Constituents of Psoralea corylifolia Fruits and Their Effects on Methicillin-Resistant Staphylococcus aureus
Abstract
:1. Introduction
2. Results and Discussion
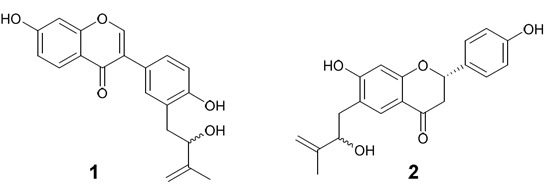
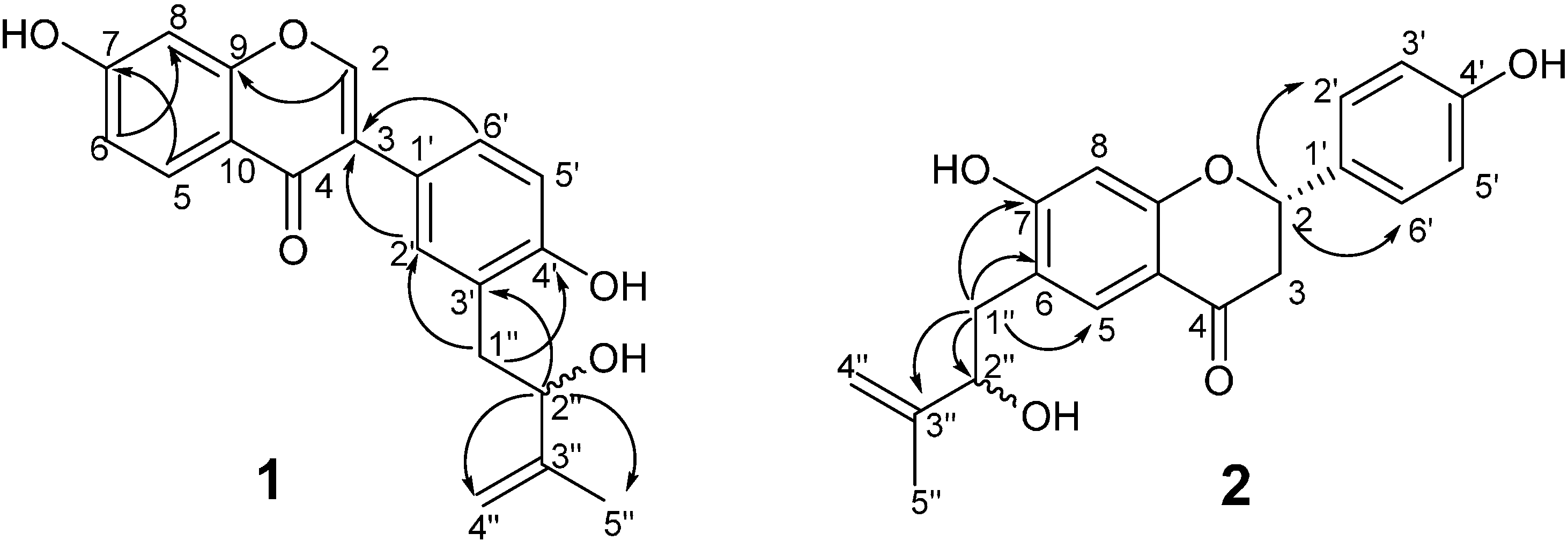
2.1. Structure Elucidation of the Novel Compounds
| No. | 1 | 2 | ||
|---|---|---|---|---|
| δH | δC | δH | δC | |
| 2 | 8.13 (s) | 153.1 | 5.41 (dd, 3.0, 13.2) | 80.5, 80.4 |
| 3 | 125.2 | 3.01 (m) | 44.8, 44.7 | |
| 2.64 (m) | ||||
| 4 | 175.7 | 190.6 | ||
| 5 | 8.05 (d, 8.4) | 128.5 | 7.60 (s) | 131.4 |
| 6 | 6.98 (dd, 2.0, 8.4) | 115.6 | 121.7, 121.6 | |
| 7 | 163.1 | 164.0 | ||
| 8 | 6.87 (d, 2.0) | 103.1 | 6.39 (s) | 104.3 |
| 9 | 158.7 | 163.2 | ||
| 10 | 118.6 | 114.9 | ||
| 1′ | 124.1 | 131.4 | ||
| 2′ | 7.38 (d, 2.0) | 132.9 | 7.39 (d, 9.0) | 128.9 |
| 3′ | 126.6 | 6.88 (d, 9.0) | 116.1 | |
| 4′ | 156.8 | 158.5 | ||
| 5′ | 6.85 (d, 8.4) | 116.7 | 6.88 (d, 9.0) | 116.1 |
| 6′ | 7.35 (dd, 2.0, 8.4) | 129.3 | 7.39 (d, 9.0) | 128.9 |
| 1′′ | 2.90 (m) | 38.9 | 2.85 (m) | 38.0 |
| 2′′ | 4.41 (m) | 77.1 | 4.38 (m) | 76.5, 76.4 |
| 3′′ | 148.5 | 148.2 | ||
| 4′′ | 4.98 (s) | 110.6 | 4.95 (s) | 110.7 |
| 4.77 (s) | 4.77 (s) | |||
| 5′′ | 1.81 (s) | 18.3 | 1.79 (s) | 18.4, 18.3 |
2.2. Antibacterial Effects of Isolated Compounds
2.2.1. Flavone and Flavanones
2.2.2. Isoflavones and Chalcones
2.2.3. Meroterpenes and Coumarins
| Compounds | MIC (μg/mL) | |
|---|---|---|
| MRSA OM481 a | MRSA OM584 a | |
| Flavone | ||
| Corylifol C (3) | 16 (4.7) b | 16 (4.7) b |
| Flavanones | ||
| Bakuflavanone (2) | >32 (>9.4) | >32 (>9.4) |
| Bavachinin (4) | >32 (>9.5) | 32 (9.5) |
| Bavachin (5) | 32 (9.9) | 32 (9.9) |
| Isoflavones | ||
| Neobavaisoflavone (6) | 16 (5.0) | 16 (5.0) |
| Corylin (7) | >32 (>10) | >32 (>10) |
| Corylifol A (8) | >32 (>8.2) | >32 (>8.2) |
| 8-Prenyldaidzein (9) | >32 (>9.9) | >32 (>9.9) |
| Bakuisoflavone (1) | >32 (>9.5) | >32 (>9.5) |
| Chalcones | ||
| Isobavachalcone (10) | 8 (2.5) | 8 (3.1) |
| Corylifol B (11) | 16 (4.7) | 8 (2.4) |
| Meroterpenes | ||
| Bakuchiol (12) | 8 (3.1) | 8 (3.1) |
| 3-Hydroxy-Δ1-bakuchiol (13) | >32 (>12) | >32 (>12) |
| 2-Hydroxy-Δ3-bakuchiol (14) | >32 (>12) | >32 (>12) |
| 12,13-Diolbakuchiol (15) | >32 (>11) | >32 (>11) |
| Coumarins | ||
| Psoralen (16) | >32 (>17) | >32 (>17) |
| Isopsoralen (17) | >32 (>17) | >32 (>17) |
2.3. Structure-Activity Relationships
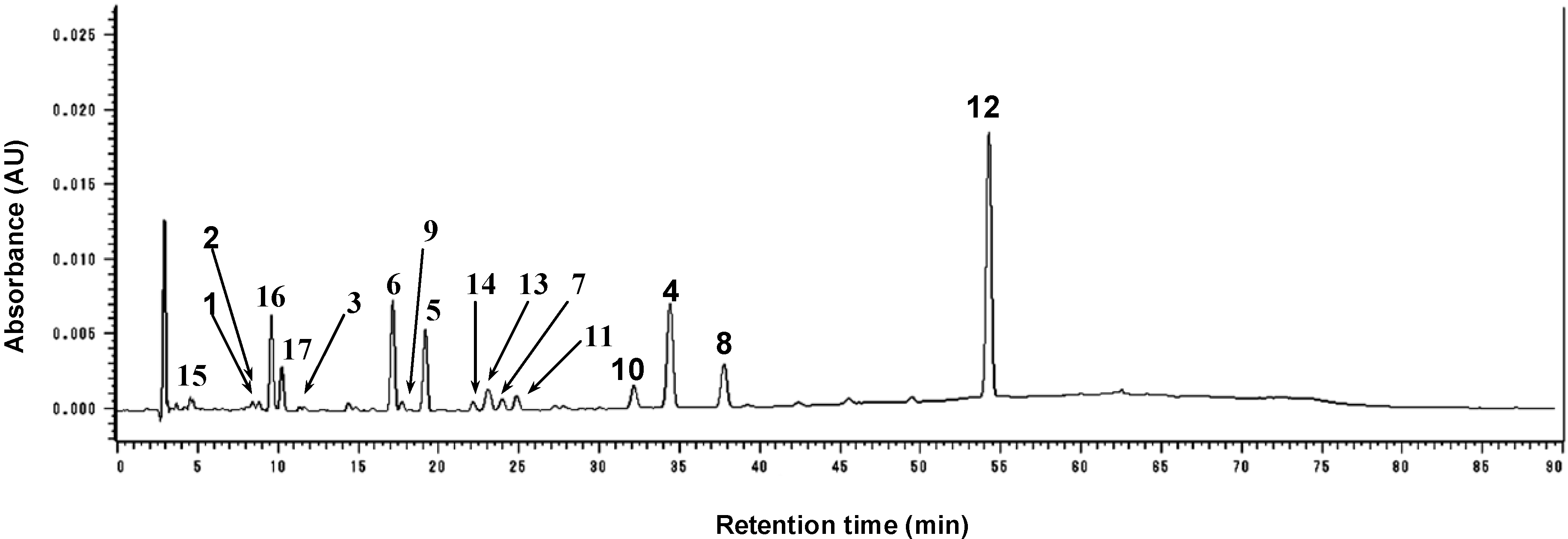
