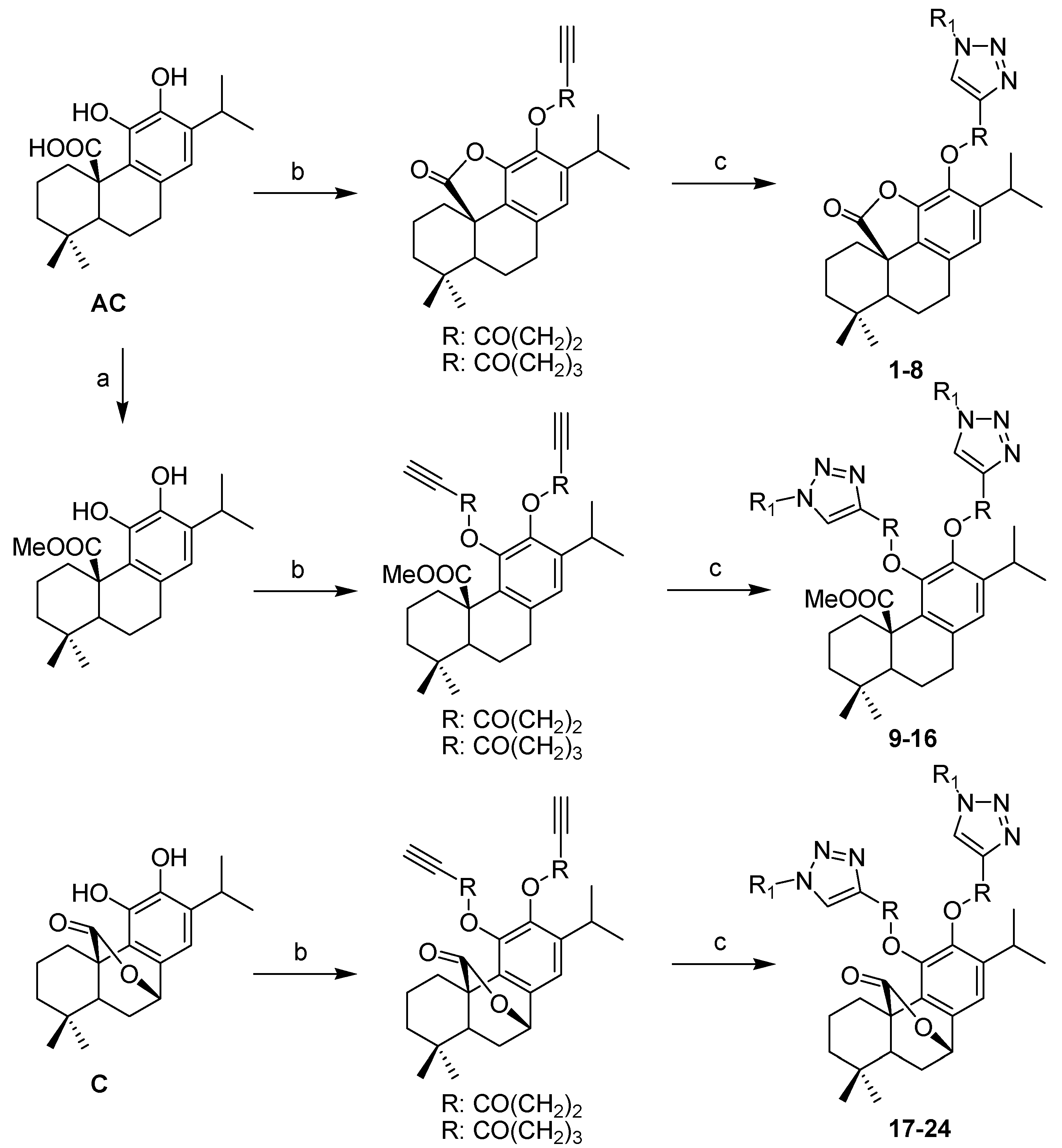
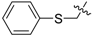
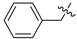
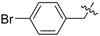
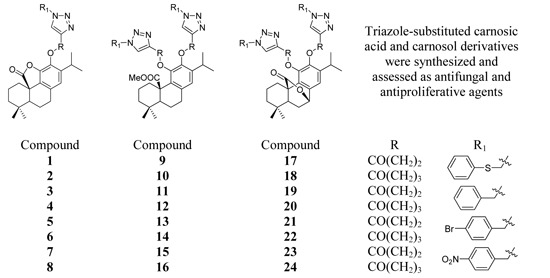
3.2.2. General Procedure for the Synthesis of Triazoles
The alkynyl esters (1 eq) and the corresponding azide (1 eq) were dissolved in t-BuOH/H2O (1:1), followed by the addition of CuSO4·5H2O (2 mol %) and sodium ascorbate (10 mol %). The mixture was stirred at room temperature for 24 h. The reaction was stopped by adding H2O, extracted with CH2Cl2, dried over anhydrous Na2SO4, concentrated and purified by column chromatography on silica gel (53%–83% yield).
