Four New Triterpenoids from Callicarpa kwangtungensis
Abstract
:1. Introduction
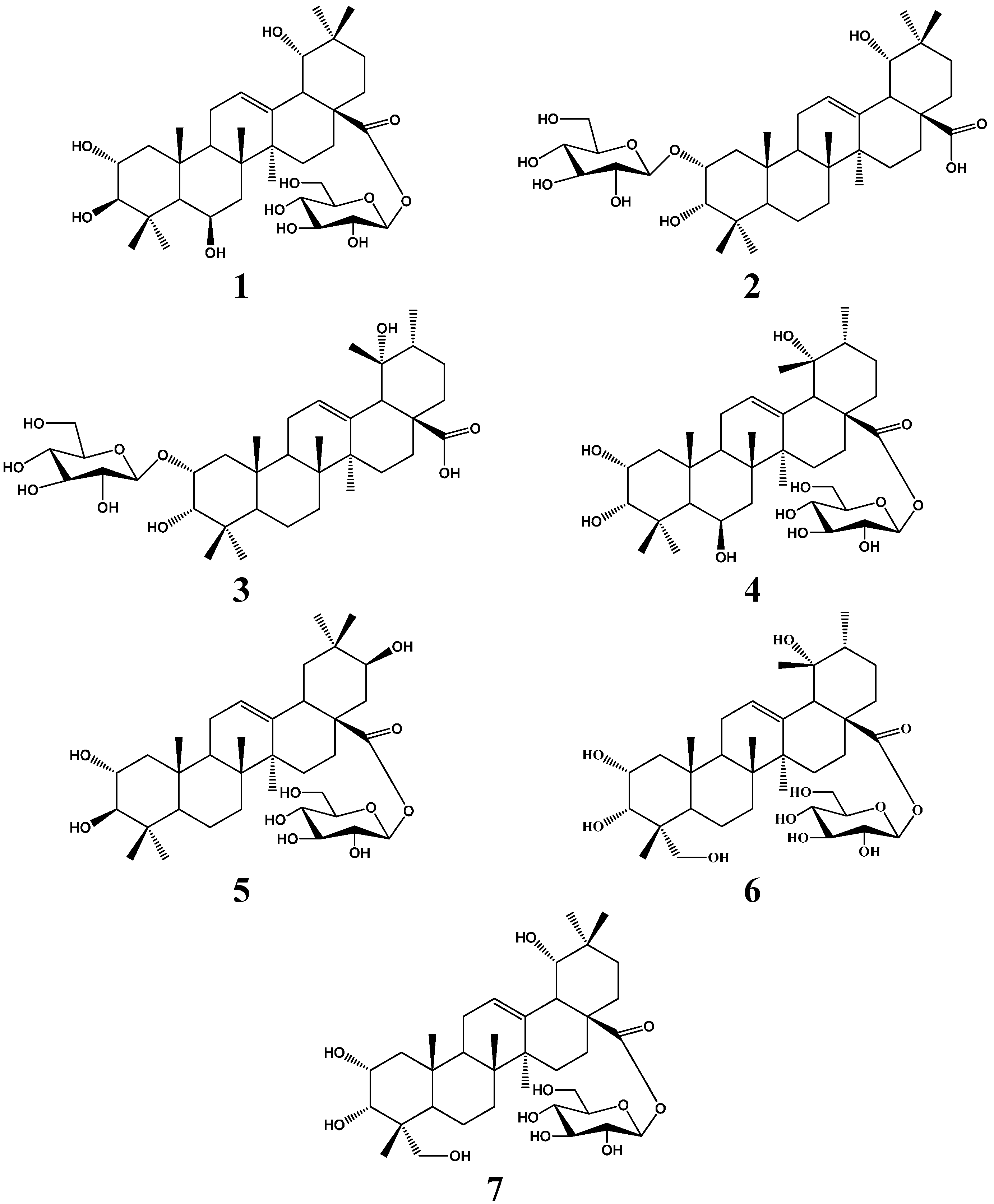
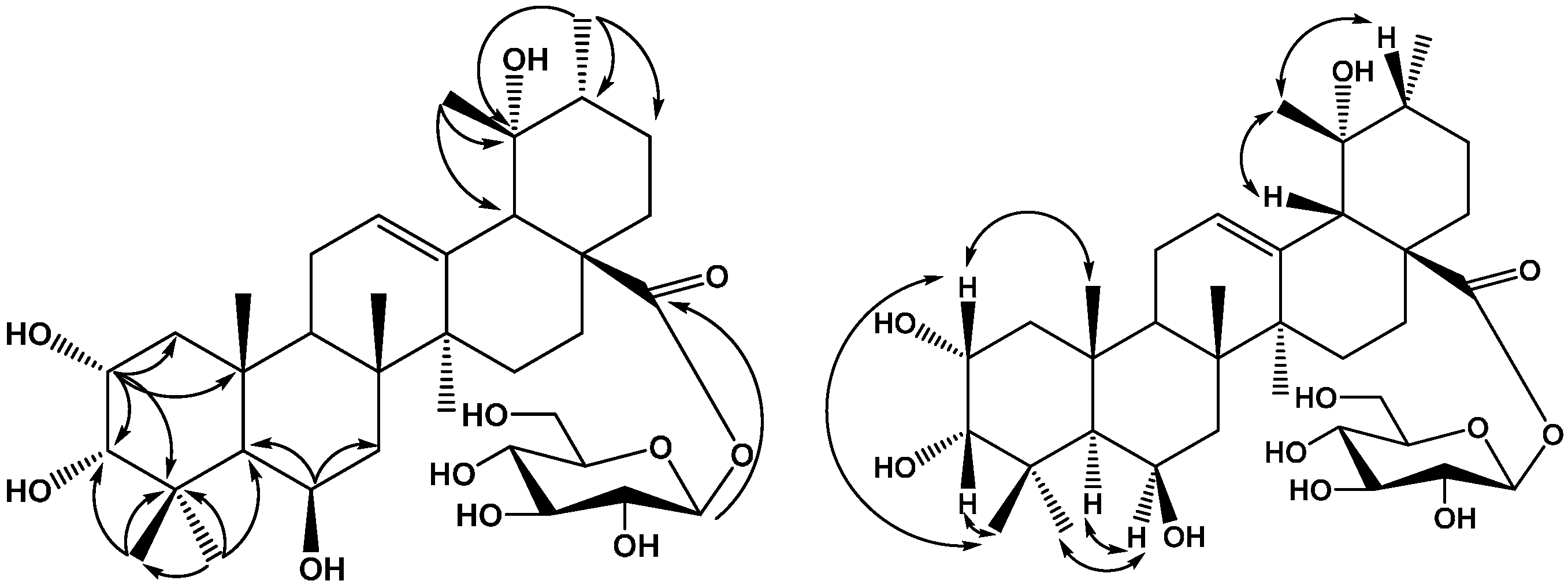
2. Results and Discussion
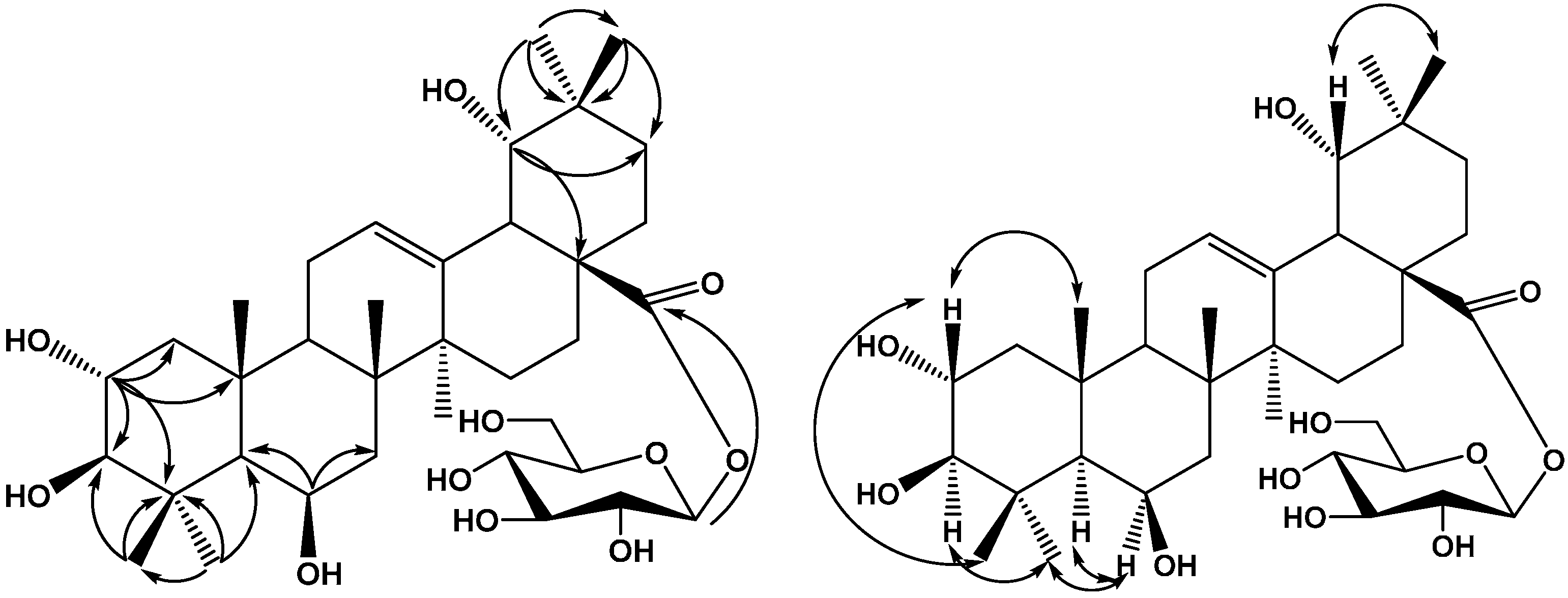
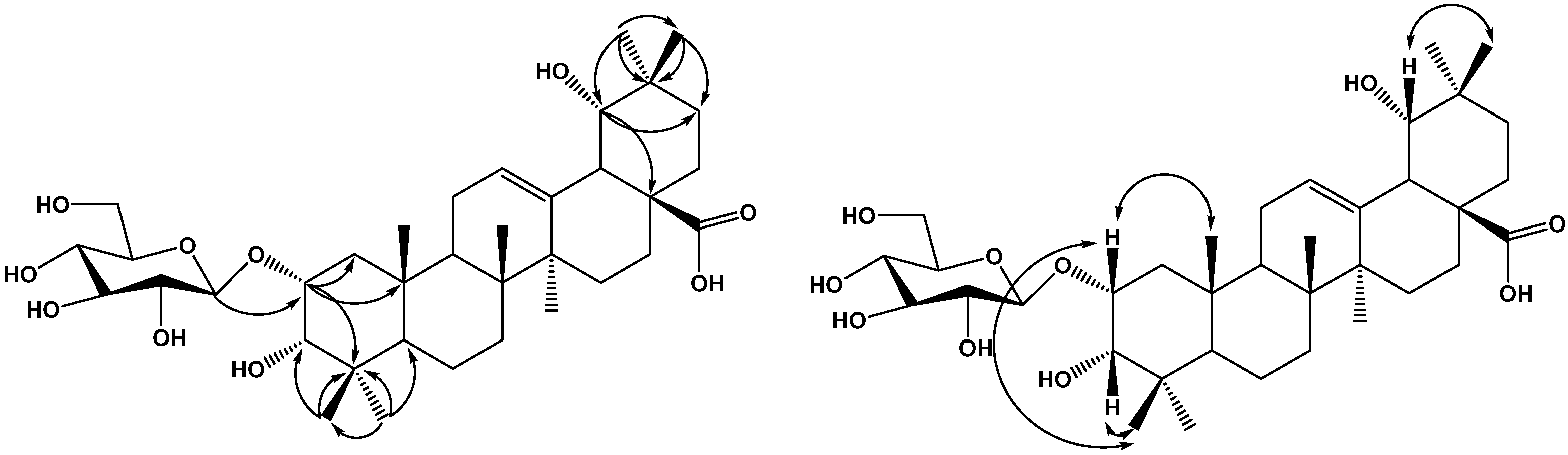
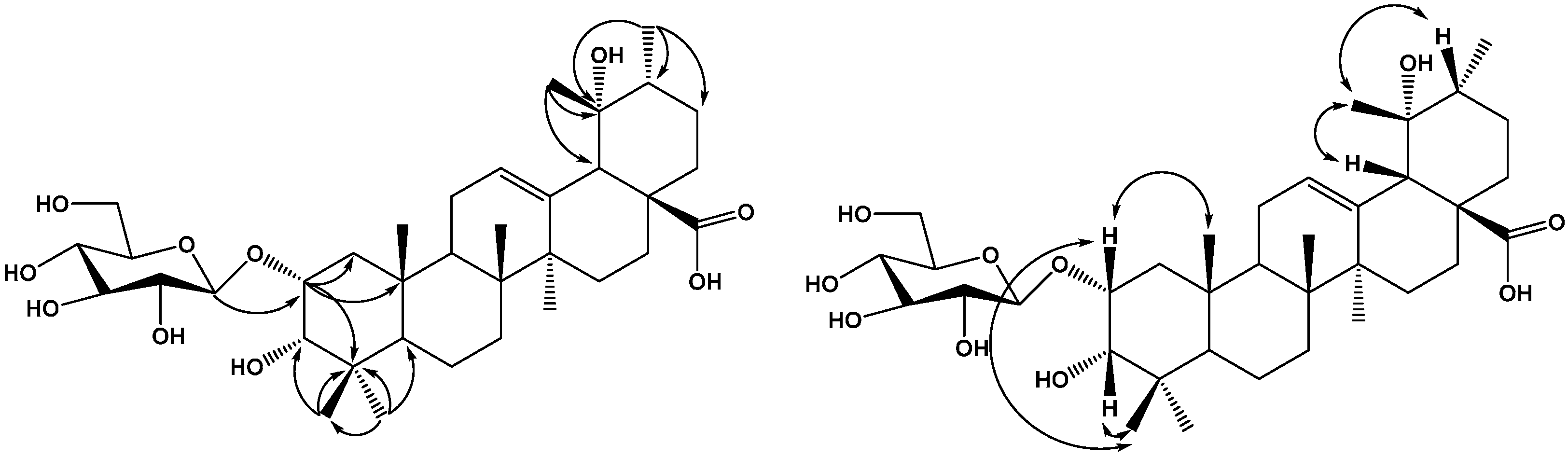
3. Experimental Section
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Acid Hydrolysis of Compounds 1–4
3.5. The Physicochemical Data of Compounds 1–7
| No. | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| C | H | C | H | C | H | C | H | |
| 1 | 50.4 | 1.40, 1H, br t, J = 12.0 Hz | 39.8 | 1.86, 1H, br t, J = 12.0 Hz | 39.9 | 1.80, 1H, br t, J = 12.0 Hz | 44.9 | 1.28, 1H, s |
| 2.35, 1H, m | 2.00, 1H, m | 1.94, 1H, m | 1.54, 1H, m | |||||
| 2 | 69.3 | 4.30, 1H, m | 76.9 | 4.46, 1H, m | 76.6 | 4.46, 1H, m | 67.1 | 4.02, 1H, m |
| 3 | 84.4 | 3.43, 1H, d, J = 9.0 Hz | 79 | 4.05,1H, d, J = 2.4 Hz | 79.1 | 4.03, 1H, d, J = 2.4 Hz | 81.5 | 3.29, 1H, d, J = 2.4 Hz |
| 4 | 40.2 | 39.2 | 39.0 | 40.1 | ||||
| 5 | 57.4 | 1.21, 1H, s | 49.5 | 1.68, 1H, m | 48.8 | 1.66, 1H, m | 49.7 | 1.28, 1H, s |
| 6 | 68.3 | 4.87, 1H, s | 19.1 | 1.34, 1H, m | 19.0 | 1.33, 1H, m | 69.2 | 4.38, 1H, s |
| 1.49 m | 1.47 m | |||||||