2.4. Quantitative Analysis of the Major Constituents in P. corylifolia Fruit Extract
| Compounds | Content (% w/w) a |
|---|---|
| Bavachinin (4) | 5.03 ± 0.100 |
| Bavachin (5) | 1.80 ± 0.059 |
| Neobavaisoflavone (6) | 2.33 ± 0.054 |
| Corylifol A (8) | 1.86 ± 0.046 |
| Isobavachalcone (10) | 3.14 ± 0.111 |
| Corylifol B (11) | 1.81 ± 0.121 |
| Bakuchiol (12) | 16.49 ± 0.455 |
| Psoralen (16) | 1.76 ± 0.052 |
| Isopsoralen (17) | 1.26 ± 0.071 |
3. Experimental Section
3.1. General Information
3.2. Isolation of Compounds from P. corylifolia
3.3. Compound Characterization
3.4. Antibacterial Assay
3.5. Quantitative Analysis of the Major Constituents in P. corylifolia Fruit Extract
| Compound Used as the External Standard | Retention Time (min) | Range of the Amounts of the External Standard Injected (µg) | Equation of Regression Line for the External Standard a | r2 b |
|---|---|---|---|---|
| 4 | 34.9 | 0.02–2.00 | y = 1.00 × 106x + 1.11 × 104 | 0.9980 |
| 5 | 19.4 | 0.50–2.00 | y = 2.00 × 106x + 0.95 × 104 | 0.9996 |
| 6 | 17.4 | 0.50–2.00 | y = 2.00 × 106x – 0.85 × 104 | 0.9992 |
| 8 | 38.3 | 0.02–2.00 | y = 1.00 × 106x + 0.08 × 104 | 1.0000 |
| 10 | 32.6 | 0.50–2.00 | y = 2.20 × 106x + 0.14 × 104 | 0.9996 |
| 11 | 25.2 | 0.02–2.00 | y = 0.49 × 106x + 0.09 × 104 | 0.9999 |
| 12 | 54.6 | 0.20–2.00 | y = 0.91 × 106x + 0.64 × 104 | 0.9992 |
| 16 | 9.7 | 0.02–2.00 | y = 2.00 × 106x + 1.23 × 104 | 0.9985 |
| 17 | 10.3 | 0.02–2.00 | y = 1.00 × 106x + 0.30 × 104 | 0.9999 |
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Guo, J.N.; Weng, X.C.; Wu, H.; Li, Q.H.; Bi, K.S. Antioxidants from a Chinese medicinal herb—Psoralea corylifolia L. Food Chem. 2005, 91, 287–292. [Google Scholar]
- Tsai, W.J.; Hsin, W.C.; Chen, C.C. Antiplatelet flavonoids from seeds of Psoralea corylifolia. J. Nat. Prod. 1996, 59, 671–672. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Ha, T.Y.; Ahn, J.; Kim, S. Estrogenic activities of Psoralea corylifolia L. seed extracts and main constituents. Phytomedicine 2011, 18, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Latha, P.G.; Evans, D.A.; Panikkar, K.R.; Jayavardhanan, K.K. Immunomodulatory and antitumour properties of Psoralea corylifolia seeds. Fitoterapia 2000, 71, 223–231. [Google Scholar] [CrossRef]
- Yin, S.; Fan, C.Q.; Wang, Y.; Dong, L.; Yue, J.M. Antibacterial prenylflavone derivatives from Psoralea corylifolia, and their structure–activity relationship study. Bioorg. Med. Chem. 2004, 12, 4387–4392. [Google Scholar] [CrossRef] [PubMed]
