Four New Cyclohexylideneacetonitrile Derivatives from the Hypocotyl of Mangrove (Bruguiera gymnorrhiza)
Abstract
:1. Introduction
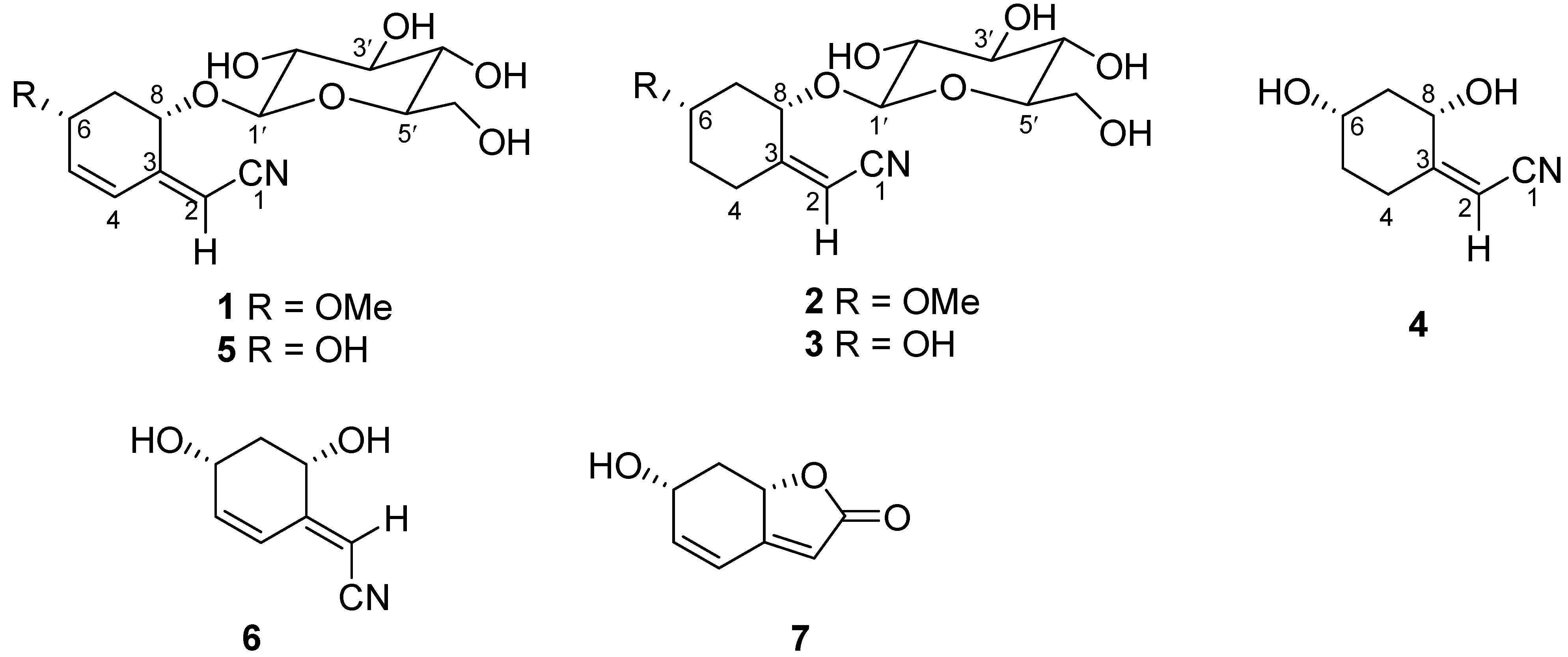
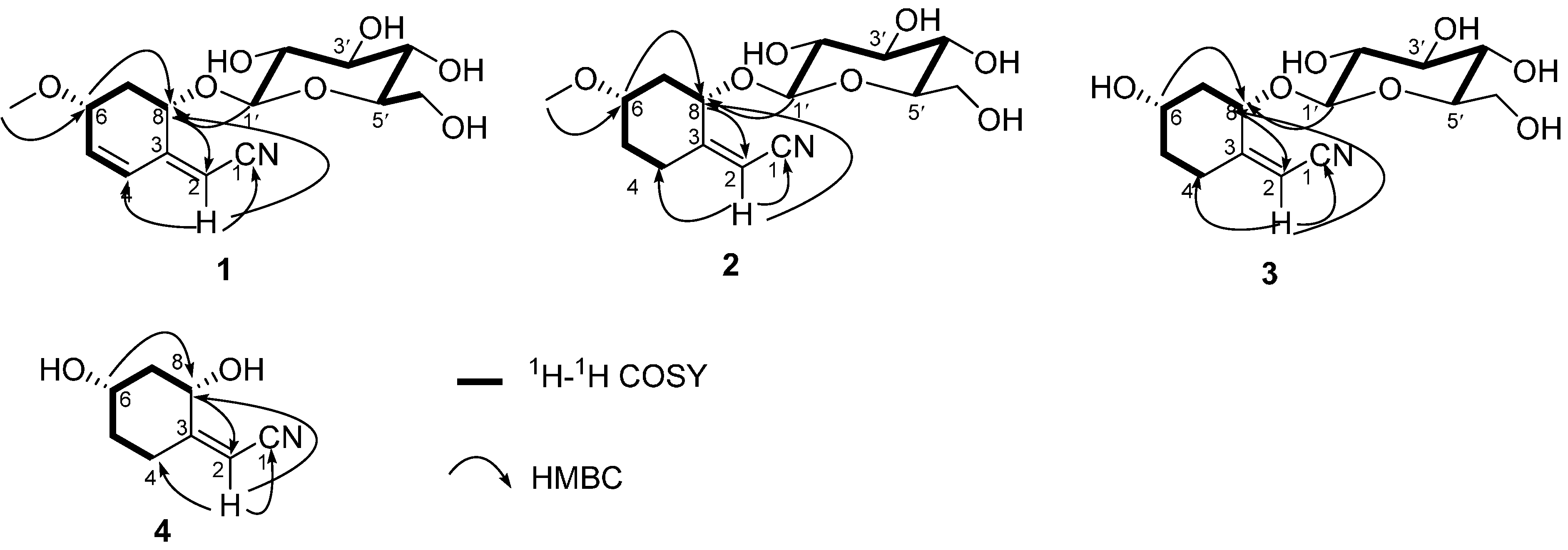
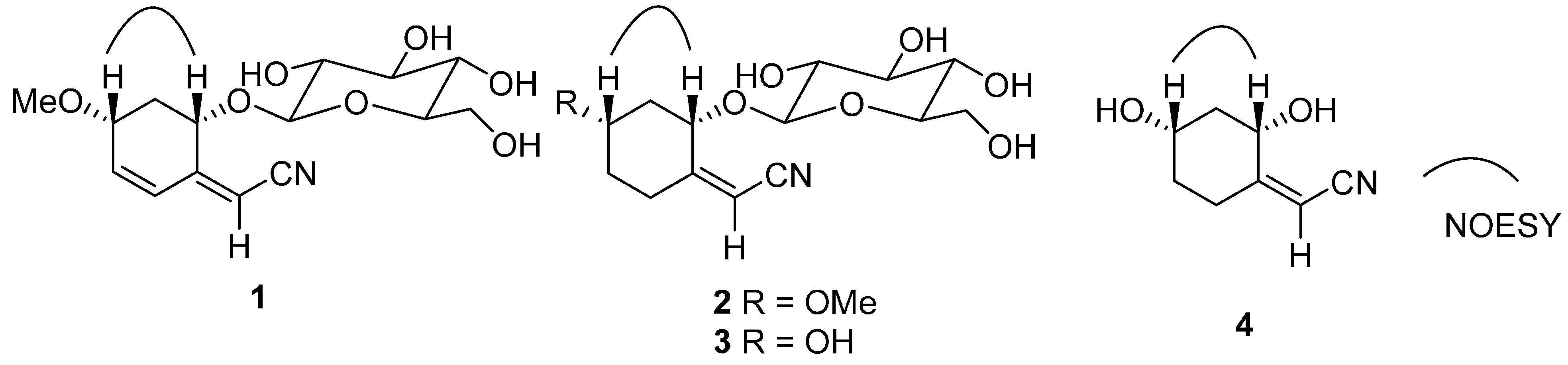
2. Results and Discussion
| No. | δC, Mult | 1 a | 2 a | 3 a | 4 b | |||
|---|---|---|---|---|---|---|---|---|
| δH (J in Hz) | δC, Mult | δH (J in Hz) | δC, Mult | δH (J in Hz) | δC, Mult | δH (J in Hz) | ||
| 1 | 117.9, C | 117.1, C | 118.7, C | 116.8, C | ||||
| 2 | 97.2, CH | 5.66 (d, 1.8) | 94.7, CH | 5.53 (d, 1.5) | 94.3, CH | 5.51 (d, 1.8) | 89.4, CH | 5.6 (s) |
| 3 | 152.2, C | 166.0, C | 166.5, C | 170.5, C | ||||
| 4a | 125.4, CH | 6.20 (d, 10.0) | 29.7, CH2 | 2.46 (d, 11.9) | 29.6, CH2 | 2.45 (d, 11.0) | 25.2, CH2 | 2.79 (d, 11.0) |
| 4b | 2.25 (dd, 11.9, 7.8) | 2.11 (dd, 11.0, 7.8) | 2.59 (dd, 11.0, 7.8) | |||||
| 5a | 144.3, CH | 6.18 (dd, 10, 2.5) | 36.4, CH2 | 1.86–1.92 (m) | 36.3, CH2 | 1.69–1.74 (m) | 33.5, CH2 | 1.87–1.93 (m) |
| 5b | 1.19–1.24 (m) | 1.36–1.42 (m) | 1.61–1.67 (m) | |||||
| 6 | 62.8, CH | 4.45 (ddd, 9.0, 5.5, 2.5) | 63.9, CH | 3.92–3.87 (m) | 67.2, CH | 3.75–3.70 (m) | 67.7, CH | 4.48–4.54 (m) |
| 7a | 37.1, CH2 | 2.39 (ddd, 12.5, 5.5, 3.5) | 36.1, CH2 | 1.87 (ddd, 11.5, 5.2, 3.5) | 41.1, CH2 | 2.09 (ddd, 11.3, 5.0, 3.8) | 42.9, CH2 | 2.18 (ddd, 10.1, 5.0, 3.3) |
| 7b | 1.62 (ddd, 12.5, 10.2, 9.0) | 1.17 (ddd, 11.5, 9.7, 8.8) | 1.55 (ddd, 11.3, 9.4, 8.5) | 1.56 (ddd, 10.1, 9.3, 8.5) | ||||
| 8 | 73.2, CH | 4.72 (dd, 10.2, 3.5) | 73.9, CH | 3.08 (dd, 9.7, 3.5) | 74.8, CH | 3.10 (dd, 9.4, 3.8) | 65.7, CH | 4.17 (dd, 9.3, 3.3) |
| 1′ | 102.3, CH | 4.38 (d, 7.8) | 102.8, CH | 4.15 (d, 7.3) | 102.6, CH | 4.27 (d, 7.6) | ||
| 2′ | 73.8, CH | 3.16 (ddd, 8.7, 7.8, 4.5) | 75.9, CH | 2.95 (ddd, 8.5, 7.3, 4.3) | 75.8, CH | 3.09 (ddd, 8.4, 7.6, 4.3) | ||
