Redox-Active Profile Characterization of Remirea maritima Extracts and Its Cytotoxic Effect in Mouse Fibroblasts (L929) and Melanoma (B16F10) Cells
Abstract
:1. Introduction
2. Results
2.1. Determination of Total Phenolic Content (TPC)
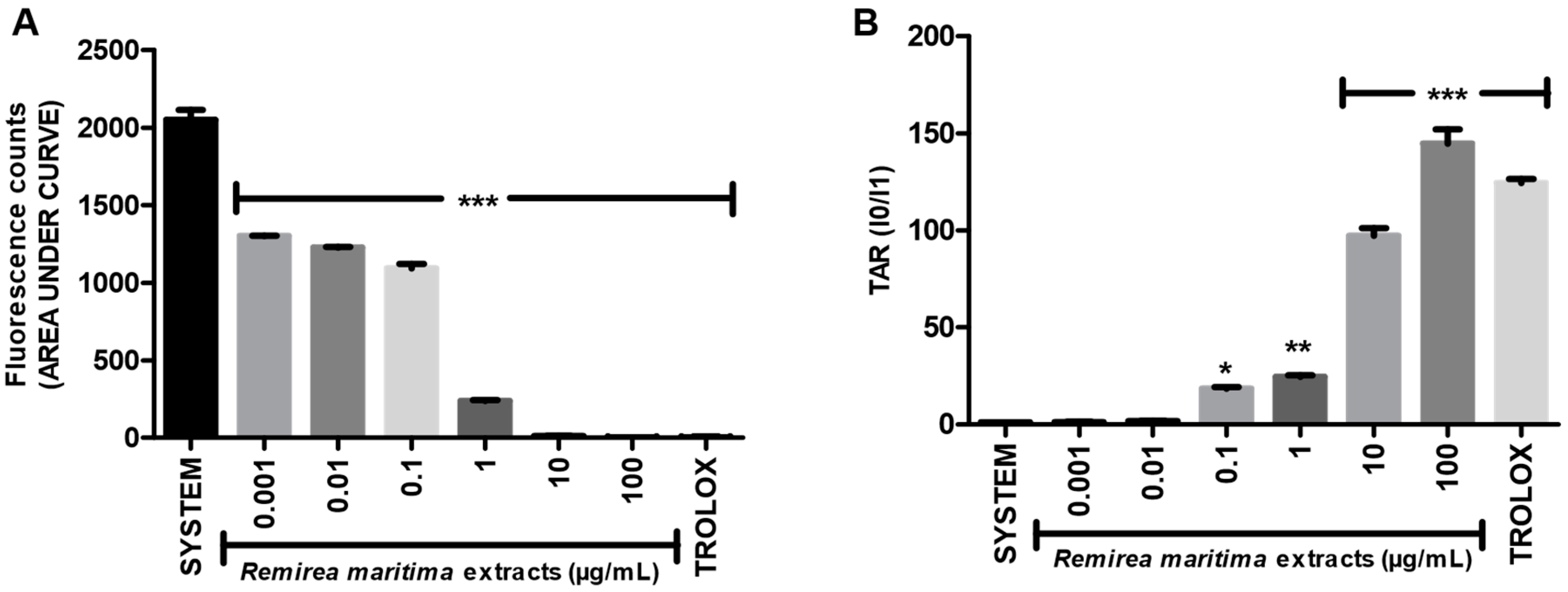
2.2. Total Reactive Antioxidant Potential (TRAP) and Total Antioxidant Reactivity (TAR)
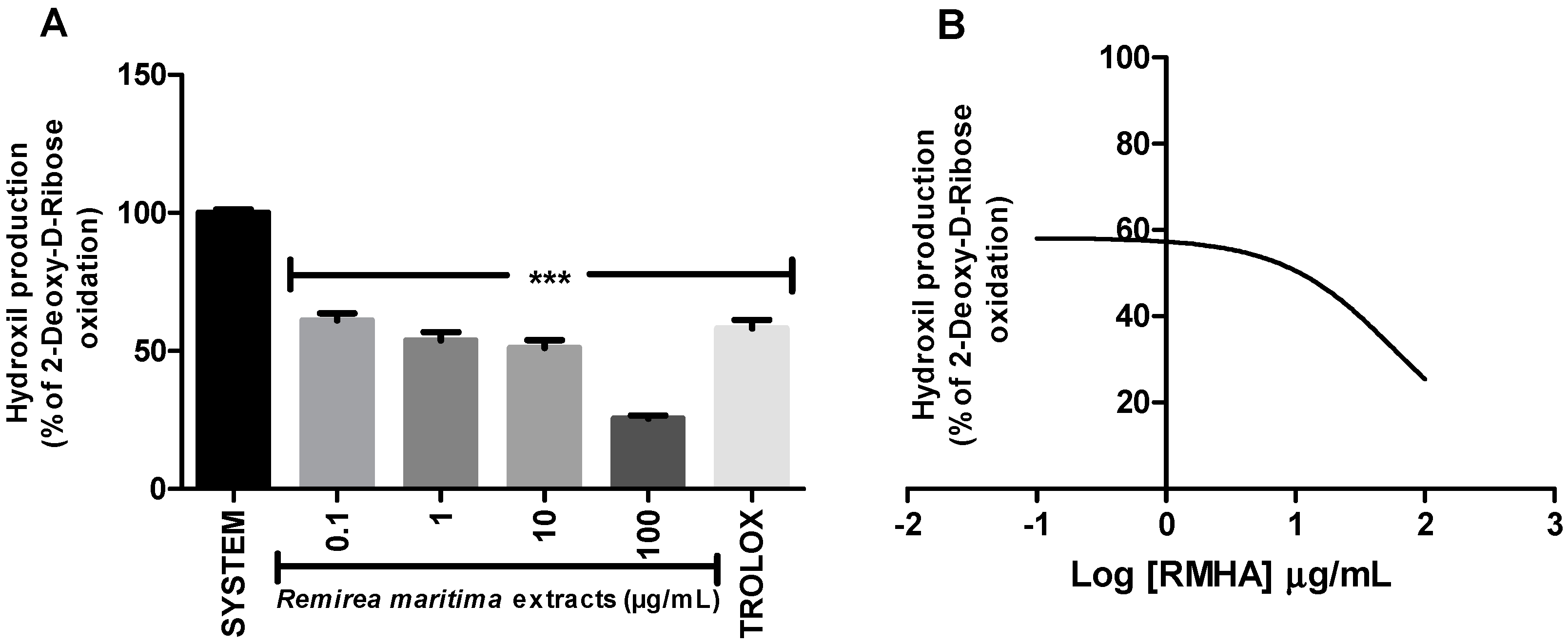
2.3. Hydroxyl Radical-Scavenging Activity
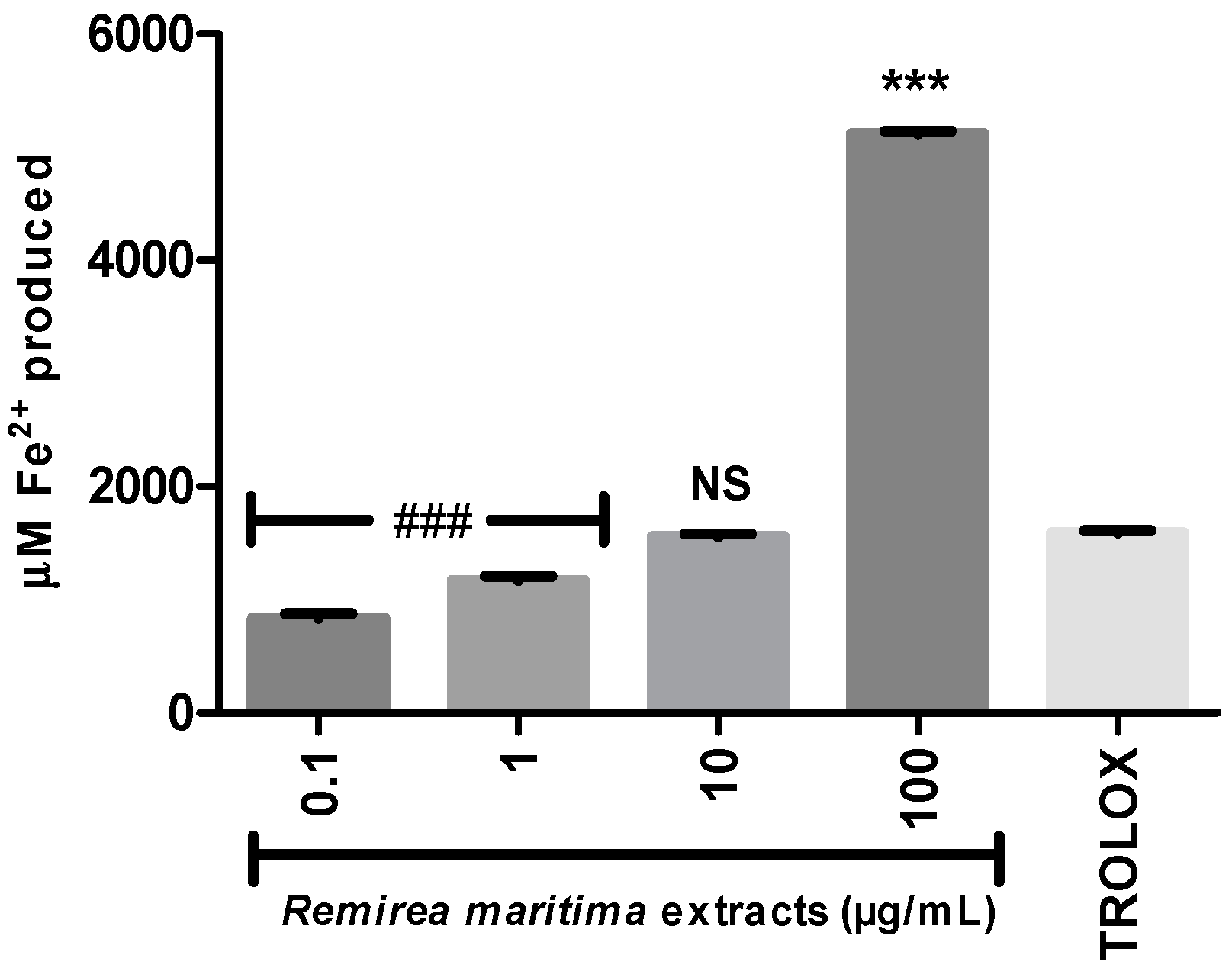
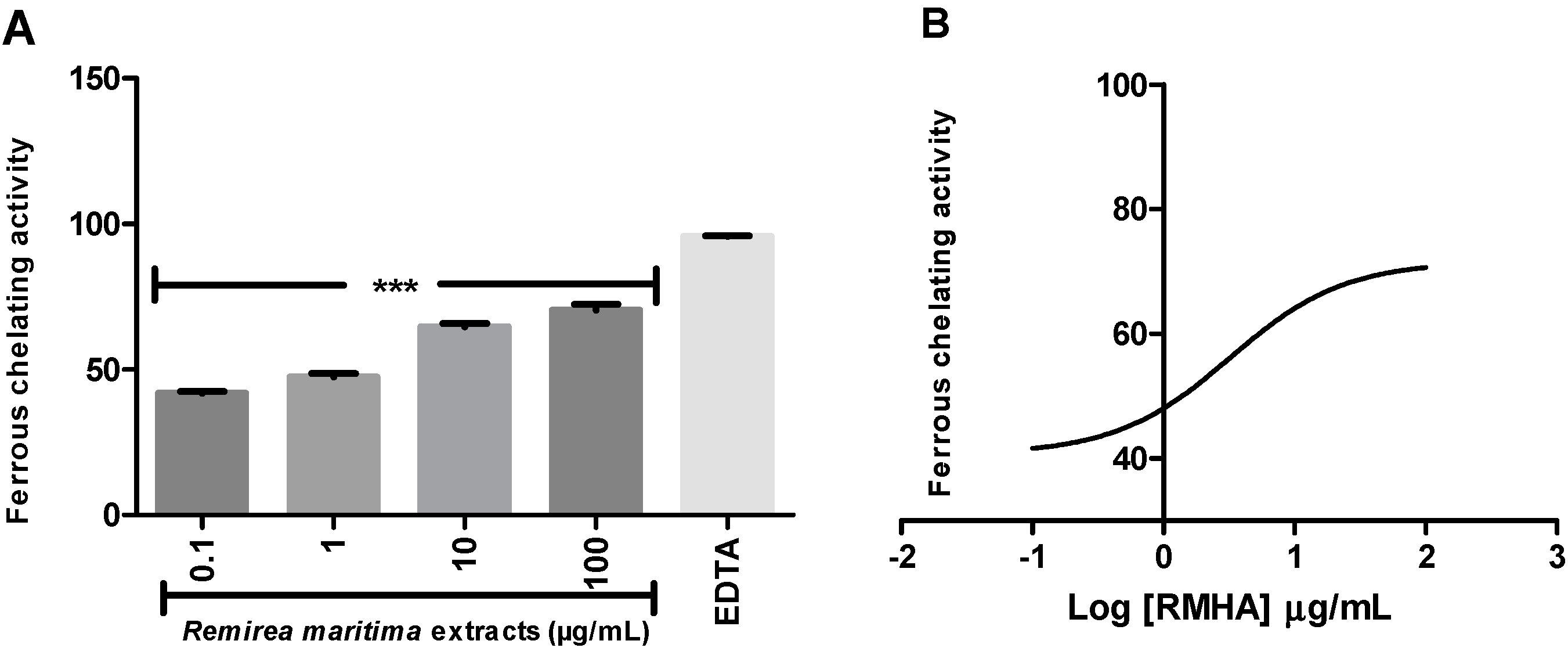
2.4. Ferric Reducing Antioxidant Power (FRAP) and Fe2+ Chelation Assay
2.5. Nitric Oxide (NO·) Scavenging Activity
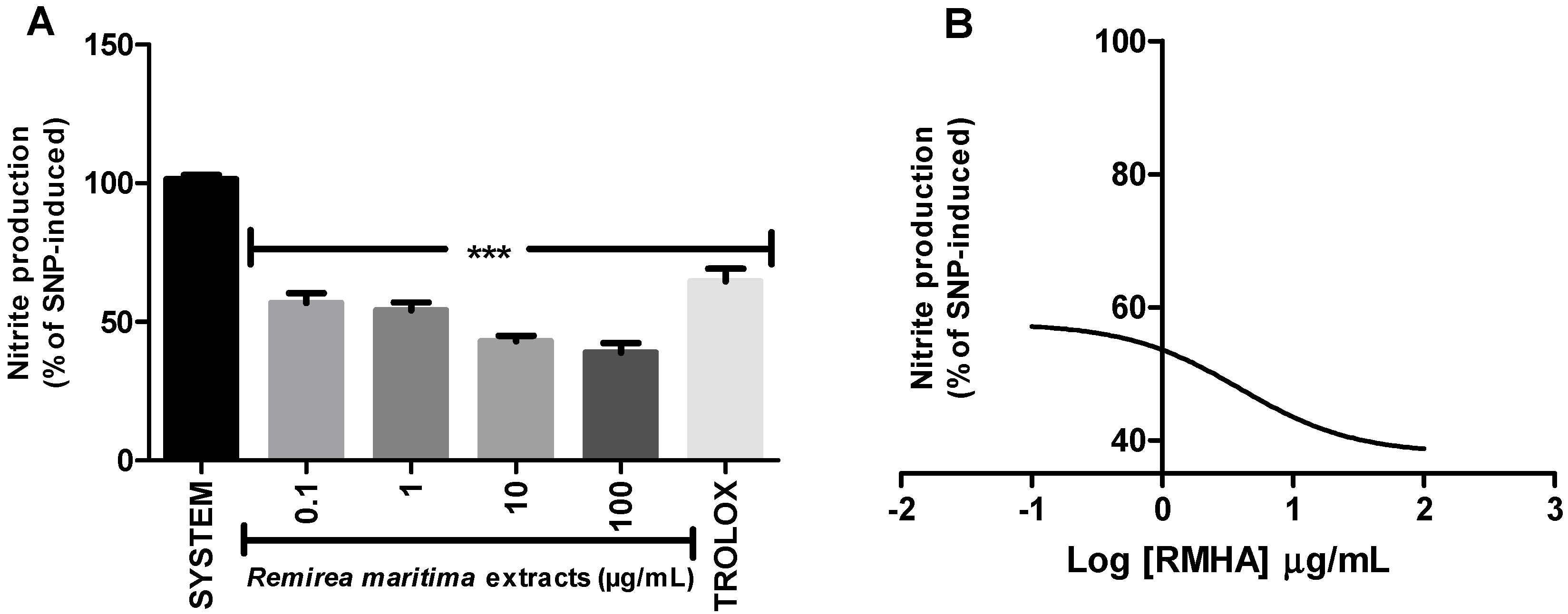
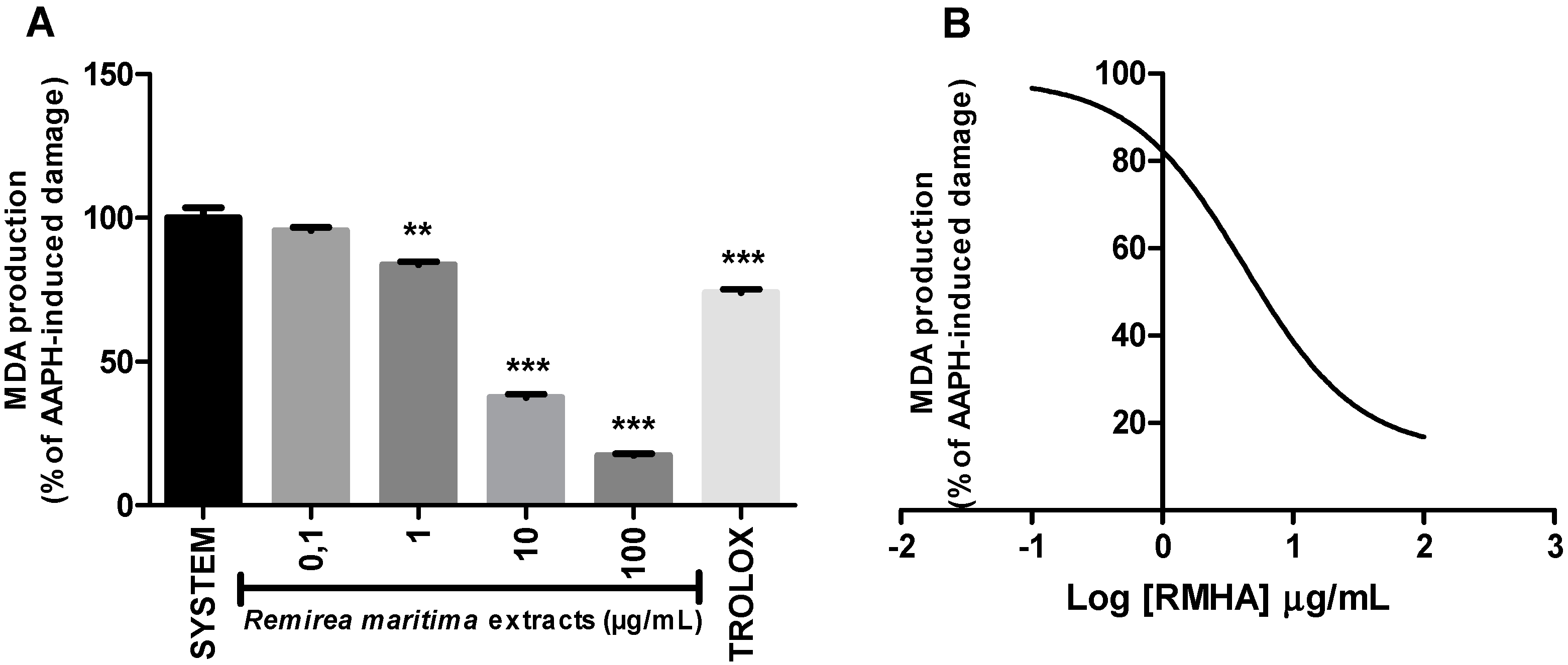
2.6. Thiobarbituric Acid Reactive Species (TBARS)
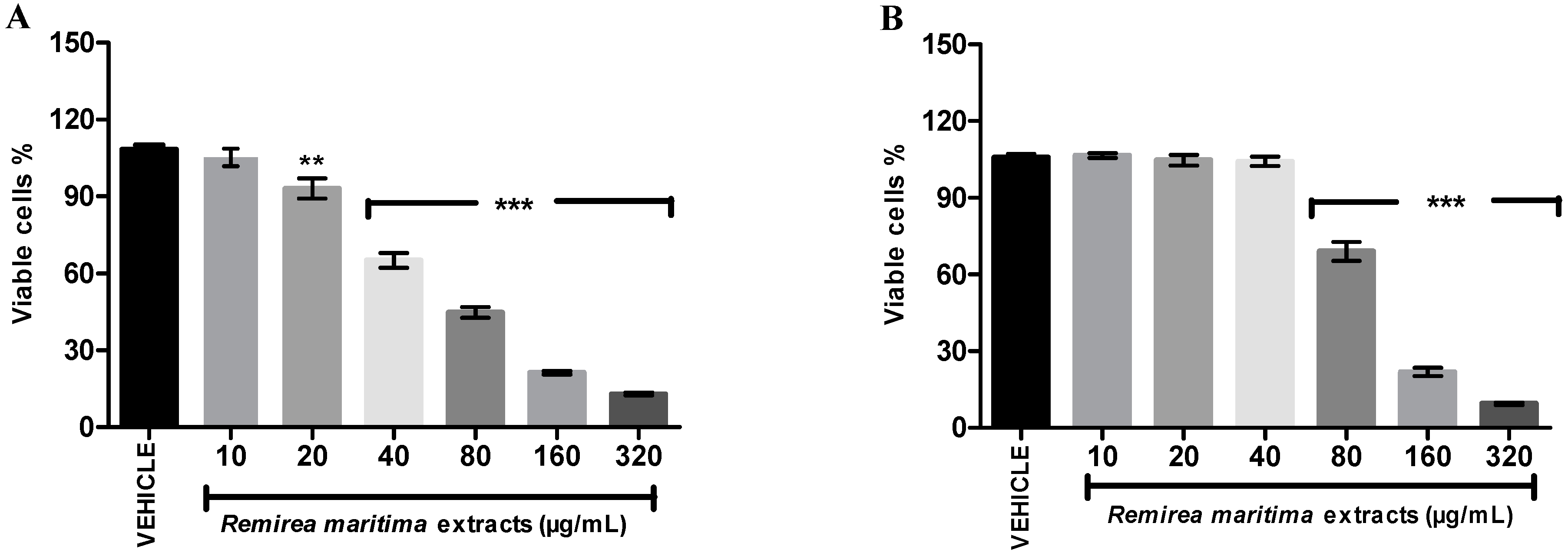
2.7. Cytotoxicity Assay
3. Discussion
4. Experimental Section
4.1. Chemicals
4.2. Plant Material and Preparation of R. maritima Extracts
4.3. Determination of Total Phenolic Content (TPC)
4.4. In Vitro Redox-Active Profile
4.4.1. Total Reactive Antioxidant Potential (TRAP) and Total Antioxidant Reactivity (TAR)
4.4.2. Hydroxyl Radical-Scavenging Activity
4.4.3. Ferric Reducing Antioxidant Power (FRAP)
4.4.4. Fe2+ Chelation Assay
4.4.5. Nitric Oxide (NO.) Scavenging Activity
4.4.6. Thiobarbituric Acid Reactive Species (TBARS)
4.5. Cells Line and Culture Conditions
In Vitro Cytotoxicity Assay
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wu, L.; Hsu, H.; Chen, Y.; Chiu, C.; Lin, Y.; Ho, J.A. Antioxidant and antiproliferative activities of red pitaya. Food Chem. 2006, 95, 319–327. [Google Scholar] [CrossRef]
- Bauman, A.E. Updating the evidence that physical activity is good for health: An epidemiological review 2000–2003. J. Sci. Med. Sport 2004, 7, 6–19. [Google Scholar] [CrossRef]
- Rosillo, M.A.; Sánchez-Hidalgo, M.; Cárdeno, A.; Aparicio-Soto, M.; Sánchez-Fidalgo, S.; Villegas, I.; Lastra, C.A. Dietary supplementation of an ellagic acid-enriched pomegranate extract attenuates chronic colonic inflammation in rats. Pharmacol. Res. 2012, 66, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Review Article. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517–520. [Google Scholar]
- Miguel, M.G.; Nunes, S.; Dandlen, S.A.; Cavaco, A.M.; Antunes, M.D. Phenols and antioxidant activity of hydro-alcoholic extracts of propolis from Algarve, South of Portugal. Food Chem. Toxicol. 2010, 48, 3418–3423. [Google Scholar] [CrossRef] [PubMed]
