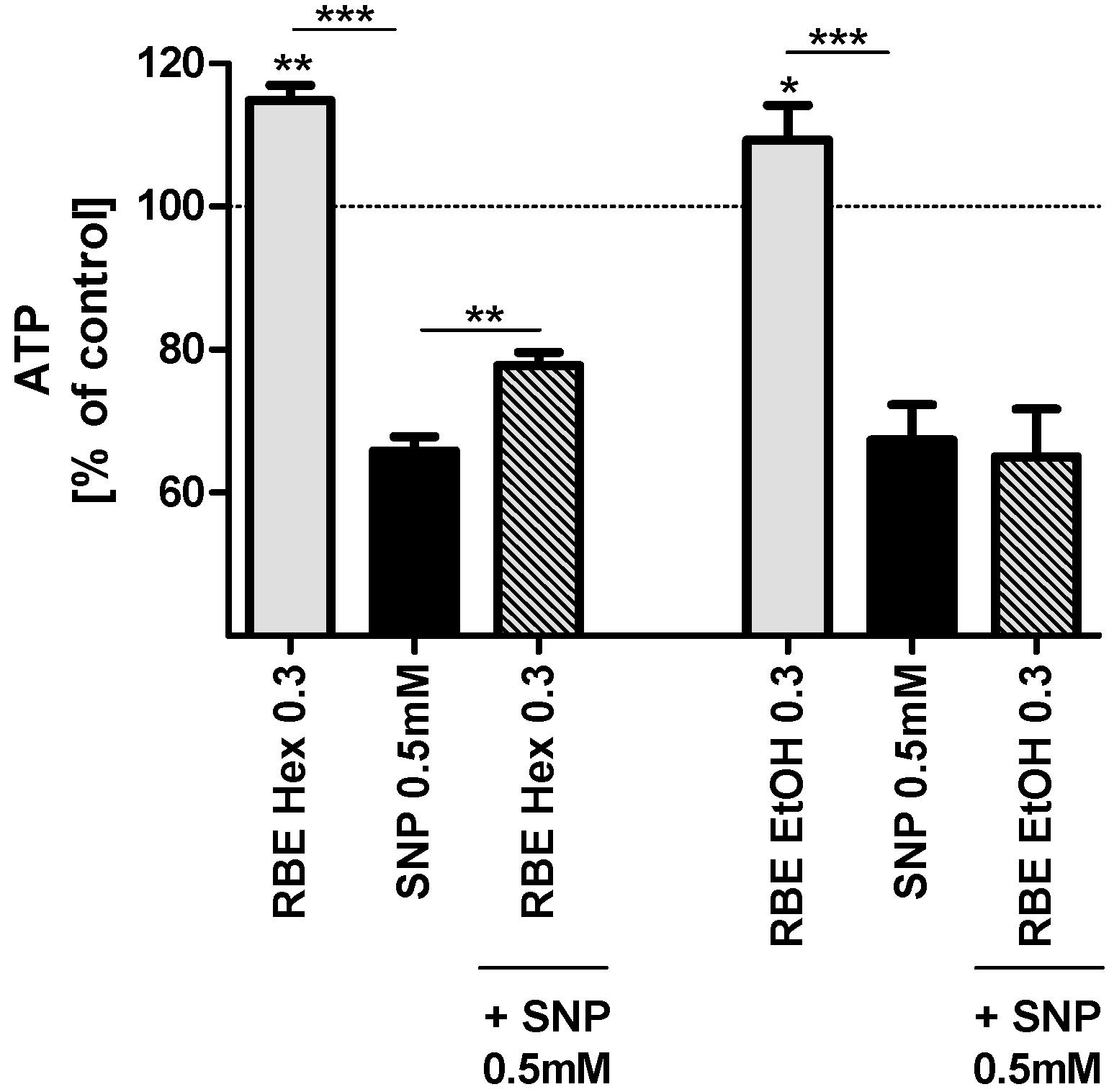
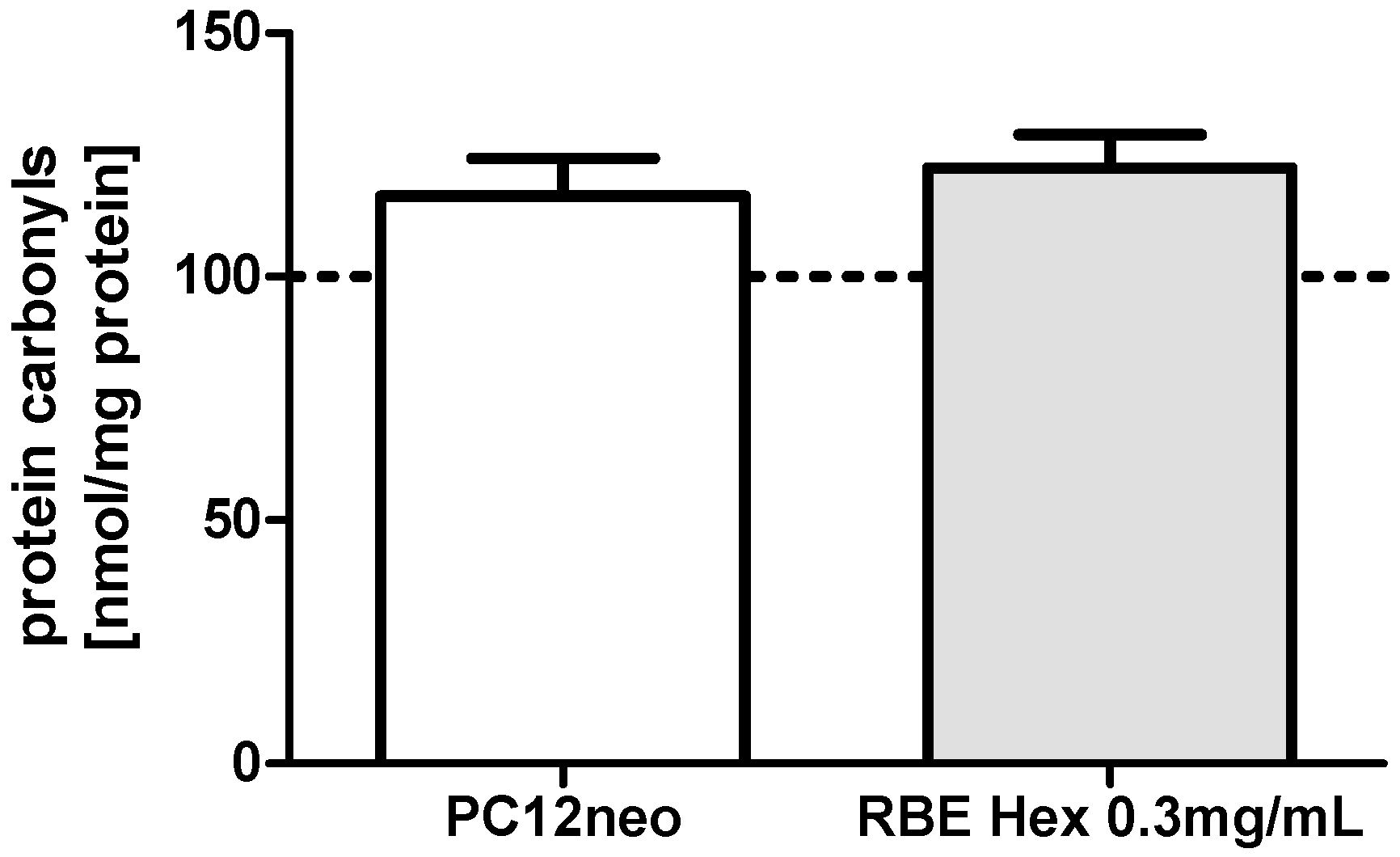
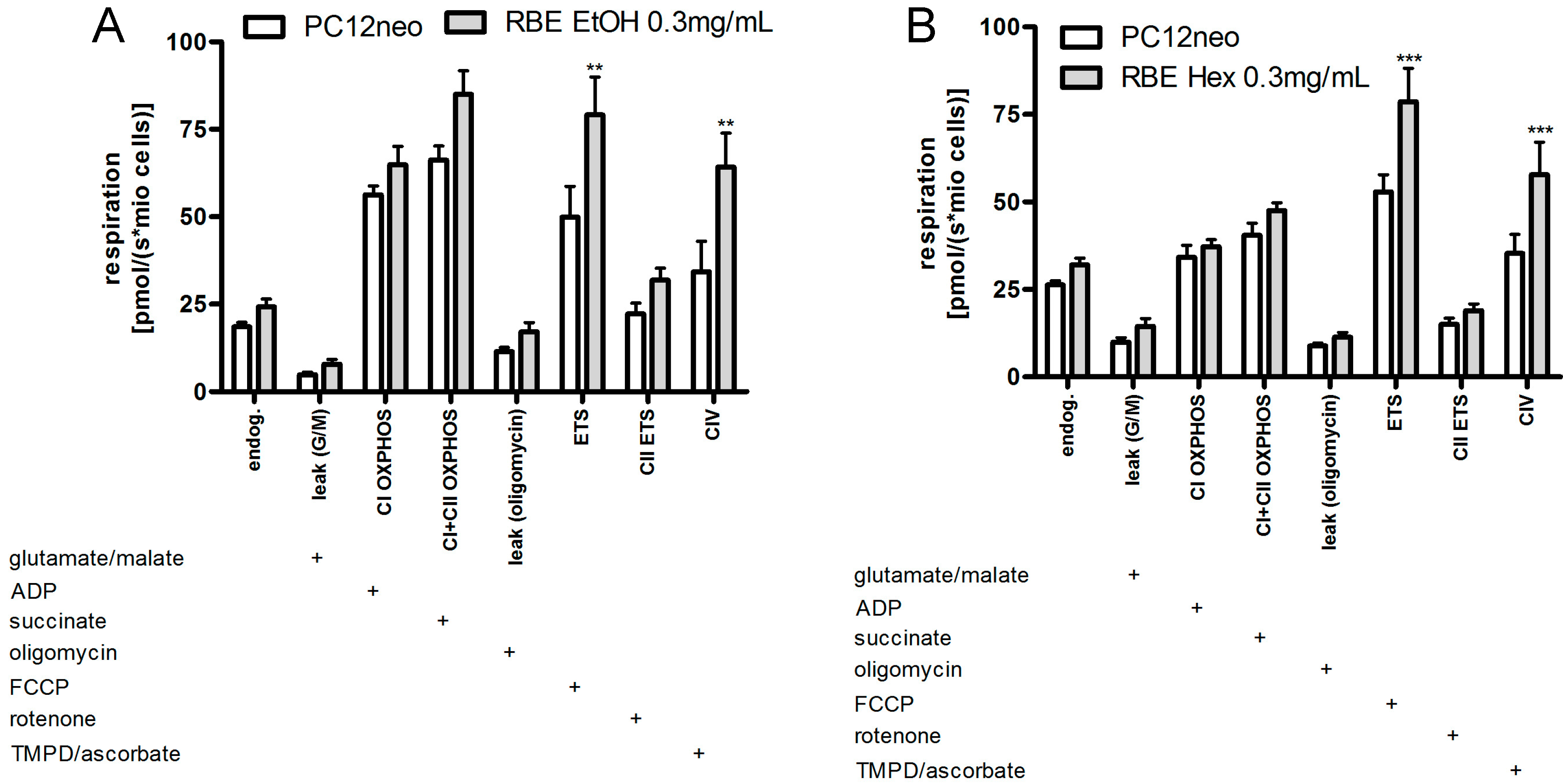
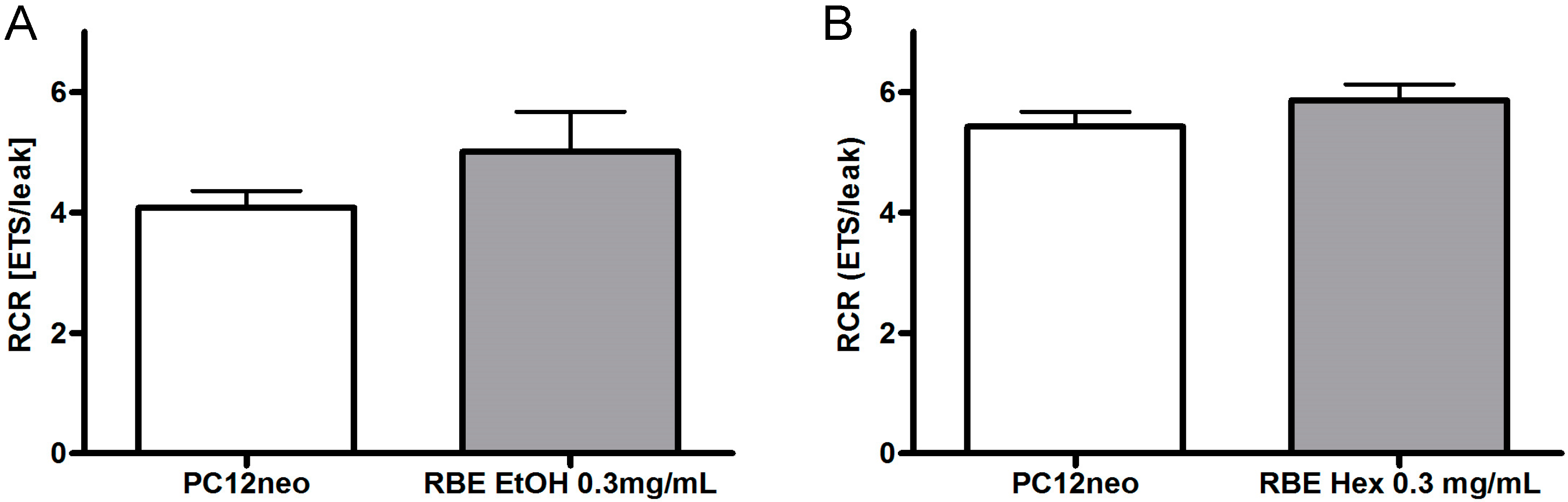
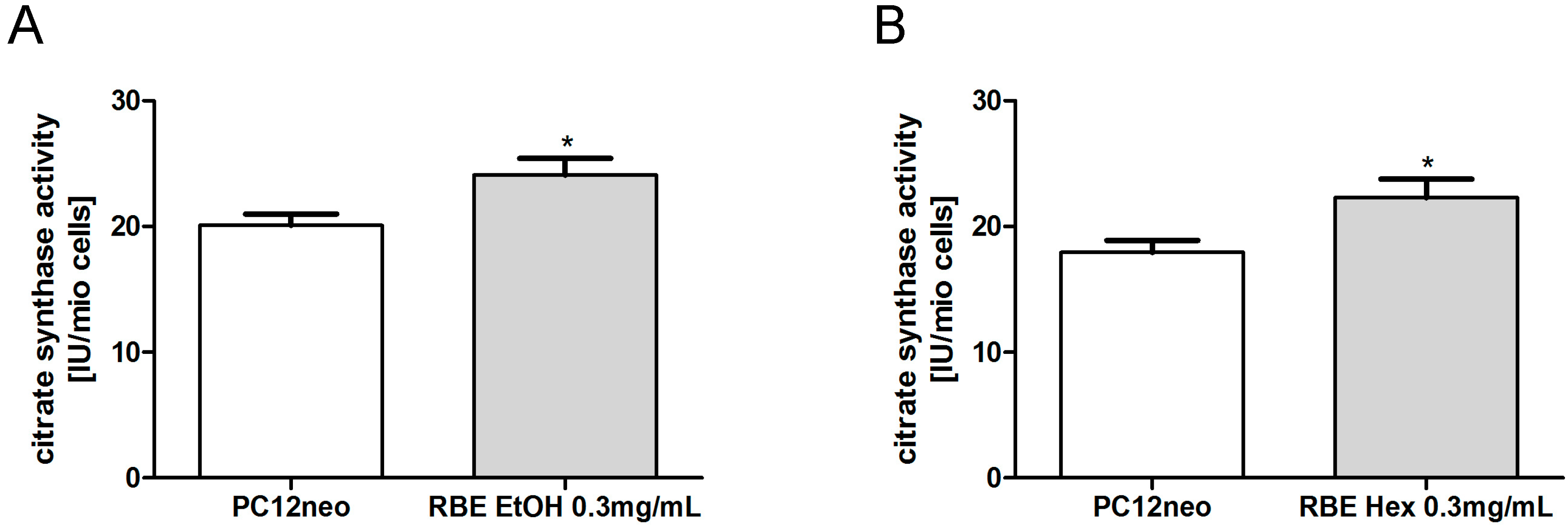
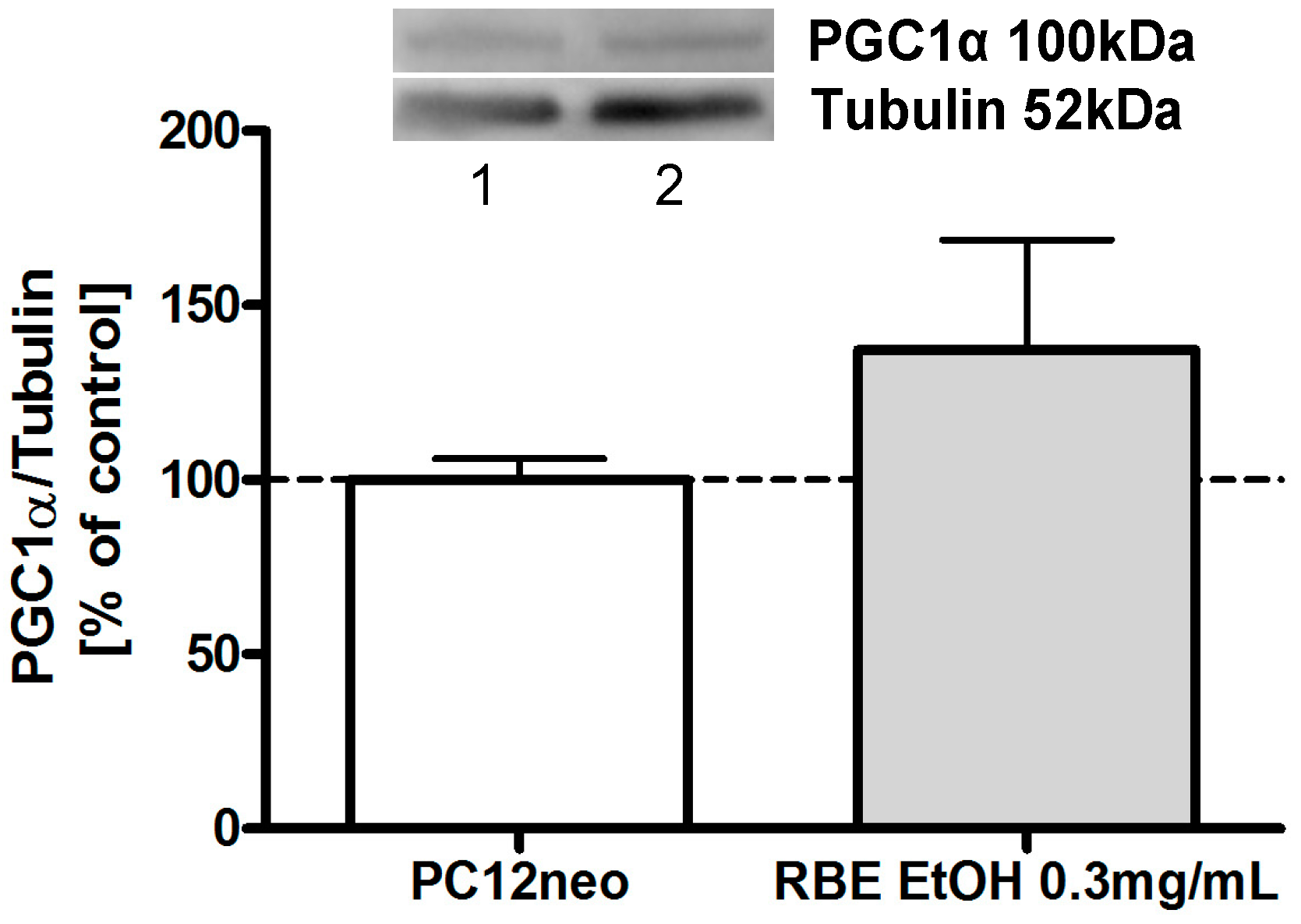
2.3. Single Substances and Fractions of RBE
| Substance (Concentration) | ATP | ATP + SNP | MMP | MMP + SNP |
|---|---|---|---|---|
| α-Tocopherol (50 nm) | p < 0.05 (↑) | - | - | - |
| α-Tocopherol (250 nM) | - | - | - | - |
| γ-Tocopherol (200 nM) | - | - | - | - |
| γ-Tocopherol (1 µM) | - | n.t. | n.t. | n.t. |
| δ-Tocopherol (70 nM) | p < 0.05 (↑) | n.t. | n.t. | n.t. |
| δ-Tocopherol (350 nM) | - | n.t. | n.t. | n.t. |
| α-Tocotrienol (40 nM) | p < 0.001 (↑) | - | - | - |
| α-Tocotrienol (200 nM) | - | n.t. | n.t. | n.t. |
| γ-Tocotrienol (1 µM) | p < 0.05 (↑) | - | - | - |
| γ-Tocotrienol (5 µM) | - | - | - | - |
| δ-Tocotrienol (200 nM) | - | - | - | - |
| δ-Tocotrienol (1 µM) | - | n.t. | n.t. | n.t. |
| Fraction | MMP | MMP + SNP (0.5 mM) | ATP | ATP + SNP (0.5 mM) |
|---|---|---|---|---|
| II | p < 0.001 (↑) | p < 0.001 (↑) | - | - |
| III | - | - | - | - |
| IV | - | - | - | - |
| V | - | - | - | - |
2.4. Discussion
3. Experimental Section
3.1. Chemicals
3.2. Cell Culture
3.3. Mitochondrial Membrane Potential
3.4. ATP Concentrations
3.5. Mitochondrial Respiration
3.6. Citrate Synthase Activity
3.7. Western Blot Analysis
3.8. HPLC Analyses of Vitamin E Congeners