| 7 | 41.3 | 2.00, 2H, m | 34.1 | 1.36, 1H, m | 33.9 | 1.35, 1H, m | 40.6 | 1.53, 2H, m |
| 1.58, 1H, m | 1.65, 1H, m | |||||||
| 8 | 42.1 | 40.7 | 41.0 | 41.7 | ||||
| 9 | 49.5 | 2.14, 1H, m | 48.7 | 2.08, 1H, m | 48.1 | 2.03, 1H, m | 49.5 | 1.90, 1H, m |
| 10 | 39.0 | 39.2 | 39.0 | 38.3 | ||||
| 11 | 24.8 | 2.33, 1H, m | 24.8 | 2.01, 1H, m | 24.6 | 2.00, 1H, s | 24.4 | 2.09, 2H, m |
| 12 | 124.5 | 5.61, 1H, s | 124.3 | 5.57, 1H, s | 128.4 | 5.56, 1H, s | 129.9 | 5.36, 1H, s |
| 13 | 144.2 | 145.3 | 140.4 | 139.9 | ||||
| 14 | 43.3 | 42.7 | 42.7 | 43.2 | ||||
| 15 | 29.6 | 1.32, 1H, m | 29.7 | 1.33, 1H, m | 29.7 | 1.25, 1H, m | 29.3 | 1.03, 1H, m |
| 2.08, 1H, m | 2.12, 1H, m | 2.33, 1H, m | 1.91, 1H, m | |||||
| 16 | 28.7 | 2.17, 1H, m | 28.9 | 2.16, 1H, m | 27.4 | 1.33, 1H, m | 27.1 | 1.26, 1H, m |
| 2.83, 1H, m | 2.80, 1H, m | 2.08, 1H, m | 1.77, 1H, m | |||||
| 17 | 47.0 | 46.6 | 49.3 | 49.6 | ||||
| 18 | 45.1 | 3.58, 1H, s | 45.3 | 3.60, 1H, m | 55.0 | 3.05, 1H, t | 55.0 | 2.54, 1H, s |
| 19 | 81.6 | 3.63, 1H, s | 81.8 | 3.63, 1H, t | 73.2 | 73.7 | ||
| 20 | 36.0 | 36.2 | 42.8 | 1.50, 1H, m | 43.0 | 1.36, 1H, m | ||
| 21 | 29.5 | 1.07, 1H, m | 29.6 | 1.25, 1H, m | 26.9 | 2.04, 1H, m | 26.6 | 1.64, 1H, m |
| 2.48, 1H, t J = 6.0 Hz | 1.35, 1H, m | 3.11, 1H, m | 2.62, 1H, m | |||||