12-O-(3-(((1-phenylthio)methyl)-1H-1,2,3-triazol-4-yl)-propanoyloxy)-11,20-epoxyabieta-8,11,13-trien-20-one (1). Pale yellow resin; +16 (c 0.227, CHCl3); IR νmax (film) 3142, 2950, 2864, 1798, 1754, 1439, 1130, 755 cm−1; 1H-NMR (CDCl3): δ 7.56 (1H, s, H-5′), 7.31–7.33 (2H, m, H-2′′ and H-6′′), 7.25–7.28 (3H, m, H-3′′; H-4′′ and H-5′′), 6.72 (1H, s, H-14), 5.63 (2H, s, CH2S), 3.16 (2H, t, J = 6.9 Hz, H-3′), 2.99 (2H, t, J = 6.9 Hz, H-2′), 2.97 (1H, m, H-15), 2.61 (2H, m, H-7), 2.24 (1H, m, H-1), 2.09 (1H, m, H-3), 2.00 (1H, m, H-2), 1.88–1.95 (2H, m, H-5 and H-6), 1.83 (1H, m, H-1), 1.70 (1H, m, H-2), 1.39 (1H, m, H-3), 1.19 (3H, d, J = 6.9 Hz, H-16), 1.16 (3H, s, H-18), 1.12 (3H, d, J = 6.9 Hz, H-17), 1.08 (3H, s, H-19), 0.87 (1H, m, H-6); 13C-NMR (CDCl3): δ 42.0 (C-1), 18.6 (C-2), 39.1 (C-3), 33.2 (C-4), 56.9 (C-5), 24.3 (C-6), 33.3 (C-7), 137.7 (C-8), 130.8 (C-9), 50.0 (C-10), 144.9 (C-11), 128.9 (C-12), 141.2 (C-13), 121.9 (C-14), 28.1 (C-15), 23.0 (C-16), 23.9 (C-17), 32.2 (C-18), 22.6 (C-19), 178.0 (C-20), 170.5 (C-1′), 33.9 (C-2′), 21.6 (C-3′), 146.8 (C-4′), 120.4 (C-5′), 54.1 (CH2S), 132.6 (C-1′′), 132.7 (2C, C-2′′ and C-6′′), 129.8 (2C, C-3′′ and C-5′′), 129.8 (C-4′′); EIMS m/z 532.2560 [M+H-CO]+ (calcd for C31H38N3O3S, 532.2634).
12-O-(4-(((1-phenylthio)methyl)-1H-1,2,3-triazol-4-yl)-butanoyloxy)-11,20-epoxyabieta-8,11,13-trien-20-one (2). Pale yellow resin; +28 (c 0.124, CHCl3); IR νmax (film) 3143, 2952, 2870, 1798, 1757, 1437, 1126, 750 cm−1; 1H-NMR (CDCl3): δ 7.41 (1H, s, H-6′), 7.31–7.33 (2H, m, H-2′′ and H-6′′), 7.25–7.28 (3H, m, H-3′′; H-4′′ and H-5′′), 6.72 (1H, s, H-14), 5.59 (2H, s, CH2S), 3.00 (1H, m, H-15), 2.83 (2H, t, J = 7.4 Hz, H-4′), 2.59–2.61 (4H, m, H-7 and H-2′), 2.24 (1H, m, H-1), 2.05–2.14 (3H, m, H-3 and H-3′), 2.00 (1H, m, H-2), 1.87–1.95 (2H, m, H-5 and H-6), 1.82 (1H, m, H-1), 1.68 (1H, m, H-2), 1.37 (1H, m, H-3), 1.20 (3H, d, J = 6.9 Hz, H-16), 1.15 (3H, d, J = 6.9 Hz, H-17), 1.14 (3H, s, H-18), 1.07 (3H, s, H-19), 0.86 (1H, m, H-6); 13C-NMR (CDCl3): δ 41.4 (C-1), 18.1 (C-2), 38.5 (C-3), 32.7 (C-4), 56.3 (C-5), 24.3 (C-6), 32.7 (C-7), 137.1 (C-8), 130.3 (C-9), 49.5 (C-10), 144.5 (C-11), 128.6 (C-12), 140.7 (C-13), 120.9 (C-14), 27.6 (C-15), 23.4 (C-16), 23.8 (C-17), 31.7 (C-18), 22.5 (C-19), 177.5 (C-20), 170.5 (C-1′), 32.7 (C-2′), 22.1 (C-3′), 24.4 (C-4′), 147.2 (C-5′), 119.8 (C-6′), 53.6 (CH2S), 129.5 (C-1′′), 132.3 (2C, C-2′′ and C-6′′), 129.3 (2C, C-3′′ and C-5′′), 131.8 (C-4′′); EIMS m/z 546.2603 [M+H]+ (calcd for C32H40N3O3S, 546.2790).