3.9. Statistics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lagouge, M.; Larsson, N.G. The role of mitochondrial DNA mutations and free radicals in disease and ageing. J. Intern. Med. 2013, 273, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, B.N.; Rao, T.S.S.; Prakasam, A.; Sambamurti, K.; Rao, K.S.J. Neuronutrition and Alzheimer’s disease. J. Alzheimer’s Dis. 2010, 19, 1123–1139. [Google Scholar]
- Mangialasche, F.; Solomon, A.; Winblad, B.; Mecocci, P.; Kivipelto, M. Alzheimer’s disease: Clinical trials and drug development. Lancet Neurol. 2010, 9, 702–716. [Google Scholar] [CrossRef]
- Lukiw, W.J. Amyloid beta (Abeta) peptide modulators and other current treatment strategies for Alzheimer’s disease (AD). Expert Opin. Emerg. Drugs 2012. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.; Eckert, A.; Kurz, C.; Eckert, G.; Leuner, K. Mitochondrial Dysfunction: Common Final Pathway in Brain Aging and Alzheimer’s Disease—Therapeutic Aspects. Mol. Neurobiol. 2010, 41, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Leuner, K.; Hauptmann, S.; Abdel-Kader, R.; Scherping, I.; Keil, U.; Strosznajder, J.B.; Eckert, A.; Muller, W.E. Mitochondrial dysfunction: The first domino in brain aging and Alzheimer’s disease? Antioxid. Redox Signal. 2007, 9, 1659–1675. [Google Scholar] [CrossRef] [PubMed]
