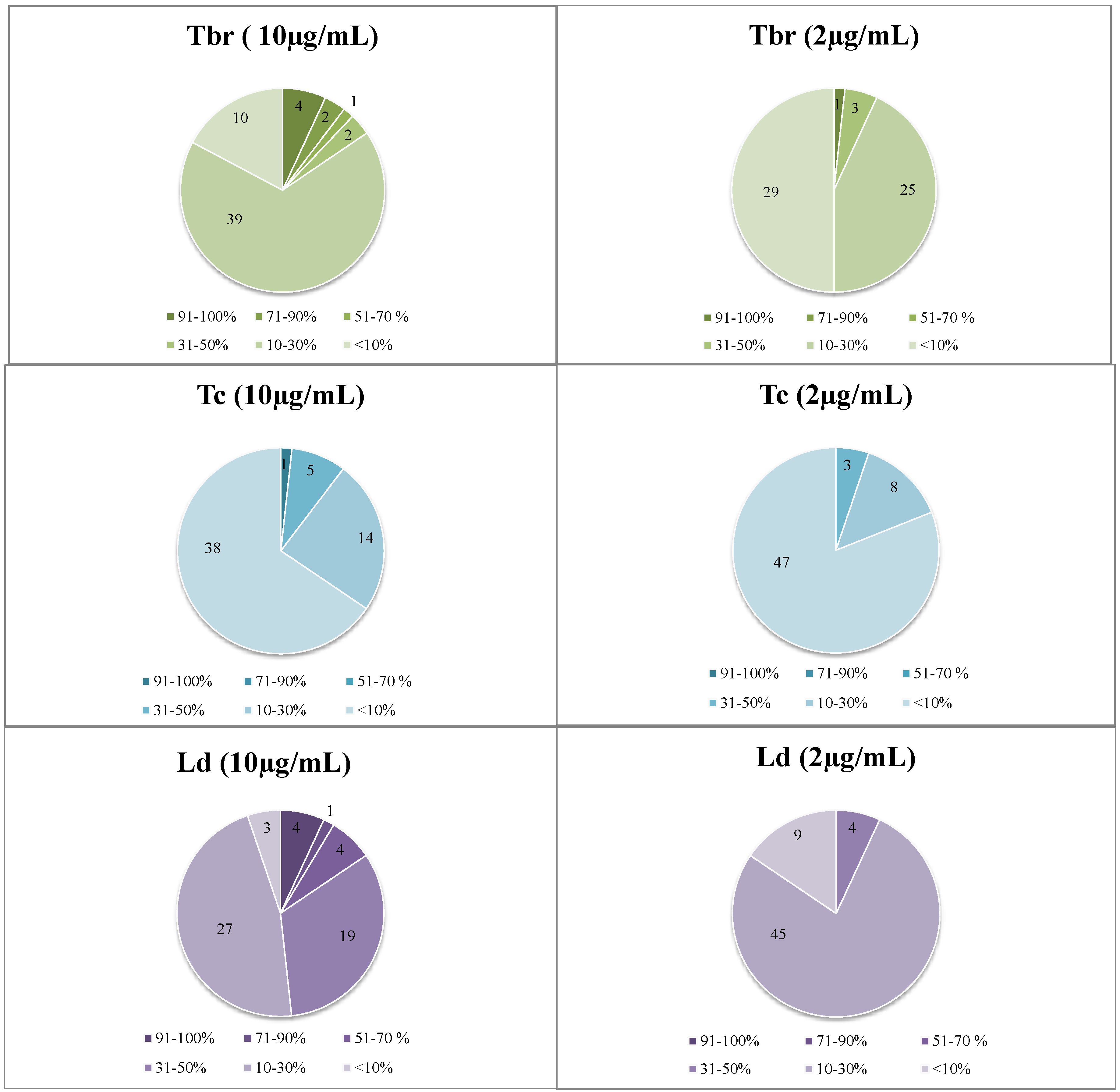2.1. Activity Screening of HMPs against Protozoan Parasites
A total number of 58 extracts were prepared from 53 HMPs and tested
in vitro at two test concentrations, 2 and 10 µg/mL, for growth inhibitory (GI) activity against the following parasites:
Trypanosoma brucei rhodesiense (
Tbr),
T. cruzi (
Tc),
Leishmania donovani (
Ld), and
Plasmodium falciparum (
Pf), according to the procedures described in
Section 3.3. The identity of the HMPs is depicted in
Table A1, Appendix. The results of these biological assays are reported in
Figure 1 (numerical data are reported in
Table A2, Appendix). In case of samples displaying activity in this assay, IC
50 values against the susceptible parasites as well as for cytotoxicity against mammalian cells (L6 rat skeletal myoblasts) were determined. The results of the IC
50 determinations and the respective selectivity indices (SI = IC
50 (L6)/IC
50 (parasite)) are reported in
Table 1. UHPLC-UV chromatograms and LC-MS data of the active extracts,
i.e., their suggested dereplicated constituents, are depicted in the section Dereplication Data, Appendix.