12-O-(3-(1-benzyl-1H-1,2,3-triazol-4-yl)-propanoyloxy)-11,20-epoxyabieta-8,11,13-trien-20-one (3). White resin; mp 154 °C; +40 (c 0.096, CHCl3); IR νmax (film) 3150, 2961, 2870, 1795, 1760, 1431, 1130, 753 cm−1; 1H-NMR (CDCl3): δ 7.51 (1H, s, H-5′), 7.32–7.34 (3H, m, H-2′′; H-4′′ and H-6′′), 7.25–7.28 (2H, m, H-3′′ and H-5′′), 6.74 (1H, s, H-14), 5.53 (2H, s, CH2Ph), 3.18 (2H, t, J = 6.9 Hz, H-3′), 3.00 (2H, t, J = 6.9 Hz, H-2′), 2.98 (1H, m, H-15), 2.63 (2H, m, H-7), 2.24 (1H, m, H-1), 2.10 (1H, m, H-3), 2.01 (1H, m, H-2), 1.88–1.96 (2H, m, H-5 and H-6), 1.84 (1H, m, H-1), 1.72 (1H, m, H-2), 1.41 (1H, m, H-3), 1.20 (3H, d, J = 6.9 Hz, H-16), 1.18 (3H, s, H-18), 1.13 (3H, d, J = 6.9 Hz, H-17), 1.10 (3H, s, H-19), 0.88 (1H, m, H-6); 13C-NMR (CDCl3): δ 42.0 (C-1), 18.6 (C-2), 39.1 (C-3), 33.2 (C-4), 56.9 (C-5), 24.3 (C-6), 33.3 (C-7), 137.7 (C-8), 130.8 (C-9), 50.0 (C-10), 144.9 (C-11), 129.8 (C-12), 141.3 (C-13), 122.2 (C-14), 28.1 (C-15), 23.0 (C-16), 23.9 (C-17), 32.2 (C-18), 22.6 (C-19), 178.0 (C-20), 170.6 (C-1′), 33.9 (C-2′), 21.5 (C-3′), 146.7 (C-4′), 120.4 (C-5′), 54.4 (CH2Ph), 135.5 (C-1′′), 129.4 (2C, C-2′′ and C-6′′), 128.4 (2C, C-3′′ and C-5′′), 128.9 (C-4′′); EIMS m/z 500.2890 [M+H]+ (calcd for C31H38N3O3, 500.2913).