- Eckert, G.P.; Renner, K.; Eckert, S.H.; Eckmann, J.; Hagl, S.; Abdel-Kader, R.M.; Kurz, C.; Leuner, K.; Muller, W.E. Mitochondrial dysfunction—A pharmacological target in Alzheimer’s disease. Mol. Neurobiol. 2012, 46, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Joshi, Y.B.; Pratico, D. Vitamin E in aging, dementia, and Alzheimer’s disease. Biofactors 2012, 38, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzi, V.; Frisardi, V.; Seripa, D.; Logroscino, G.; Imbimbo, B.P.; D’Onofrio, G.; Addante, F.; Sancarlo, D.; Cascavilla, L.; Pilotto, A.; et al. Mediterranean diet in predementia and dementia syndromes. Curr. Alzheimer Res. 2011, 8, 520–542. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Alvarez-Suarez, J.M.; Battino, M. Strawberry and human health: Effects beyond antioxidant activity. J. Agric. Food Chem. 2014, 62, 3867–3876. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Oh, M.S. Herbal medicines for the prevention and treatment of Alzheimer’s disease. Curr. Pharm. Des. 2012, 18, 57–75. [Google Scholar] [PubMed]
- Hagl, S.; Kocher, A.; Schiborr, C.; Eckert, S.H.; Ciobanu, I.; Birringer, M.; El-Askary, H.; Helal, A.; Khayyal, M.T.; Frank, J.; et al. Rice bran extract protects from mitochondrial dysfunction in guinea pig brains. Pharmacol. Res. 2013, 76, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Hagl, S.; Grewal, R.; Ciobanu, I.; Helal, A.; Khayyal, M.T.; Muller, W.E.; Eckert, G.P. Rice bran extract compensates mitochondrial dysfunction in a cellular model of early Alzheimer’s disease. J. Alzheimer’s Dis. 2015, 43, 927–938. [Google Scholar]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Hutter, E.; Unterluggauer, H.; Garedew, A.; Jansen-Durr, P.; Gnaiger, E. High-resolution respirometry—A modern tool in aging research. Exp. Gerontol. 