- Androutsopoulos, V.P.; Ruparelia, K.; Arroo, R.R.J.; Tsatsakis, A.M.; Spandidos, D.A. CYP1mediatedantiproliferative activity of dietary flavonoids in MDA-MB-468 breast cancer cells. Toxicology 2009, 264, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Jin, F.; Liu, H.; Wang, Y.; Jiang, Y. Metabonomic study on the antitumor effect of flavonoid derivative 3d in HepG2 cells and its action mechanism. Talanta 2014, 118, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Moon, J.Y.; Kim, H.; Lee, D.S.; Cho, M.; Choi, H.K.; Kim, Y.S.; Mosaddik, A.; Cho, S.K. Antioxidant and antiproliferative activities of mango (Mangifera indica L.) flesh and peel. Food Chem. 2010, 121, 429–436. [Google Scholar] [CrossRef]
- Malta, L.G.; Tessaro, E.P.; Eberlin, M.; Pastore, G.M.; Liu, R.H. Assessment of antioxidant and antiproliferative activities and the identification of phenolic compounds of exotic Brazilian fruits. Food Res. Int. 2013, 53, 417–425. [Google Scholar] [CrossRef]
- Becker, K.; Schroecksnadel, S.; Gostner, J.; Zaknun, C.; Schennach, H.; Überall, F.; Fuchs, D. Comparison of in vitro tests for antioxidant and immunomodulatory capacities of compounds. Phytomedicine 2014, 21, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Lasekan, O. Volatile constituents of roasted tigernut oil (Cyperus esculentus L.). J. Sci. Food Agric. 2013, 93, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Allan, R.D.; Wells, R.J.; Macleod, I.K. Flavanone quinones from Cyperus species. Tetrahedron Lett. 1973, 1, 7–8. [Google Scholar] [CrossRef]
- Siani, A.C.; Silva, A.M.P.; Nakamura, M.J.; Carvalho, M.V.; Henriques, M.G.O.; Ramos, M.F.S.; Kaiser, C.R. Chemical Composition and Anti-Inflammatory Activity of the Hydrodistillate from Mariscus pedunculatu. J. Braz. Chem. Soc. 2001, 12, 354–359. [Google Scholar] [CrossRef]
- Rabelo, A.S.; Oliveira, I.D.; Guimarães, A.G.; Quintans, J.S.S.; Prata, A.P.N.; Gelain, D.P.; Venceslau, E.M.; Santos, J.P.A.; Quintans-Júnior, L.J.; Bonjardim, L.R.; et al. Antinociceptive, anti-inflammatory and antioxidant activities of aqueous extract from Remirea maritima (Cyperaceae). J. Ethnopharmacol. 2013, 145, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Dória, G.A.A.; Menezes, P.P.; Lima, B.S.; Vasconcelos, B.S.; Silva, F.A.; Henriques, R.M.; Melo, M.G.D.; Alves, A.V.F.; Moraes, M.O.; Pessoa, C.O.; et al. In vivo antitumor effect, induction of apoptosis and safety of Remirea maritima Abul. (Cyperaceae) extracts. Phytomedicine 2015, in press. [Google Scholar]
- Ceriello, A.; Motz, E. Is oxidative stress the pathogenic mechanism under-lying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Huynh, K.; Bernardo, B.C.; McMullen, J.R.; Ritchie, R.H. Diabetic cardiomyopathy: Mechanisms and new treatment strategies targeting antioxidant signaling pathways. Pharmacol. Ther. 2014, 142, 375–415. [Google Scholar] [CrossRef] [PubMed]