12-O-(4-(1-benzyl-1H-1,2,3-triazol-4-yl)-butanoyloxy)-11,20-epoxyabieta-8,11,13-trien-20-one (4). White resin; mp 162 °C; +51 (c 0.106, CHCl3); IR νmax (film) 3141, 2950, 2868, 1793, 1754, 1440, 1128, 753 cm−1; 1H-NMR (CDCl3): δ 7.43 (1H, s, H-6′), 7.33–7.35 (3H, m, H-2′′; H-4′′ and H-6′′), 7.26–7.29 (2H, m, H-3′′ and H-5′′), 6.73 (1H, s, H-14), 5.49 (2H, s, CH2Ph), 3.01 (1H, m, H-15), 2.84 (2H, t, J = 7.4 Hz, H-4′), 2.59–2.62 (4H, m, H-7 and H-2′), 2.25 (1H, m, H-1), 2.06–2.15 (3H, m, H-3 and H-3′), 2.00 (1H, m, H-2), 1.88–1.96 (2H, m, H-5 and H-6), 1.83 (1H, m, H-1), 1.68 (1H, m, H-2), 1.38 (1H, m, H-3), 1.21 (3H, d, J = 6.9 Hz, H-16), 1.15 (3H, d, J = 6.9 Hz, H-17), 1.14 (3H, s, H-18), 1.08 (3H, s, H-19), 0.86 (1H, m, H-6); 13C-NMR (CDCl3): δ 41.5 (C-1), 18.1 (C-2), 38.7 (C-3), 33.1 (C-4), 56.8 (C-5), 24.3 (C-6), 32.3 (C-7), 137.5 (C-8), 130.7 (C-9), 49.9 (C-10), 144.5 (C-11), 129.4 (C-12), 140.9 (C-13), 121.8 (C-14), 27.6 (C-15), 23.0 (C-16), 23.9 (C-17), 32.2 (C-18), 22.6 (C-19), 177.6 (C-20), 170.5 (C-1′), 32.7 (C-2′), 22.1 (C-3′), 24.3 (C-4′), 147.5 (C-5′), 119.6 (C-6′), 53.5 (CH2Ph), 135.3 (C-1′′), 129.5 (2C, C-2′′ and C-6′′), 128.4 (2C, C-3′′ and C-5′′), 129.1 (C-4′′); EIMS m/z 514.3124 [M+H]+ (calcd for C32H40N3O3, 514.3070).
12-O-(3-(1-(4-bromobenzyl)-1H-1,2,3-triazol-4-yl)-propanoyloxy)-11,20-epoxyabieta-8,11,13-trien-20-one (5). White resin; +61 (c 0.131, CHCl3); IR νmax (film) 3150, 2961, 2867, 1798, 1762, 1442, 1129, 763 cm−1; 1H-NMR (CDCl3): δ 7.54 (1H, s, H-5′), 7.41 (2H, d, J = 8.4 Hz, H-3′′ and H-5′′), 7.12 (2H, d, J = 8.4 Hz, H-2′′ and H-6′′), 6.71 (1H, s, H-14), 5.47 (2H, s, CH2PhBr), 3.17 (2H, t, J = 6.8 Hz, H-3′), 2.98 (2H, t, J = 6.8 Hz, H-2′), 2.96 (1H, m, H-15), 2.60 (2H, m, H-7), 2.18 (1H, m, H-1), 2.06 (1H, m, H-3), 1.97 (1H, m, H-2), 1.86–1.94 (2H, m, H-5 and H-6), 1.81 (1H, m, H-1), 1.70 (1H, m, H-2), 1.38 (1H, m, H-3), 1.17 (3H, d, J = 6.9 Hz, H-16), 1.14 (3H, s, H-18), 1.10 (3H, d, J = 6.9 Hz, H-17), 1.07 (3H, s, H-19), 0.86 (1H, m, H-6); 13C-NMR (CDCl3): δ 42.0 (C-1), 18.6 (C-2), 39.0 (C-3), 33.2 (C-4), 56.8 (C-5), 24.3 (C-6), 33.3 (C-7), 137.7 (C-8), 130.7 (C-9), 50.0 (C-10), 144.8 (C-11), 129.7 (C-12), 141.2 (C-13), 122.3 (C-14), 28.1 (C-15), 23.0 (C-16), 23.9 (C-17), 32.2 (C-18), 22.6 (C-19), 178.1 (C-20), 170.6 (C-1′), 34.0 (C-2′), 21.6 (C-3′), 146.8 (C-4′), 120.5 (C-5′), 53.6 (CH2PhBr), 134.7 (C-1′′), 130.0 (2C, C-2′′ and C-6′′), 132.5 (2C, C-3′′ and C-5′′), 123.0 (C-4′′); EIMS m/z 578.2042 [M+H]+ (calcd for C31H37BrN3O3, 578.2018).