2006, 41, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Gnaiger, E. Bioenergetics at low oxygen: Dependence of respiration and phosphorylation on oxygen and adenosine diphosphate supply. Respir. Physiol. 2001, 128, 277–297. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.A.; Zak, R.; Pette, D. Chronic stimulation of rat skeletal muscle induces coordinate increases in mitochondrial and nuclear mRNAs of cytochrome-c-oxidase subunits. Eur. J. Biochem. 1989, 179, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Marcos, P.J.; Auwerx, J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 2011, 93. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Garcia, C.; Gavino, G.; Baragaño-Mosqueda, M.; Hevia, P.; Gavino, V.C. Correlation of tocopherol, tocotrienol, γ-oryzanol and total polyphenol content in rice bran with different antioxidant capacity assays. Food Chem. 2007, 102, 1228–1232. [Google Scholar] [CrossRef]
- Mukai, K.; Ouchi, A.; Abe, T.; Murata, K.; Nakagawa, K.; Miyazawa, T. Kinetic study of the scavenging reaction of the aroxyl radical by seven kinds of rice bran extracts in ethanol solution. Development of an aroxyl radical absorption capacity (ARAC) assay method. J. Agric. Food Chem. 2014, 62, 11901–11909. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Chiu, T.H. Phytochemicals characterization of solvent extracts from taro-scented japonica rice bran. J. Food Sci. 2011, 76, C656–C662. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.; Chin, X.W.D.; Schrader, C.; Eckert, G.P.; Rimbach, G. Do tocotrienols have potential as neuroprotective dietary factors? Aging Res. Rev. 2012, 11, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.L. An update on vitamin E, tocopherol and tocotrienol-perspectives. Molecules 2010, 15, 2103–2113. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.; Muller, W.E.; Eckert, G.P. Tocotrienols: Constitutional effects in aging and disease. J. Nutr. 2005, 135, 151–154. [Google Scholar] [PubMed]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienol: The Natural Vitamin E to Defend the Nervous System? Ann. N. Y. Acad. Sci. 2004, 1031, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Osakada, F.; Hashino, A.; Kume, T.; Katsuki, H.; Kaneko, S.; Akaike, A. Alpha-tocotrienol provides the most potent neuroprotection among vitamin E analogs on cultured striatal neurons. Neuropharmacology 2004, 47, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Rukmini, C.; Raghuram, T.C. Nutritional and biochemical aspects of the hypolipidemic action of rice bran oil: A review. J. Am. Coll. Nutr. 1991, 10, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Eckert, G.; Franke, C.; Nöldner, M.; Rau, O.; Wurglics, M.; Schubert-Zsilavecz, M. Plant derived omega-3-fatty acids protect mitochondrial function in the brain. Eur. J. Pharmacol. 