- Montezano, A.C.; Touyz, R.M. Review: Molecular mechanisms of hypertension—Reactive oxygen species and antioxidants: A basic science update for the clinician. Can. J. Cardiol. 2012, 28, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Seifried, H.E.; Anderson, D.E.; Fisher, E.I.; Milner, J.A. A review of the interaction among dietary antioxidants and reactive oxygen species. J. Nutr. Biochem. 2007, 18, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Q.; Kosten, T.R.; Zhang, X.Y. Free radicals, antioxidant defense systems, and schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 46, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Becker, L.B. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc. Res. 2004, 61, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.R.; Barrett, J.C. Reactive oxygen species as double-edgeds words in cellular processes: Low-dose cell signaling versus high-dose toxicity. Hum. Exp. Toxicol. 2002, 21, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, I.; Liu, W.; Akhand, A.A.; Takeda, K.; Kawamoto, Y.; Kato, M.; Suzuki, H. 4Hydroxynonenal triggers multi step signal transduction cascades for suppresion of cellular functions. Mol. Asp. Med. 2003, 24, 231–238. [Google Scholar] [CrossRef]
- Kardeh, S.; Ashkani-Esfahani, S.; Alizadeh, A.M. Review Paradoxical action of reactive oxygen species in creation and therapy of cancer. Eur. J. Pharmacol. 2014, 735, 150–168. [Google Scholar] [CrossRef] [PubMed]
- Kowaltowski, A.J.; Souza-Pinto, N.C.; Castilho, R.F.; Vercesi, A.E. Mitochondria and reactive oxygen species. Free Radic. Biol. Med. 2009, 47, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Witaicenis, A.; Seito, L.N.; Chagas, A.S.; Junior, L.D.A.; Luchini, A.C.; Rodrigues-Orsi, P.; Cestari, S.H.; Stasi, L.C. Antioxidantand intestinal anti-inflammatory effects of plant-derived coumarin derivatives. Phytomedicine 2014, 21, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, V.J. The paradox of reactive oxygen species: Injury, signaling, or both? Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 284, L24–L25. [Google Scholar] [CrossRef] [PubMed]
- Gongora, M.C.; Qin, Z.; Laude, K.; Laude, K.; Kim, H.W.; Folz, J.R.; Dikalov, S.; Fukai, T.; Harrison, D.G. Role of extracellular superoxide dismutase in hypertension. Hypertension 2006, 48, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Brusotti, G.; Ngueyem, T.A.; Biesuz, R.; Caccialanza, G. Optimum extraction process of polyphenols from Bridelia grandis stem bark using experimental design. J. Sep. Sci. 2010, 33, 1692–1697. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch. Biochem. Biophys. 