- Chopra, B.; Dhingra, A.K.; Dhar, K.L. Antimicrobial activity of Psoralea corylifolia Linn. (Baguchi) seeds extracts by organic solvents and supercritical fluids. Int. J. Pharm. Clin. Res. 2013, 5, 13–16. [Google Scholar]
- Agnoletti, F.; Mazzolini, E.; Bacchin, C.; Bano, L.; Berto, G.; Rigoli, R.; Muffato, G.; Coato, P.; Tonon, E.; Drigo, I. First reporting of methicillin-resistant Staphylococcus aureus (MRSA) ST398 in an industrial rabbit holding and in farm-related people. Vet. Microbiol. 2014, 170, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Hatano, T.; Shintani, Y.; Aga, Y.; Shiota, S.; Tsuchiya, T.; Yoshida, T. Phenolic constituents of licorice. VIII. Structures of glicophenone and glicoisoflavanone, and effects of licorice phenolics on methicillin-resistant Staphylococcus aureus. Chem. Pharm. Bull. 2000, 48, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.W.; Cai, X.F.; Dat, N.T.; Hong, S.S.; Han, A.R.; Seo, E.K.; Hwang, B.Y.; Nan, J.X.; Lee, D.; Lee, J.J. Bisbakuchiols A and B, novel dimeric meroterpenoids from Psoralea corylifolia. Tetrahedron Lett. 2007, 48, 8861–8864. [Google Scholar]
- Wang, X.; Wang, Y.; Yuan, J.; Sun, Q.; Liu, J.; Zheng, C. An efficient new method for extraction, separation and purification of psoralen and isopsoralen from Fructus Psoraleae by supercritical fluid extraction and high-speed counter-current chromatography. J. Chromatogr. A 2004, 1055, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.D.; Li, G.W.; Chen, L.; Zhang, Z.J.; Yin, J.J.; Wu, T.; Cheng, Z.H.; Wei, X.H.; Wang, Z.G. Isolation of antioxidants from Psoralea corylifolia fruits using high-speed counter-current chromatography guided by thin layer chromatography-antioxidant autographic assay. J. Chromatogr. A 2010, 1217, 5470–5476. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.Y.; Lee, J.H.; Curtis-Long, M.J.; Cho, J.K.; Kim, J.Y.; Lee, W.S.K.; Park, H. Glycosidase inhibitory phenolic compounds from the seed of Psoralea corylifolia. Food Chem. 2010, 121, 940–945. [Google Scholar] [CrossRef]
- Zhao, L.H.; Huang, C.Y.; Shan, Z.; Xiang, B.R.; Mei, L.H. Fingerprint analysis of Psoralea corylifolia L. by HPLC and LC–MS. J. Chromatogr. B 2005, 821, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Hakamatsuka, T.; Ebizuka, Y.; Sankawa, U. Induced isoflavonoids from copper chloride-treated stems of Pueraria lobata. Phytochemistry 1991, 30, 1481–1482. [Google Scholar] [CrossRef]