2010, 61, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Afshordel, S.; Hagl, S.; Werner, D.; Rohner, N.; Kogel, D.; Bazan, N.G.; Eckert, G.P. Omega-3 polyunsaturated fatty acids improve mitochondrial dysfunction in brain aging—Impact of Bcl-2 and NPD-1 like metabolites. Prostaglandins Leukot. Essent. Fat. Acids 2015, 92C, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Connor, W.E. Importance of n-3 fatty acids in health and disease. Am. J. Clin. Nutr. 2000, 71, 171S–175S. [Google Scholar] [PubMed]
- Bhilwade, H.N.; Tatewaki, N.; Nishida, H.; Konishi, T. Squalene as novel food factor. Curr. Pharm. Biotechnol. 2010, 11, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, J.J.; Pamplona, R.; Ramirez-Tortosa, M.C.; Granados-Principal, S.; Perez-Lopez, P.; Naudi, A.; Portero-Otin, M.; Lopez-Frias, M.; Battino, M.; Quiles, J.L. Age-related changes in brain mitochondrial DNA deletion and oxidative stress are differentially modulated by dietary fat type and coenzyme Q10. Free Radic. Biol. Med. 2011, 50, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Barzanti, V.; Battino, M.; Baracca, A.; Cavazzoni, M.; Cocchi, M.; Noble, R.; Maranesi, M.; Turchetto, E.; Lenaz, G. The effect of dietary lipid changes on the fatty acid composition and function of liver, heart and brain mitochondria in the rat at different ages. Br. J. Nutr. 1994, 71, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, M.; Nakamura, S.; Makita, F.; Ohwada, S.; Miyamoto, Y.; Morishita, Y. Antitumor effect of RBS (rice bran saccharide) on