| 3′ | 77.4, CH | 3.39 (ddd, 8.7, 8.7 4.2) | 77.6, CH | 3.10 (ddd, 8.7, 8.5, 4.5) | 78.8, CH | 3.11 (ddd, 8.4, 8.3, 4.5) | ||
| 4′ | 70.6, CH | 3.01 (ddd, 8.7, 8.7, 5.4) | 70.4, CH | 3.04 (ddd, 8.7, 8.7, 5.2) | 71.9, CH | 3.01 (ddd, 8.3, 7.8, 5.2) | ||
| 5′ | 77.3, CH | 3.11 (ddd, 8.7, 6.8, 6.2) | 77.5, CH | 2.94 (ddd, 8.7, 6.4, 2.4) | 78.6, CH | 3.05 (ddd, 7.8, 6.4, 2.2) | ||
| 6′a | 61.8, CH2 | 3.45 (ddd, 11.8, 6.2, 5.2) | 61.4, CH2 | 3.39 (ddd, 11.3, 6.2, 5.1) | 63.0, CH2 | 3.39 (ddd, 11.0, 6.2, 5.1) | ||
| 6′b | 3.98 (ddd, 11.8, 6.8, 2.3) | 3.65 (ddd, 11.3, 6.8, 2.2) | 3.65 (ddd, 11.0, 6.5, 2.2) | |||||
| 6-OMe | 49.2, CH3 | 49.2, CH3 | ||||||
| 6-OH | 4.79 (d, 3.5) | |||||||
| 2′-OH | 4.97 (d, 4.5) | 5.09 (d, 4.3) | 5.02 (d, 4.3) | |||||
| 3′-OH | 4.94 (d, 4.2) | 5.04 (d, 4.5) | 5.01 (d, 4.5) | |||||
| 4′-OH | 4.93 (d, 5.4) | 5.02 (d, 5.2) | 4.99 (d, 5.2) | |||||
| 6′-OH | 4.17 (d, 5.2) | 4.15 (d, 5.1) | 4.13 (d, 5.1) | |||||
| Compounds | EC50 (μg/mL) | CC50 (μg/mL) | SI (CC50/EC50) |
|---|---|---|---|
| 1 | 23.8 ± 0.5 | 178.2 ± 13.3 | 7.5 |
| 2 | 30.7 ± 1.0 | 164.9 ± 10.4 | 5.4 |
| 3 | 19.4 ± 0.3 | 182.0 ± 6.8 | 9.4 |
| 4 | 8.7 ± 1.1 | 72.3 ± 4.1 | 8.3 |
| 5 | 5.1 ± 0.2 | 57.8 ± 3.9 | 11.3 |
| 6 | 7.6 ± 0.5 | 83.9 ± 2.3 | 11.0 |
| 7 | 80.7 ± 5.8 | 214.8 ± 14.7 | 2.7 |
| Lamivudine | 0.1 ± 0.03 | / | / |
3. Experimental Section
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Acid Hydrolysis of 1–3
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Homhual, S.; Zhang, H.J.; Bunyapraphatsara, N.; Kondratyuk, T.P.; Santarsiero, B.D.; Mesecar, A.D.; Herunsalee, A.; Chaukul, W.; Pezzuto, J.M.; Fong, H.H. Bruguiesulfurol, a new sulfur compound from Bruguiera gymnorrhiza. Planta Med. 2006, 72, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Homhual, S.; Bunyapraphatsara, N.; Kondratyuk, T.; Herunsalee, A.; Chaukul, W.; Pezzuto, J.M.; Fong, H.H.S.; Zhang, H.J. Bioactive