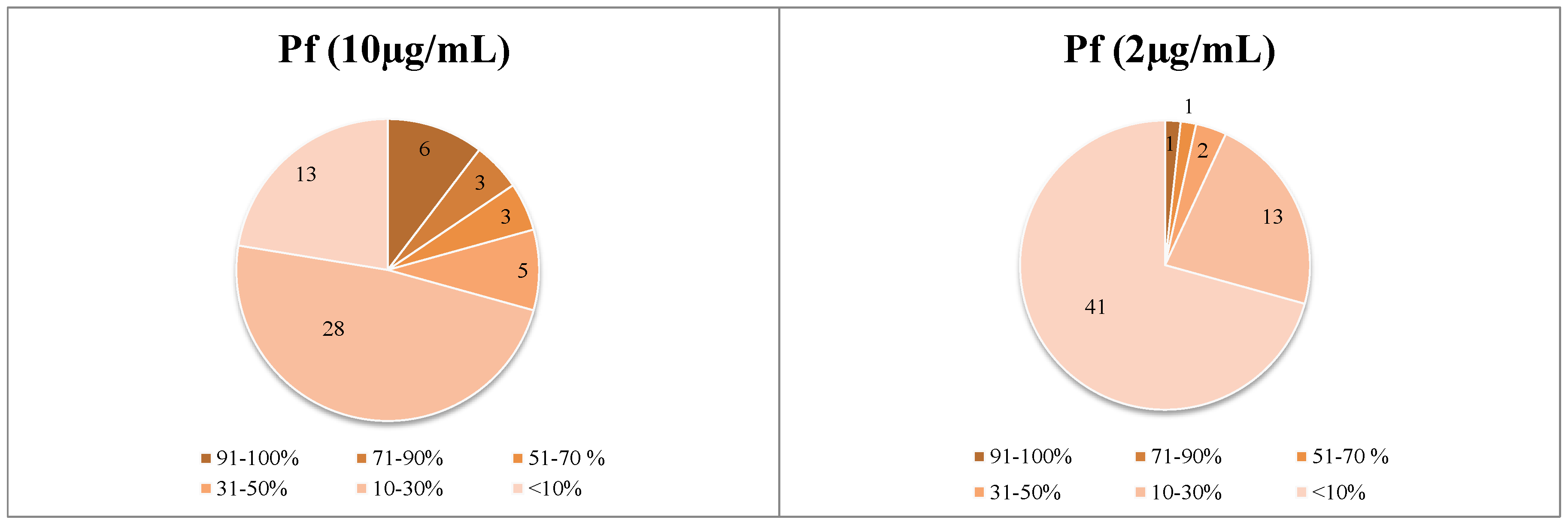
Figure 1.
Distribution of the dataset of 58 extracts over the four biological activities under study at the two tested concentrations. Data represent percent growth inhibition of the various parasites at 10 and 2 µg/mL.
Figure 1.
Distribution of the dataset of 58 extracts over the four biological activities under study at the two tested concentrations. Data represent percent growth inhibition of the various parasites at 10 and 2 µg/mL.
Table 1.
IC50 values of active extracts against the most susceptible parasites and for cytotoxicity against L6 cells. All data are expressed in µg/mL and represent the mean of two independent determinations. Highlighted in light green: 2.5 < IC50 < 10 µg/mL. Dark green: IC50 < 2.5 µg/mL. Orange: SI > 10. Yellow: 10 > SI > 7.5.
Table 1.