ENNG-induced carcinogenesis. Biotherapy 1992, 4, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Greene, L.A.; Tischler, A.S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. USA 1976, 73, 2424–2428. [Google Scholar] [CrossRef] [PubMed]
- Eckert, A.; Steiner, B.; Marques, C.; Leutz, S.; Romig, H.; Haass, C.; Muller, W.E. Elevated vulnerability to oxidative stress-induced cell death and activation of caspase-3 by the Swedish amyloid precursor protein mutation. J. Neurosci. Res. 2001, 64, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Stadlmann, S.; Renner, K.; Pollheimer, J.; Moser, P.L.; Zeimet, A.G.; Offner, F.A.; Gnaiger, E. Preserved coupling of oxidative phosphorylation but decreased mitochondrial respiratory capacity in IL-1beta-treated human peritoneal mesothelial cells. Cell Biochem. Biophys. 2006, 44, 179–186. [Google Scholar] [CrossRef]
- Hutter, E.; Renner, K.; Pfister, G.; Stockl, P.; Jansen-Durr, P.; Gnaiger, E. Senescence-associated changes in respiration and oxidative phosphorylation in primary human fibroblasts. Biochem. J. 2004, 380, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.V.; Strobl, D.; Ruttmann, E.; Königsrainer, A.; Margreiter, R.; Gnaiger, E. Evaluation of Mitochondrial Respiratory Function in Small Biopsies of Liver. Anal. Biochem. 