dammarane triterpenes from the mangrove plant Bruguiera gymnorrhiza. J. Nat. Prod. 2006, 69, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Huang, X.S.; Sattler, I.; Dahse, H.M.; Fu, H.Z.; Lin, W.H.; Grabley, S. New diterpenoids from the marine mangrove Bruguiera gymnorrhiza. J. Nat. Prod. 2004, 67, 1620–1623. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Huang, X.S.; Sattler, I.; Moellmann, U.; Fu, H.Z.; Lin, W.H.; Grabley, S. New aromatic compounds from the marine mangrove Bruguiera gymnorrhiza. Planta Med. 2005, 71, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.Q.; Guo, Y.W. Gymnorrhizol, an unusual macrocyclic polydisulfide from the Chinese mangrove Bruguiera gymnorrhiza. Tetrahedron Lett. 2004, 45, 5533–5535. [Google Scholar] [CrossRef]
- Panyadee, A.; Sahakitpichan, P.; Ruchirawat, S.; Kanchanapoom, T. 5-Methyl ether flavone glucosides from the leaves of Bruguiera gymnorrhiza. Phytochem. Lett. 2015, 11, 215–219. [Google Scholar] [CrossRef]
- Gao, C.H.; Yi, X.X.; Xie, W.P.; Chen, Y.N.; Xu, M.B.; Su, Z.W.; Yu, L.; Huang, R.M. New antioxidative secondary metabolites from the fruits of a Beibu Gulf mangrove, Avicennia marina. Mar. Drugs 2014, 12, 4353–4360. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.X.; Chen, Y.; Xie, W.P.; Xu, M.B.; Chen, Y.N.; Gao, C.H.; Huang, R.M. Four new jacaranone analogs from the fruits of a Beibu Gulf mangrove Avicennia marina. Mar. Drugs 2014, 12, 2515–2525. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Yasutomi, K.; Mori, I. Structure of a new cyanoglucoside from Ilex warburgii Loesn. Chem. Lett. 1983, 1, 149–150. [Google Scholar] [CrossRef]
- Yogo, M.; Ishiguro, S.; Murata, H.; Furukawa, H. Coclauril, a nonglucosidic 2-cyclohexen-1-ylideneacetonitrile, from Cocculus lauriforius DC. Chem. Pharm. Bull. 1990, 38, 225–226. [Google Scholar] [CrossRef]