12-O-(4-(1-(4-bromobenzyl)-1H-1,2,3-triazol-4-yl)-butanoyloxy)-11,20-epoxyabieta-8,11,13-trien-20-one (6). White resin; pale yellow resin; +54 (c 0.088, CHCl3); IR νmax (film) 3144, 2943, 2862, 1796, 1766, 1446, 1126, 753 cm−1; 1H-NMR (CDCl3): δ 7.42 (1H, s, H-6′), 7.40 (2H, d, J = 8.4 Hz, H-3′′ and H-5′′), 7.10 (2H, d, J = 8.4 Hz, H-2′′ and H-6′′), 6.70 (1H, s, H-14), 5.44 (2H, s, CH2PhBr), 3.00 (1H, m, H-15), 2.86 (2H, t, J = 7.4 Hz, H-4′), 2.59–2.62 (4H, m, H-7 and H-2′), 2.26 (1H, m, H-1), 2.07–2.15 (3H, m, H-3 and H-3′), 2.03 (1H, m, H-2), 1.88–1.96 (2H, m, H-5 and H-6), 1.83 (1H, m, H-1), 1.69 (1H, m, H-2), 1.38 (1H, m, H-3), 1.20 (3H, d, J = 6.9 Hz, H-16), 1.16 (3H, d, J = 6.9 Hz, H-17), 1.15 (3H, s, H-18), 1.10 (3H, s, H-19), 0.87 (1H, m, H-6); 13C-NMR (CDCl3): δ 41.7 (C-1), 18.5 (C-2), 38.8 (C-3), 32.1 (C-4), 56.4 (C-5), 24.5 (C-6), 32.3 (C-7), 137.7 (C-8), 130.7 (C-9), 50.0 (C-10), 144.7 (C-11), 129.6 (C-12), 140.9 (C-13), 121.9 (C-14), 27.9 (C-15), 23.1 (C-16), 23.8 (C-17), 32.1 (C-18), 22.6 (C-19), 177.6 (C-20), 170.4 (C-1′), 32.8 (C-2′), 22.2 (C-3′), 24.4 (C-4′), 147.3 (C-5′), 119.8 (C-6′), 53.4 (CH2PhBr), 134.3 (C-1′′), 130.1 (2C, C-2′′ and C-6′′), 132.7 (2C, C-3′′ and C-5′′), 122.6 (C-4′′); EIMS m/z 592.2296 [M+H]+ (calcd for C32H39BrN3O3, 592.2175).
12-O-(3-(1-(4-nitrobenzyl)-1H-1,2,3-triazol-4-yl)-propanoyloxy)-11,20-epoxyabieta-8,11,13-trien-20-one (7). Colorless resin; +24 (c 0.107, CHCl3); IR νmax (film) 3139, 2954, 2870, 1791, 1758, 1436, 1124, 752 cm−1; 1H-NMR (CDCl3): δ 8.13 (2H, d, J = 8.7 Hz, H-3′′ and H-5′′), 7.68 (1H, s, H-5′), 7.38 (2H, d, J = 8.7 Hz, H-2′′ and H-6′′), 6.72 (1H, s, H-14), 5.66 (2H, s, CH2PhNO2), 3.20 (2H, t, J = 6.8 Hz, H-3′), 2.98 (2H, t, J = 6.8 Hz, H-2′), 2.96 (1H, m, H-15), 2.60 (2H, m, H-7), 2.16 (1H, m, H-1), 2.04 (1H, m, H-3), 1.96 (1H, m, H-2), 1.85–1.94 (2H, m, H-5 and H-6), 1.81 (1H, m, H-1), 1.69 (1H, m, H-2), 1.38 (1H, m, H-3), 1.17 (3H, d, J = 6.9 Hz, H-16), 1.12 (3H, s, H-18), 1.10 (3H, d, J = 6.9 Hz, H-17), 1.06 (3H, s, H-19), 0.84 (1H, m, H-6); 13C-NMR (CDCl3): δ 42.0 (C-1), 18.5 (C-2), 39.0 (C-3), 33.1 (C-4), 56.8 (C-5), 24.3 (C-6), 33.2 (C-7), 137.8 (C-8), 130.7 (C-9), 50.0 (C-10), 144.7 (C-11), 129.7 (C-12), 141.2 (C-13), 122.8 (C-14), 28.1 (C-15), 22.9 (C-16), 23.8 (C-17), 32.2 (C-18), 22.5 (C-19), 78.2 (C-20), 170.5 (C-1′), 34.0 (C-2′), 21.7 (C-3′), 147.1 (C-4′), 120.6 (C-5′), 53.2 (CH2PhNO2), 142.8 (C-1′′), 129.0 (2C, C-2′′ and C-6′′), 124.4 (2C, C-3′′ and C-5′′), 148.3 (C-4′′); EIMS m/z 545.2931 [M+H]+ (calcd for C31H37N4O5, 545.2764).