2008, 476, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Nour, V.; Stampar, F.; Veberic, R.; Jakopic, J. Anthocyanins profile, total phenolics and antioxidant activity of black currant ethanolic extracts as influenced by genotype and ethanol concentration. Food Chem. 2013, 141, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Mandave, P.C.; Pawar, P.K.; Ranjekar, P.K.; Mantri, N.; Kuvalekar, A.A. Comprehensive evaluation of in vitro antioxidant activity, total phenols and chemical profiles of two commercially important strawberry varieties. Sci. Hortic. 2014, 172, 124–134. [Google Scholar] [CrossRef]
- Pace, B.; Renna, M.F.; Renna, M.; Serio, F.; Attolico, G. Multiple regression models and computer vision systems to predict antioxidant activity and total phenols in pigmented carrots. J. Food Eng. 2013, 117, 74–81. [Google Scholar] [CrossRef]
- Andrade, C.A.; Costa, C.K.; Bora, K.; Miguel, M.D.; Miguel, O.G.; Kerber, V.A. Determination of the phenolic content and evaluation of the antioxidant activity of Acacia podallyriifolia A. Cunn. ex. G. Don., Leguminosae-mimosoideae. Rev. Bras. Farmacogn. 2007, 17, 231–235. [Google Scholar] [CrossRef]
- Souza, C.M.M.; Silva, H.R.; Vieira, G.M., Jr.; Ayres, M.C.C.; Costa, C.L.S.; Araújo, D.S.; Cavalcante, L.C.D.; Barros, E.D.S.; Araújo, P.B.M. Total phenolics and antioxidant activity of five medicinal plants. Quim. Nova 2007, 30, 351–355. [Google Scholar]
- Maisuthisakul, P.; Suttajit, M.; Pongsawatmanit, R. Assessment of phenolic content and free radical-scavenging capacity of some Thai indigenous plants. Food Chem. 2007, 100, 1409–1418. [Google Scholar] [CrossRef]
- Serafini, M.R.; Santos, R.C.; Guimarães, A.G.; Santos, J.P.A.; Santos, A.D.C.; Alves, I.A.; Gelain, D.P.; Nogueira, P.C.L.; Quintans-Júnior, L.J.; Bonjardim, L.R.; et al. Morinda citrifolia Linn Leaf Extract Possesses Antioxidant Activities and Reduces Nociceptive Behavior and Leukocyte Migration. J. Med. Food 2011, 14, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Beevi, S.S.; Narasu, M.L.; Gowda, B.B. Polyphenolics profile, antioxidant and radical scavenging activity of leaves and stem of Raphanus sativus L. Plant Foods Hum. Nutr. 2010, 65, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.R.; Kwak, J. Chemical composition and antioxidant activity in different tissues of Brassica vegetables. Molecules 2015, 20, 1228–1243. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.G.; Behr, G.A.; Zanotto-Filho, A.; Lorenzi, R.; Pasquali, M.A.; Ravazolo, L.G.; Bordignon, C.L., Jr.; Silva, F.A.; Aboy, A.L.; Bassani, V.L.; et al. Antioxidant activities and free radical scavenging potential of Bauhinia microstachya (RADDI) MACBR (Caesalpinaceae) extracts linked to their polyphenol content. Biol. Pharm. Bull. 2007, 30, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.S.; Abdalla, D.A.P. Lipid peroxidatiom: Mechanisms and evaluation in biological samples. Braz. J. Pharm. Sci. 2001, 37, 293–303. [Google Scholar]