- Takahashi, K.; Matsuzawa, S.; Takani, M. Studies on constituents of medicinal plants: 20. The constituent of vines of Menispermum dauricum DC. Chem. Pharm. Bull. 1978, 26, 1677–1681. [Google Scholar] [CrossRef]
- Nahrstedt, A.; Wray, V. Structural revision of a putative cyanogenic glucoside from Ilex aquifolium. Phytochemistry 1990, 29, 3934–3936. [Google Scholar] [CrossRef]
- Nakanishi, T.; Nishi, M.; Somekawa, M.; Murata, H.; Mizuno, M.; Iinuma, M.; Tanaka, T.; Murata, J.; Lang, F.A.; Inada, A. Structures of new and known cyanoglucosides from a North-American plant, Purshia tridentata DC. Chem. Pharm. Bull. 1994, 42, 2251–2255. [Google Scholar] [CrossRef]
- Geng, C.A.; Huang, X.Y.; Lei, L.G.; Zhang, X.M.; Chen, J.J. Chemical constituents of Saniculiphyllum guangxiense. Chem. Biodivers. 2012, 9, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Ikeda, T.; Kaku, N.; Zhu, X.H.; Okawa, M.; Yokomizo, K.; Uyeda, M.; Nohara, T. A new lignan glycoside and phenylethanoid glycosides from Strobilanthes cusia BREMEK. Chem. Pharm. Bull. 2004, 52, 1242–1245. [Google Scholar] [CrossRef] [PubMed]
- Kirk, D.N. The chiroptical properties of carbonyl-compounds. Tetrahedron 1986, 42, 777–818. [Google Scholar] [CrossRef]
- Burnett, R.D.; Kirk, D.N. Chiroptical studies. 101. An empirical-analysis of circular-dichroism data for steroidal and related transoid α,β-unsaturated ketones. J. Chem. Soc. Perkin Trans. 1 1981, 1460–1468. [Google Scholar] [CrossRef]
- Prakash, I.; Chaturvedula, V.S.P. Structures of some novel α-glucosyl diterpene glycosides from the glycosylation of steviol glycosides. Molecules 2014, 19, 20280–20294. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Wang, M.C.; Xu, M.; Wang, Y.; Tang, H.F.; Sun, X.L. Cytotoxic oleanane-type triterpenoid saponins from the rhizomes of Anemone rivularis var. flore-minore. Molecules 2014, 19, 2121–2134. [Google Scholar] [CrossRef] [PubMed]