| 22 | 33.4 | 2.00, 1H, m | 33.8 | 2.04, 1H, m | 39.2 | 2.08, 1H, m | 38.8 | 1.66, 1H, m |
| 2.08, 1H, m | 2.17, 1H, m | 2.16, 1H, m | 1.79, 1H, m | |||||
| 23 | 29.6 | 1.49, 3H, s | 29.9 | 1.23, 3H, s | 29.8 | 1.22, 3H, s | 29.6 | 1.07, 3H, s |
| 24 | 19.0 | 1.79, 3H, s | 22.8 | 0.88, 3H, s | 22.8 | 0.86, 3H, s | 24.4 | 1.25, 3H, s |
| 25 | 18.9 | 1.79, 3H, s | 17.0 | 0.97, 3H, s | 17.0 | 0.96, 3H, s | 18.5 | 1.38, 3H, s |
| 26 | 19.8 | 1.83, 3H, s | 18.1 | 1.06, 3H, s | 17.7 | 1.09, 3H, s | 18.7 | 1.04, 3H, s |
| 27 | 25.3 | 0.99, 3H, s | 25.3 | 1.54, 3H, s | 25.1 | 1.62, 3H, s | 24.7 | 1.33, 3H, s |
| 28 | 177.7 | 181.3 | 181.1 | 178.5 | ||||
| 29 | 29.2 | 1.17, 3H, s | 29.3 | 1.19, 3H, s | 27.5 | 1.44, 3H, s | 27.2 | 1.22, 3H, s |
| 30 | 25.3 | 1.66, 3H, s | 25.3 | 1.54, 3H, s | 17.3 | 1.14, 3H, d, J = 6.6 Hz | 16.6 | 0.94, 3H, d, J = 6.6 Hz |
| Glc | ||||||||
| 1 | 96.4 | 6.36, 1H, d, J = 7.8 Hz | 104.2 | 5.16, 1H, d, J = 7.2 Hz | 104.1 | 5.14, 1H, d, J = 7.8 Hz | 95.9 | 5.32, 1H, d, J = 7.8 Hz |
| 2 | 74.6 | 4.24, 1H, t, J = 7.8 Hz | 75.8 | 4.07,1H, t, J = 7.2 Hz | 75.9 | 4.07, 1H, t, J = 7.8 Hz | 73.9 | 3.35, 1H, t, J = 7.8 Hz |
| 3 | 79.7 | 4.02, 1H, d, J = 9.0 Hz | 78.3 | 4.28,1H, d, J = 9.0 Hz | 78.3 | 4.29, 1H, d, J = 8.4 Hz | 78.3 | 3.35, 1H, d, J = 8.4 Hz |