- Khaomek, P.; Ichino, C.; Ishiyama, A.; Sekiguchi, H.; Namatame, M.; Ruangrungsi, N.; Saifah, E.; Kiyohara, H.; Otoguro, K.; Omura, S.; et al. In vitro antimalarial activity of prenylated flavonoids from Erythrina fusca. J. Nat. Med. 2008, 62, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Labbe, C.; Faini, F.; Coll, J.; Connolly, J. Bakuchiol derivatives from the leaves of Psoralea glandulosa. Phytochemistry 1996, 42, 1299–1303. [Google Scholar] [CrossRef]
- Chen, H.L.; Du, X.L.; Tang, W.; Zhou, Y.; Zuo, J.P.; Feng, H.J.; Li, Y.C. Synthesis and structure–immunosuppressive activity relationships of bakuchiol and its derivatives. Bioorg. Med. Chem. 2008, 16, 2403–2411. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Takasugi, M.; Anetai, M. Psoralen and other linear furanocoumarins as phytoalexins in Glehnia littoralis. Phytochemistry 1998, 47, 13–16. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, H.; Park, E.; Shim, S.H. Inhibition of human 20S proteasome by compounds from seeds of Psoralea corylifolia. Bull. Korean Chem. Soc. 2009, 30, 1867–1869. [Google Scholar]
- De Rijke, E.; Zappey, H.; Ariese, F.; Gooijer, C. Flavonoids in Leguminosae: Analysis of extracts of T. pratense L., T. dubium L., T. repens L., and L. corniculatus L. leaves using liquid chromatography with UV, mass spectrometric and fluorescence detection. Anal. Bioanal. Chem. 2004, 378, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Liu, S.; He, S.Z.; He, W.H.; Luo, X.M.; Zhang, S.; Xiao, Z.H.; Qiu, X.M.; Yin, H. A new prenylated flavanone from Derris trifoliata Lour. Molecules 2012, 17, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, V.K.; Nayak, U.R.; Dev, S. Some new flavonoids from Psoralea corylifolia. Tetrahedron Lett. 1968, 9, 2401–2406. [Google Scholar] [CrossRef]
- Hatano, T.; Uebayashi, H.; Ito, H.; Shiota, S.; Tsuchiya, T.; Yoshida, T. Phenolic constituents of Cassia seeds and antibacterial effect of some naphthalenes and anthraquinones on methicillin-resistant Staphylococcus aureus. Chem. Pharm. Bull. 1999, 47, 1121–1127. [Google Scholar] [CrossRef]