- Davies, S.S.; Guo, L. Lipid peroxidation generates biologically active phospholipids including oxidatively N-modified phospholipids. Review. Chem. Phys. Lipids 2014, 181, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, J.; Han, W.; Mai, W.; Wang, L.; Li, Q.; Lin, M.; Bai, M.; Zhang, L.; Chen, D. Antioxidant Ability and Mechanism of Rhizoma Atractylodes macrocephala. Molecules 2012, 17, 13457–13472. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Gao, W.; Zhang, M.; Li, C.; Wang, A.; Su, Y.; Ji, T. Composition and Antioxidant Activity of the Anthocyanins of the Fruit of Berberis heteropoda Schrenk. Molecules 2014, 19, 19078–19096. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Chen, J.; Cai, Y.; Lei, Y.; Chen, L.; Pei, L.; Zhou, D.; Liang, X.; Ruan, J. Antioxidant, free radical scavenging, anti-inflammatory and hepatoprotective potential of the extract from Parathelypteris nipponica (Franch. et Sav.) Ching. J. Ethnopharmacol. 2010, 130, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M.; Sahli, A.A.A.A.; Alaraidh, I.A.; Al-Homaidan, A.A.; Mostafa, E.M.; EL-Gaaly, G.A. Assessment of antioxidant activities in roots of Miswak (Salvadora persica) plants grown at two different locations in Saudi Arabia. Saudi J. Biol. Sci. 2015, 22, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Conde-Hernández, L.A.; Guerrero-Beltrán, J.A. Total phenolics and antioxidant activity of Piper auritum and Porophyllum ruderale. Food Chem. 2014, 142, 455–460. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). Cancer 2012. Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx (accessed on 1 November 2014).
- Badisa, R.B.; Mina, D.A.; Latinwo, L.M.; Solima, K.F.A. Selective Anticancer Activity of Neurotoxin 1-Methyl-4-Phenylpyridinium on Non-Small Cell Lung Adenocarcinoma A549 Cells. Anticancer Res. 2014, 34, 5447–5452. [Google Scholar] [PubMed]
- Caruso, Í.P.; Vilegas, W.; Souza, F.P.; Fossey, M.A.; Cornélio, M.L. Binding of antioxidant flavone isovitexin to human serum albumin investigated by experimental and computational assays. J. Pharm. Biomed. 2014, 98, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.T.; Chen, C.T.; Chieng, K.T.; Huang, S.H.; Chiang, B.H.; Wang, L.F.; Kuo, H.S.; Lin, C.M. Inhibitory effects of a rice hull constituent on tumor necrosis factor alpha, prostaglandin E2, and cyclooxygenase-2 production in lipopolysaccharide-activated mouse macrophages. Ann. N. Y. Acad Sci. 2005, 1042, 387–395. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, T.; Wu, W.; Li, D.; Xu, T.; Zhu, H.; Pan, D.; Zhu, S.; Liu, Y. Anti-oxidant and antiapoptotic effects of luteolin on mice peritoneal macrophages stimulated by angiotensin II. Int. J. Immunopharmacol. 2014, 20, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Chiu, F.L.; Lin, J.K. Downregulation of androgen receptor expression by luteolin causes inhibition of cell proliferation and induction of apoptosis in human prostate cancer cells and xenografts. Prostate 2008, 68, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lazaro, M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Seelinger, G.; Merfort, I.; Schempp, C.M. Anti-oxidant, anti-inflammatoryand anti-allergic activities of luteolin. Planta Med. 2008, 74, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Horváthová, K.; Chalupa, I.; Sebová, L.; Tóthová, D.; Vachálková, A. Protective effect of quercetin and luteolin in human melanoma HMB-2 cells. Mutat. Res. 2005, 565, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.K.; Hikegaki, M.; Abreu, J.A.S.; Alcici, N.M.F. Estudo da preparação dos extratos de própolis e suas aplicações. Food Sci. Technol. 1998, 18, 313–318. [Google Scholar] [CrossRef]
- Attoub, S.; Hassan, A.H.; Vanhoecke, B.; Iratni, R.; Takahashi, T.; Gaben, A.M.; Bracke, M.; Awad, S.; John, A.; Kamalboor, H.A.; et al. Inhibition of cell survival, invasion, tumor growth and histone deacetylase activity by the dietary flavonoid luteolin in human epithelioid cancer cells. Eur. J. Pharmacol. 2011, 651, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Can, O.D.; Ozkay, U.D.; Ucel, U.I. Anti-depressant-like effect of vitexin in BALB/c mice and evidence for the involvement of monoaminergic mechanisms. Eur. J. Pharmacol. 2013, 699, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Li, H.; Yi, J.; Zhang, J.; Che, H.; Cao, J.; Yang, L.; Zhu, C.; Jiang, W. Antioxidant properties of the mung bean flavonoids on alleviating heat stress. PLoS ONE 2011, 6, e21071. [Google Scholar] [CrossRef] [PubMed]
- Gorzalczany, S.; Marrassini, C.; Mino, J.; Acevedo, C.; Ferraro, G. Antinociceptive activity of ethanolic extract and isolated compounds of Urtica circularis. J. Ethnopharmacol. 2011, 134, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Özkay, Ü.D.; Can, Ö.D. Anti-nociceptive effect of vitexin mediated by the opioid system in mice. Pharmacol. Biochem. Behav. 