| 4 | 71.7 | 4.39, 1H, t, J = 9.0 Hz | 72.3 | 4.30,1H, t, J = 9.0 Hz | 72.2 | 4.31, 1H, t, J = 8.4 Hz | 71.3 | 3.40, 1H, t, J = 8.4 Hz |
| 5 | 79.3 | 4.30, 1H, t, J = 9.0 Hz | 78.8 | 4.32, 1H, t, J = 9.0 Hz | 78.9 | 4.33, 1H, t, J = 8.4 Hz | 78.6 | 4.03, 1H, dd, J = 8.4 Hz J = 4.2 Hz |
| 6 | 62.6 | 4.41, 1H, t, J = 9.6 Hz | 63.3 | 4.37, 1H, dd, J = 4.8 Hz J = 12.0 Hz | 63.2 | 4.37, 1H, dd, J = 8.4 Hz J = 11.4 Hz | 62.4 | 3.70, 1H, dd, J = 4.2 Hz J = 12.0 Hz |
| 4.44, 1H, m | 4.54, 1H, dd, J = 2.4 Hz J = 12.0 Hz | 4.54, 1H, dd, J = 2.4 Hz J = 11.4 Hz | 3.81, 1H, dd, J = 1.8 Hz J = 12.0 Hz | |||||
| Position | 5 | 6 | 7 | |||
|---|---|---|---|---|---|---|
| 1 | 46.7 | 0.95 (1H, m) | 42.7 | 1.90 (1H, m) | 48 | 1.23 (1H, m) |
| 2.25 (1H, m) | 2.00 (1H, m) | 2.08 (1H, m) | ||||
| 2 | 67.2 | 3.31 (1H, m) | 66.7 | 4.08 (1H, m) | 69.4 | 4.10 (1H, m) |
| 3 | 83.1 | 2.95 (1H, d, J = 9.6 Hz) | 79.9 | 3.78 (1H, d, J = 2.4 Hz) | 78.8 | 3.76 (1H, d, J = 3.6 Hz) |
| 4 | 39 | 42.6 | 44.2 | |||
| 5 | 55.6 | 0.93 (1H, m) | 44 | 1.41 (1H, s) | 48.5 | 1.33 (1H, m) |
| 1.23 (1H, m) | 19 | 1.21 (1H, m) | ||||
| 6 | 18.5 | 1.38 (1H, m) | 1.34 (1H, m) | 19.3 | 1.48 (2H, m) | |
| 1.25 (1H, m) | 33.7 | 1.25 (1H, m) | ||||