- Osório, T.M.; Monache, F.D.; Chiaradia, L.D.; Mascarello, A.; Stumpf, T.R.; Zanett, C.R.; Silveira, D.B.; Barardi, C.R.M.; de Fatima Albino Smania, E.; Viancelli, A.; et al. Antibacterial activity of chalcones, hydrazones and oxadiazoles against methicillin-resistant Staphylococcus aureus. Bioorg. Med. Chem. Lett. 2012, 22, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Katsura, H.; Tsukiyama, R.; Suzuki, A.; Kobayashi, M. In vitro antimicrobial activities of bakuchiol against oral microorganisms. Antimicrob. Agents Chemother. 2001, 45, 3009–3013. [Google Scholar] [CrossRef] [PubMed]
- Eerdunbayaer; Orabi, M.A.; Aoyama, H.; Kuroda, T.; Hatano, T. Structures of two new flavonoids and effects of licorice phenolics on vancomycin-resistant Enterococcus species. Molecules 2014, 19, 3883–3897. [Google Scholar] [CrossRef] [PubMed]
- Eerdunbayaer; Orabi, M.A.; Aoyama, H.; Kuroda, T.; Hatano, T. Structures of new phenolics isolated from licorice, and the effectiveness of licorice phenolics on vancomycin-resistant Enterococci. Molecules 2014, 19, 13027–13041. [Google Scholar] [CrossRef] [PubMed]
- One of the authors (T.H.) of the papers 26 and 27 regretted that the locations of the methoxyl group and the hydroxy group in the structure of glycyrol shown in these papers were wrong, and the methoxyl group should be at C-1 and the hydroxyl group at C-3, respectively.
- Mehta, G.; Nayak, U.R.; Dev, S. Meroterpenoids—I: Psoralea corylifolia Linn.—1. Bakuchiol, a novel monoterpene phenol. Tetrahedron 1973, 29, 1119–1125. [Google Scholar] [CrossRef]
- Sample Availability: Samples of compounds 6, 12, 16 and 17 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Taniguchi, S.; Kuroda, T.; Hatano, T. Constituents of Psoralea corylifolia Fruits and Their Effects on Methicillin-Resistant Staphylococcus aureus. Molecules 2015, 20, 12500-12511. https://doi.org/10.3390/molecules200712500
Cui Y, Taniguchi S, Kuroda T, Hatano T. Constituents of Psoralea corylifolia Fruits and Their Effects on Methicillin-Resistant Staphylococcus aureus. Molecules. 2015; 20(7):12500-12511. https://doi.org/10.3390/molecules200712500
Chicago/Turabian StyleCui, Yanmei, Shoko Taniguchi, Teruo Kuroda, and Tsutomu Hatano. 2015. "Constituents of Psoralea corylifolia Fruits and Their Effects on Methicillin-Resistant Staphylococcus aureus" Molecules 20, no. 7: 12500-12511. https://doi.org/10.3390/molecules200712500
APA StyleCui, Y., Taniguchi, S., Kuroda, T., & Hatano, T. (2015). Constituents of Psoralea corylifolia Fruits and Their Effects on Methicillin-Resistant Staphylococcus aureus. Molecules, 20(7), 12500-12511. https://doi.org/10.3390/molecules200712500