2013, 109, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Knipping, K.; Garssen, J.; van’t Land, B. An evaluation of the inhibitory effects against rota-virus infection of edible plant extracts. Virol. J. 2012, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, E.; Nassiri-Asl, M.; Shafeei, M.; Sheikhi, M. Neuroprotective effects of vitexin, a flavonoid, on pentylenetetrazole-induced seizure in rats. Chem. Biol. Drug Des. 2012, 80, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Zhang, Y.; Deng, J.; Zeng, G.; Zhang, Y. Purified vitexin compound 1 suppresses tumor growth and induces cell apoptosis in a mouse model of human choriocarcinoma. Int. J. Gynecol. Cancer 2012, 22, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.J.; Liu, Y.E.; Cao, J.G.; Zeng, G.Y.; Shen, C.; Li, Y.L.; Zhou, M.C.; Chen, Y.; Pu, W.; Potters, L.; et al. Vitexins, nature-derived lignan compounds, induce apoptosis and suppress tumor growth. Clin. Cancer Res. 2009, 15, 5161–5169. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- López-Alarcón, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, L.S.; Zeidán-Chuliá, F.; Yatsu, F.K.J.; Schnorr, C.E.; Moresco, K.S.; Kolling, E.A.; Gelain, D.P.; Bassani, V.L.; Moreira, J.C.F. Guarana (Paullinia cupana Mart.) prevents β-amyloid aggregation, generation of advanced glycation-end products (AGEs), and acrolein-induced cytotoxicity on human neuronal-like cells. Phytother. Res. 2014, 28, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Dresch, M.T.; Rossato, S.B.; Kappel, V.D.; Biegelmeyer, R.; Hoff, M.L.; Mayorga, P.; Zuanazzi, J.A.; Henriques, A.T.; Moreira, J.C. Optimization and validation of an alternative method to evaluate total reactive antioxidant potential. Anal. Biochem. 2009, 385, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Lissi, E.A.; Salim-Hanna, M.; Pascual, C.; Del Castillo, M.D. Evaluation of total antioxidant potential (TRAP) and total antioxidant reactivity (TAR) from luminol enhanced chemiluminescence measurements. Free Radic. Biol. Med. 1995, 18, 153–158. [Google Scholar] [CrossRef]
- Lopes, G.K.; Schulman, H.M.; Hermes-Lima, M. Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ions. Biochim. Biophys. Acta 1999, 1472, 142–152. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, Y.; Gao, H.; Yuan, J.; Feng, F.; Cao, W.; Zheng, J. Protective effect of extract of Crataegus pinnatifida pollen on DNA damage response to oxidative stress. Food Chem. Toxicol. 2013, 59, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Hazra, B. Evaluation of nitric oxide scavenging activity, in vitro and ex vivo, of selected medicinal plants traditionally used in inflammatory diseases. Phytother. Res. 2006, 20, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Draper, H.H.; Hadley, M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990, 186, 421–431. [Google Scholar] [PubMed]