| 7 | 32.8 | 1.42(1H, m) | 1.40 (1H, m) | 33.9 | 1.70 (2H, m) | |
| 8 | 39 | 41.2 | 40.8 | |||
| 9 | 47.8 | 1.93 (1H, m) | 48.3 | 2.06 (1H, m) | 49 | 2.03 (1H, m) |
| 10 | 37.7 | 38.2 | 39.1 | |||
| 11 | 23.3 | 2.16 (1H, m) | 25 | 2.00 (1H, m) | 24.8 | 2.01 (1H, m) |
| 12 | 122.3 | 4.76 (1H, d, J = 4.5 Hz) | 128.7 | 5.56 (1H, br s) | 1283.6 | 5.54 (1H, br s) |
| 13 | 143.7 | 139.6 | 144.8 | |||
| 14 | 41.4 | 42.7 | 42.7 | |||
| 15 | 28.5 | 0.98 (1H, m) | 29.7 | 1.34 (1H, m) | 29.7 | 1.31 (1H, m) |
| 1.83 (1H, m) | 2.10 (1H, m) | 2.04 (1H, m) | ||||
| 16 | 24.3 | 1.06 (1H, m) | 27.1 | 2.14 (1H, m) | 26.7 | 2.08 (1H, m) |
| 1.68 (1H, m) | 2.62 (1H, m) | 2.74 (1H, m) | ||||
| 17 | 47 | 49.1 | 47 | |||
| 18 | 41.8 | 2.52 (1H, s) | 54.9 | 2.52 (1H, s) | 45.1 | 3.52 (1H, s) |
| 19 | 46.6 | 1.06 (1H, m) | 73.1 | 81.5 | 3.57 (1H, s) | |
| 2.16 (1H, m) | ||||||
| 20 | 36 | 42.1 | 1.43 (1H, m) | 36 | ||
| 21 | 71.4 | 3.53 (1H, m) | 26.6 | 2.00 (1H, m) | 29.5 | 1.88 (2H, m) |
| 3.13 (1H, m) | ||||||
| 22 | 41.5 | 1.91 (1H, m) | 38.9 | 2.04 (1H, m) | 33.5 | 2.04 (1H, m) |
| 2.22 (1H, m) | 2.14 (1H, m) | 2.10 (1H, m) | ||||
| 23 | 29.5 | 0.84 (3H, s) | 71 | 3.75 (1H, d, 10.8 Hz) | 67 | 3.57 (1H, d, 10.2 Hz) |
| 3.92 (1H, d, 10.8 Hz) | 3.73 (1H, d, 10.2 Hz) | |||||