IC50 values of active extracts against the most susceptible parasites and for cytotoxicity against L6 cells. All data are expressed in µg/mL and represent the mean of two independent determinations. Highlighted in light green: 2.5 < IC50 < 10 µg/mL. Dark green: IC50 < 2.5 µg/mL. Orange: SI > 10. Yellow: 10 > SI > 7.5.
| ID | Tbr | Tc | Ld | Pf | L6 | SI (Tbr) | SI (Tc) | SI (Ld) | SI (Pf) |
|---|
| Melarsoprol | 0.004 | | | | | | | | |
| Benznidazole | | 0.533 | | | | | | | |
| Miltefosine | | | 0.075 | | | | | | |
| Chloroquine | | | | 0.002 | | | | | |
| Podophyllotoxin | | | | | 0.007 | | | | |
| 2 | n.d. | n.d. | 5.34 | 9.25 | 67.4 | | | 12.6 | 7.3 |
| 3 | n.d. | n.d. | n.d. | 8.21 | 59.4 | | | | 7.2 |
| 14 | 5.87 | n.d. | 2.14 | 0.59 | 5.28 | 0.90 | | 2.5 | 8.9 |
| 22 | n.d. | n.d. | n.d. | 10.08 | 47.0 | | | | 4.7 |
| 25 | n.d. | n.d. | 5.65 | 2.00 | 54.6 | | | 9.7 | 27.3 |
| 26 | n.d. | 5.87 | 2.24 | 4.13 | 41.6 | | 7.09 | 18.6 | 10.1 |
| 40 | n.d. | n.d. | 3.17 | 2.28 | 47.7 | | | 15.1 | 21.0 |
| 45 | n.d. | n.d. | n.d. | 5.83 | 52.3 | | | | 9.0 |
| 49 | n.d. | n.d. | 4.53 | n.d. | 50.5 | | | 11.1 | |
| 20B * | 4.03 | n.d. | n.d. | n.d. | 0.01 | 0.003 | | | |
| 28B * | 3.79 | n.d. | n.d. | n.d. | 53.7 | 14.2 | | | |
| 38 | n.d. | n.d. | n.d. | 2.72 | 55.0 | | | | 20.3 |
| 39 | 1.86 | n.d. | n.d. | n.d. | 32.3 | 17.3 | | | |
| 55 | 1.12 | n.d. | n.d. | n.d. | 12.1 | 10.9 | | | |
Sixteen HMPs showed growth inhibition (GI) activity in vitro against at least one of the pathogens (>50% GI at 10 µg/mL), six extracts of which displayed high activity (IC50 < 2.5 µg/mL).
Generally, Plasmodium falciparum was found to be the most sensitive parasite to the tested HMPs and T. cruzi the least sensitive, against which activities hardly ever exceeded 45% of inhibition.
In particular, promising results were obtained with Arnica montana and Salvia officinalis as the most active preparations against the etiologic agent of East African Human Trypanosomiasis (sleeping sickness). Moreover, the highest antimalarial activities were determined for the extracts of Curcuma longa, Silybum marianum, and Hypericum perforatum. It is noteworthy that the preparation of Valeriana officinalis showed antileishmanial activity with an IC50 value of 2.1 µg/mL and was the only preparation that also displayed moderate activity against T. cruzi. Beside these “first line samples”, some preparations presenting moderate activity (IC50 values 2.5–6 µg/mL) and showing high SI values, i.e., ID_38, can still be considered interesting samples which will be subject to following studies.
2.2. Antitrypanosomal Activity
The extract of
Arnica montana L. (Asteraceae) (IC
50 = 1.12 µg/mL and SI = 10.86) was included in the study as a herbal positive-control, since the anti-trypansosomal activity of its main sesquiterpene lactone constituents was previously described by our group [
8,
9]. The tincture under study was analyzed by UHPLC/ESI-QqTOF-MSMS and found to contain the main sesquiterpene lactones of the helenalin type known from this plant (see
Figure A6 and
Table A8, Appendix). Thus, the positive result found with this
Arnica tincture in the present research is in agreement with the strong antitrypanosomal activity of its major constituents.