- Korba, B.E.; Gerin, J.L. Use of a standardized cell-culture assay to assess activities of nucleoside analogs against Hepatitis B virus replication. Antivir. Res. 1992, 19, 55–70. [Google Scholar] [CrossRef]
- Sells, M.A.; Zelent, A.Z.; Shvartsman, M.; Acs, G. Replicative intermediates of Hepatitis B virus in HepG2 cells that produce infectious virions. J. Virol. 1988, 62, 2836–2844. [Google Scholar] [PubMed]
- Acs, G.; Sells, M.A.; Purcell, R.H.; Price, P.; Engle, R.; Shapiro, M.; Popper, H. Hepatitis B virus produced by transfected HepG2 cells causes hepatitis in chimpanzees. Proc. Natl. Acad. Sci. USA 1987, 84, 4641–4644. [Google Scholar] [CrossRef] [PubMed]
- Korba, B.E.; Gerin, J.L. Antisense oligonucleotides are effective inhibitors of Hepatitis B virus replication in vitro. Antivir. Res. 1995, 28, 225–242. [Google Scholar] [CrossRef]
- Seigler, D.S.; Pauli, G.F.; Frohlich, R.; Wegelius, E.; Nahrstedt, A.; Glander, K.E.; Ebinger, J.E. Cyanogenic glycosides and menisdaurin from Guazuma ulmifolia, Ostrya virginiana, Tiquilia plicata, and Tiquilia canescens. Phytochemistry 2005, 66, 1567–1580. [Google Scholar] [CrossRef] [PubMed]
- Damtoft, S.; Jensen, S.R. Cyclohexyl butenolide glucosides from Epacridaceae. Phytochemistry 1995, 40, 157–159. [Google Scholar] [CrossRef]
- Manga, S.S.E.; Messanga, B.B.; Sondengam, B.L. 7,8-Dihydrobenzofuranones from Ouratea reticulata. Fitoterapia 2001, 72, 706–708. [Google Scholar] [CrossRef]
- Sosa, A.; Winternitz, F.; Wylde, R.; Pavia, A.A. Structure of a cyanoglucoside of Lithospermum purpureo-caeruleum. Phytochemistry 1977, 16, 707–709. [Google Scholar] [CrossRef]
- Murakami, A.; Ohigashi, H.; Tanaka, S.; Hirota, M.; Irie, R.; Takeda, N.; Tatematsu, A.; Koshimizu, K. Bitter cyanoglucosides from Lophira alata. Phytochemistry 1993, 32, 1461–1466. [Google Scholar] [CrossRef]