| Table 2. Cont. 24 | 17.3 | 1.06 (3H, s) | 18.2 | 0.90 (3H, s) | 14.7 | 0.99 (3H, s) |
| 25 | 16.7 | 1.00 (3H, s) | 17.6 | 1.09 (3H, s) | 17,8 | 1.22 (3H, s) |
| 26 | 17 | 0.91 (3H, s) | 18 | 1.26 (3H, s) | 18.2 | 1.10 (3H, s) |
| 27 | 25.8 | 1.41 (3H, s) | 27.5 | 1.65 (3H, s, ) | 25.9 | 1.56 (3H, s) |
| 28 | 176.6 | 177.9 | 177.8 | |||
| 29 | 29.7 | 1.12 (3H, s) | 24.7 | 1.39 (3H, s) | 29.4 | 1.15 (3H, s) |
| 30 | 20.1 | 1.09 (3H, s) | 17.2 | 1.07 (3H, d, J = 6.6 Hz) | 25.1 | 1.17 (3H, s) |
| Glc | ||||||
| 1′ | 96.6 | 5.44 (1H, d, J = 7.8 Hz) | 96.4 | 6.30 (1H, d, J = 7.8 Hz) | 96.4 | 6.30 (1H, d, J = 7.8 Hz) |
| 2′ | 74 | 3.33 (1H, m) | 74.6 | 4.22 (1H, m) | 74.6 | 4.21 (1H, m) |
| 3′ | 78.9 | 3.43 (1H, m) | 79.8 | 4.02 (1H, m) | 79.9 | 3.98 (1H, m) |
| 4′ | 71.3 | 3.3 (1H, m) | 71.7 | 4.33 (1H, m) | 71.6 | 4.32 (1H, m) |
| 5′ | 79.4 | 3.31 (1H, m) | 79.5 | 4.27 (1H, m) | 79.8 | 4.25 (1H, m) |
| 6′ | 61.9 | 3.64 (1H, m) | 62.7 | 4.35 (1H, m) | 62.7 | 4.37 (1H, m) |
| 3.85 (1H, m) | 4.37 (1H, m) | 4.40 (1H, m) | ||||
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chinese Academy of Sciences, Flora of China Editorial Committee. Flora of China, 1st ed.; Science Press: Beijing, China, 1982; Volume 65, p. 25. [Google Scholar]