- Melo, M.G.D.; Santos, J.P.A.; Serafini, M.R.; Caregnato, F.F.; Pasquali, M.A.B.; Rabelo, T.K.; Rocha, R.F.; Quintans, L.J., Jr.; Araújo, A.A.S.; Silva, F.A.; et al. Redox properties and cytoprotective actions of atranorin, a lichen secondary metabolite. Toxicol. in Vitro 2011, 25, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Borenfreund, E.; Babich, H.; Martin-Alguacil, N. Comparisons of two in vitro cytotoxicity assays-the neutral red (NR) and tetrazoliummtt tests. Toxicol. in Vitro 1988, 2, 1–6. [Google Scholar] [CrossRef]
- Cao, T.; Saw, T.Y.; Heng, B.C.; Liu, H.; Yap, A.U.J.; Ng, M.L. Comparison of different test models for the assessment of cytotoxicity of composite resins. J. Appl. Toxicol. 2005, 25, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the Remirea maritima extract are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dória, G.A.A.; Santos, A.R.; Bittencourt, L.S.; Bortolin, R.C.; Menezes, P.P.; Vasconcelos, B.S.; Souza, R.O.; Fonseca, M.J.V.; Santos, A.D.C.; Saravanan, S.; et al. Redox-Active Profile Characterization of Remirea maritima Extracts and Its Cytotoxic Effect in Mouse Fibroblasts (L929) and Melanoma (B16F10) Cells. Molecules 2015, 20, 11699-11718. https://doi.org/10.3390/molecules200711699
Dória GAA, Santos AR, Bittencourt LS, Bortolin RC, Menezes PP, Vasconcelos BS, Souza RO, Fonseca MJV, Santos ADC, Saravanan S, et al. Redox-Active Profile Characterization of Remirea maritima Extracts and Its Cytotoxic Effect in Mouse Fibroblasts (L929) and Melanoma (B16F10) Cells. Molecules. 2015; 20(7):11699-11718. https://doi.org/10.3390/molecules200711699
Chicago/Turabian StyleDória, Grace Anne A., Anderson R. Santos, Leonardo S. Bittencourt, Rafael C. Bortolin, Paula P. Menezes, Bruno S. Vasconcelos, Rebeca O. Souza, Maria José V. Fonseca, Alan Diego C. Santos, Shanmugam Saravanan, and et al. 2015. "Redox-Active Profile Characterization of Remirea maritima Extracts and Its Cytotoxic Effect in Mouse Fibroblasts (L929) and Melanoma (B16F10) Cells" Molecules 20, no. 7: 11699-11718. https://doi.org/10.3390/molecules200711699
APA StyleDória, G. A. A., Santos, A. R., Bittencourt, L. S., Bortolin, R. C., Menezes, P. P., Vasconcelos, B. S., Souza, R. O., Fonseca, M. J. V., Santos, A. D. C., Saravanan, S., Silva, F. A., Gelain, D. P., Moreira, J. C. F., Prata, A. P. N., Quintans-Júnior, L. J., & Araújo, A. A. S. (2015). Redox-Active Profile Characterization of Remirea maritima Extracts and Its Cytotoxic Effect in Mouse Fibroblasts (L929) and Melanoma (B16F10) Cells. Molecules, 20(7), 11699-11718. https://doi.org/10.3390/molecules200711699