- Silva, T.M.S.; Lins, A.C.D.; Sarmento-Filha, M.J.; Ramos, C.S.; Agra, M.D.; Camara, C.A. Riachin, a new cyanoglucoside from Bauhinia pentandra and its antioxidant activity. Chem. Nat. Compd. 2013, 49, 685–690. [Google Scholar] [CrossRef]
- Abbassy, M.A.; Abdelgaleil, S.A.M.; Belal, A.S.H.; Rasoul, M.A.A.A. Insecticidal, antifeedant and antifungal activities of two glucosides isolated from the seeds of Simmondsia chinensis. Ind. Crop. Prod. 2007, 26, 345–350. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, X.-X.; Deng, J.-G.; Gao, C.-H.; Hou, X.-T.; Li, F.; Wang, Z.-P.; Hao, E.-W.; Xie, Y.; Du, Z.-C.; Huang, H.-X.; et al. Four New Cyclohexylideneacetonitrile Derivatives from the Hypocotyl of Mangrove (Bruguiera gymnorrhiza). Molecules 2015, 20, 14565-14575. https://doi.org/10.3390/molecules200814565
Yi X-X, Deng J-G, Gao C-H, Hou X-T, Li F, Wang Z-P, Hao E-W, Xie Y, Du Z-C, Huang H-X, et al. Four New Cyclohexylideneacetonitrile Derivatives from the Hypocotyl of Mangrove (Bruguiera gymnorrhiza). Molecules. 2015; 20(8):14565-14575. https://doi.org/10.3390/molecules200814565
Chicago/Turabian StyleYi, Xiang-Xi, Jia-Gang Deng, Cheng-Hai Gao, Xiao-Tao Hou, Fei Li, Zhi-Ping Wang, Er-Wei Hao, Yan Xie, Zheng-Cai Du, Hui-Xue Huang, and et al. 2015. "Four New Cyclohexylideneacetonitrile Derivatives from the Hypocotyl of Mangrove (Bruguiera gymnorrhiza)" Molecules 20, no. 8: 14565-14575. https://doi.org/10.3390/molecules200814565
APA StyleYi, X.-X., Deng, J.-G., Gao, C.-H., Hou, X.-T., Li, F., Wang, Z.-P., Hao, E.-W., Xie, Y., Du, Z.-C., Huang, H.-X., & Huang, R.-M. (2015). Four New Cyclohexylideneacetonitrile Derivatives from the Hypocotyl of Mangrove (Bruguiera gymnorrhiza). Molecules, 20(8), 14565-14575. https://doi.org/10.3390/molecules200814565