- Zhou, B.T.; Li, X.Z.; Xu, P.S.; Ye, W.C.; Wang, M.S. Chemical constituents from the upground of Callicarpa kwangtungensis Chun (I). Cent. South Pharm. 2004, 2, 238–239. [Google Scholar]
- Zhou, B.T.; Li, X.Z.; Xu, P.S.; Ouyang, J.D.; Zhang, Y. Chemical constituents from the upground of Callicarpa kwangtungensis Chun (II). J. TCM Univ. Hunan 2005, 25, 20–22. [Google Scholar]
- Wang, Y.J.; Yang, Y.F.; Gao, D. Advances in studies on chemical constituents in plants of Callicarpa Linn and their bioactivities. Chin. Tradit. Herb. Drugs 2008, 33, 133–138. [Google Scholar]
- Xie, E.L.; Zhou, G.P.; Ji, T.F.; Wu, J.; Yuan, G.P. A Novel Phenylpropanoid Glycoside from Callicarpa kwangtungensis Chun. Chin. Chem. Lett. 2009, 20, 833–835. [Google Scholar] [CrossRef]
- Yuan, M.M.; Zhong, R.J.; Chen, G.; Zhou, G.P.; Fu, H.Z.; Yan, Q.W. Two new triterpenoids from Callicarpa kwangtungensis. J. Asian Nat. Prod. Res. 2015, 17, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ye, W.C.; Yin, Z.Q. Triterpene saponins from Adinandra nitida. Acta Pharm. Sin. 2008, 43, 504–508. [Google Scholar]
- Tabopda, T.K.; Ngoupayo, J.; Tanoli, S.A.K. Antimicrobial Pentacyclic Triterpenoids from Terminalia superba. Planta Med. 2009, 75, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Ngounou, F.N.; Choudhary, M.I.; Malik, S.; Zareen, S.; Ali, R.; Lontsi, D.; Sondengam, B.L. Two saponins from Pteleopsis hylodendron. Phytochemistry 1999, 52, 917–921. [Google Scholar] [CrossRef]