With an IC
50 value of 1.86 µg/mL against
Tbr, the extract ID_39 of
Salvia officinalis L. (
Lamiaceae) appears to be a promising hit for further evaluation. The antitrypanosomal and antimalarial activity of other species of the genus
Salvia have been previously reported, however, the majority of hitherto isolated and active compounds also showed nonselective toxicity [
10,
11,
12,
13,
14]. Our tested extract presented a favorable value of SI = 17.3. Therefore, the fractionation of this extract and the evaluation of the resulting biologically active fractions appears interesting. In-depth studies with the aim to identify its antitrypanosomal constituent(s) based on multivariate data analyses in a similar manner, as it was recently described by Ellendorf
et al. [
15], and to target isolation of the relevant natural products are in progress.
The only preparation that showed activity against
T. cruzi (IC
50 = 5.86 µg/mL and SI = 7.9) was the ethanolic extract ID_26 of
Valeriana officinalis L. (Caprifoliaceae—including all former Valerianaceae—[
16]). To the best of our knowledge, no previous reports exist on any antitrypanosomal activity of this plant. Even though the biological values of this sample somewhat exceeded our general criteria range for promising activity (IC
50 < 4 µg/mL and SI > 10), which is probably due to the generally lower sensitivity of this intracellular parasite to drugs, it appears interesting to study extract ID_26 further for the antitrypanosomal activity of its single constituents. Valerian is very widespread in Europe, Asia, and North America and is easy to cultivate, so it might become a promising source of new therapeutics against
T. cruzi infections.
2.4. Antiplasmodial Activity
Extract ID_14, obtained from a commercial preparation of turmeric,
Curcuma longa L. (
Zingiberaceae), showed promising antiplasmodial activity with an IC
50 value of only 0.59 µg/mL, which is significantly lower than previously published data on this plant [
19]. In the literature, the rhizomes of different species of
Curcuma are mentioned to be used in traditional medicine, attributing the therapeutic properties largely to their polyphenolic curcuminoid constituents [
19]. Specifically, the most abundant curcuminoid, curcumin, exhibited
in vitro and
in vivo antimalarial activity with IC
50 values ranging from 1.84–3.5 µg/mL [
20,
21] and an absence of significant toxicity. Furthermore, Arteminisin (ART)-based combination therapies with curcumin have been investigated as a new hope for malaria therapy. Curcumin was found to synergize with ART but also to prime the immune system to protect against recrudescence in mice infected with
Plasmodium berghei [
22]. Based on the significantly lower IC
50 value of the present extract compared to the published data, experiments are in progress to investigate whether curcumin/curcuminoids are the only active compounds of our extract or whether further synergistic contributions can come from other constituents of the total extract.
The sample ID_25, representing a preparation of Silymarin, a standardized mixture of closely related flavolignans obtained from milk thistle (
Silybum marianum (L.) Gaertn. (Asteraceae)), showed promising antiplasmodial activity with IC
50 = 2 µg/mL and SI = 27.3. Milk thistle is one of the oldest medicinal plants and is nowadays used for the treatment of liver damage, hepatitis, and cirrhosis. Moreover, Silybin dihemisuccinate, a derivative of silybin, is used as a clinical antidote for acute
Amanita mushroom poisoning [
23]. In spite of a large number of studies on the pharmacological activity of milk thistle existing in the literature, there is no information, to date, on the antiplasmodial activity of this plant or its constituents, apart from some tests carried out with the synthetized flavolignans 2,3-dehydrosilibinin and 8-(1;1)-DMA-kaempferide [
24]. Since the IC
50 value and SI found in our study were quite favorable and the therapeutic window of the extract is known to be wide, we consider
S. marianum extract a promising and a useful hit to combat
Pf. The isolation of the different flavolignans present in the extract and their biological activity tests are in progress.
The ethanolic extract ID_40 of
Hypericum perforatum L. (Hypericaceae) showed high activity against
Pf with an IC
50 value of 2.27 µg/mL and with favorable selectivity values. Five phloroglucinol derivatives of
H. erectum were reported to possess antiplasmodial activity [
25], however, they were not found in our active extract (see
Figure A5 and
Table A7, Appendix). Hyperforin, which was present in our sample, and some derivatives were also reported active against
Pf. For instance, the lithium salt of Hyperforin showed IC
50 = 2.1 µM [
26]. Thus, bioguided fractionation and biological evaluation of isolated compounds compared to the total extract are in progress in order to evaluate whether synergism or the additive effects of single constituents may account for the conspicuous activity of the total extract. Thus, we would justify the use of the complete extract as antiplasmodial instead of the single compounds.