2002, 305, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Grebenstein, N.; Frank, J. Rapid baseline-separation of all eight tocopherols and tocotrienols by reversed-phase liquid-chromatography with a solid-core pentafluorophenyl column and their sensitive quantification in plasma and liver. J. Chromatogr. A 2012, 1243, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the rice bran extract are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagl, S.; Berressem, D.; Bruns, B.; Sus, N.; Frank, J.; Eckert, G.P. Beneficial Effects of Ethanolic and Hexanic Rice Bran Extract on Mitochondrial Function in PC12 Cells and the Search for Bioactive Components. Molecules 2015, 20, 16524-16539. https://doi.org/10.3390/molecules200916524
Hagl S, Berressem D, Bruns B, Sus N, Frank J, Eckert GP. Beneficial Effects of Ethanolic and Hexanic Rice Bran Extract on Mitochondrial Function in PC12 Cells and the Search for Bioactive Components. Molecules. 2015; 20(9):16524-16539. https://doi.org/10.3390/molecules200916524
Chicago/Turabian StyleHagl, Stephanie, Dirk Berressem, Bastian Bruns, Nadine Sus, Jan Frank, and Gunter P. Eckert. 2015. "Beneficial Effects of Ethanolic and Hexanic Rice Bran Extract on Mitochondrial Function in PC12 Cells and the Search for Bioactive Components" Molecules 20, no. 9: 16524-16539. https://doi.org/10.3390/molecules200916524
APA StyleHagl, S., Berressem, D., Bruns, B., Sus, N., Frank, J., & Eckert, G. P. (2015). Beneficial Effects of Ethanolic and Hexanic Rice Bran Extract on Mitochondrial Function in PC12 Cells and the Search for Bioactive Components. Molecules, 20(9), 16524-16539. https://doi.org/10.3390/molecules200916524