- Zhang, J.; Li, B.Q.; Feng, F. Chemical constituents from Callicarpa nudiflora and their hemostatic acitivity. Chin. Tradit. Herb. Drugs 2010, 35, 3297–3301. [Google Scholar]
- Sample Availability: Samples of the compounds 1–7 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, G.-P.; Yu, Y.; Yuan, M.-M.; Ji, T.; Fu, H.-Z.; Zhong, R.-J. Four New Triterpenoids from Callicarpa kwangtungensis. Molecules 2015, 20, 9071-9083. https://doi.org/10.3390/molecules20059071
Zhou G-P, Yu Y, Yuan M-M, Ji T, Fu H-Z, Zhong R-J. Four New Triterpenoids from Callicarpa kwangtungensis. Molecules. 2015; 20(5):9071-9083. https://doi.org/10.3390/molecules20059071
Chicago/Turabian StyleZhou, Guo-Ping, Yan Yu, Ming-Ming Yuan, Tengfei Ji, Hui-Zheng Fu, and Rui-Jian Zhong. 2015. "Four New Triterpenoids from Callicarpa kwangtungensis" Molecules 20, no. 5: 9071-9083. https://doi.org/10.3390/molecules20059071
APA StyleZhou, G.-P., Yu, Y., Yuan, M.-M., Ji, T., Fu, H.-Z., & Zhong, R.-J. (2015). Four New Triterpenoids from Callicarpa kwangtungensis. Molecules, 20(5), 9071-9083. https://doi.org/10.3390/molecules20059071