12-O-(4-(1-(4-nitrobenzyl)-1H-1,2,3-triazol-4-yl)-butanoyloxy)-11,20-epoxyabieta-8,11,13-trien-20-one (8). Colorless resin; +30 (c 0.072, CHCl3); IR νmax (film) 3133, 2950, 2864, 1795, 1761, 1442, 1130, 755 cm−1; 1H-NMR (CDCl3): δ 8.15 (2H, d, J = 8.7 Hz, H-3′′ and H-5′′), 7.56 (1H, s, H-6′), 7.39 (2H, d, J = 8.7 Hz, H-2′′ and H-6′′), 6.70 (1H, s, H-14), 5.63 (2H, s, CH2PhNO2), 2.98 (1H, m, H-15), 2.84 (2H, t, J = 7.4 Hz, H-4′), 2.57–2.60 (4H, m, H-7 and H-2′), 2.24 (1H, m, H-1), 2.06–2.14 (3H, m, H-3 and H-3′), 2.00 (1H, m, H-2), 1.87–1.94 (2H, m, H-5 and H-6), 1.82 (1H, m, H-1), 1.69 (1H, m, H-2), 1.37 (1H, m, H-3), 1.19 (3H, d, J = 6.9 Hz, H-16), 1.15 (3H, d, J = 6.9 Hz, H-17), 1.13 (3H, s, H-18), 1.06 (3H, s, H-19), 0.84 (1H, m, H-6); 13C-NMR (CDCl3): δ 41.3 (C-1), 18.6 (C-2), 38.8 (C-3), 32.4 (C-4), 56.6 (C-5), 24.3 (C-6), 32.5 (C-7), 137.1 (C-8), 130.7 (C-9), 49.9 (C-10), 144.6 (C-11), 128.9 (C-12), 140.8 (C-13), 121.7 (C-14), 27.7 (C-15), 23.4 (C-16), 23.8 (C-17), 32.2 (C-18), 22.5 (C-19), 177.5 (C-20), 170.6 (C-1′), 32.7 (C-2′), 22.3 (C-3′), 24.3 (C-4′), 147.1 (C-5′), 119.4 (C-6′), 53.4 (CH2 PhNO2), 142.4 (C-1′′), 129.0 (2C, C-2′′ and C-6′′), 129.6 (2C, C-3′′ and C-5′′), 148.0 (C-4′′); EIMS m/z 559.3044 [M+H]+ (calcd for C32H39N4O5, 559.2920).
Methyl(11,12-O-(3-(((1-phenylthio)methyl)-1H-1,2,3-triazol-4-yl)-propanoyloxy)-abieta-8,11,13-triene)-20-oate (9). Pale yellow resin; +18 (c 0.053, CHCl3); IR νmax (film) 3147, 2967, 2873, 1769, 1743, 1458, 1112, 760 cm−1; 1H-NMR (CDCl3): δ 7.51, 7.49 (each 1H, s, H-5′), 7.26–7.30 (10H, m, 2×SPh), 6.92 (1H, s, H-14), 5.54 (4H, brs, 2×CH2S), 3.45 (3H, s, COOMe), 3.22 (1H, brd, J = 13.0 Hz, H-1), 2.97–3.04 (4H, m, 2×H-3′), 2.90–2.95 (2H, m, H-7), 2.73–2.88 (5H, m, H-15 and 2×H-2′), 2.28 (1H, m, H-6), 2.06 (1H, m, H-2), 1.83 (1H, m, H-6), 1.43–1.52 (3H, m, H-2; H-3 and H-5), 1.19–1.27 (2H, m, H-1 and H-3), 1.13 (3H, d, J = 6.9 Hz, H-16), 1.03 (3H, d, J = 6.9 Hz, H-17), 0.95 (3H, s, H-18), 0.72 (3H, s, H-19); 13C-NMR (CDCl3): δ 34.6 (C-1), 20.4 (C-2), 40.9 (C-3), 33.8 (C-4), 53.4 (C-5), 19.6 (C-6), 31.7 (C-7), 136.6 (C-8), 131.5 (C-9), 47.5 (C-10), 141.1 (C-11), 138.3 (C-12), 139.6 (C-13), 124.9 (C-14), 27.1 (C-15), 22.4 (C-16), 22.9 (C-17), 32.3 (C-18), 19.7 (C-19), 175.1 (C-20), 51.6 (OMe), 170.2, 169.9 (C-1′), 32.8, 32.7 (C-2′), 20.9, 20.6 (C-3′), 146.7, 146.3 (C-4′), 121.3 (2C, 2×C-5′), 53.5 (2C, 2×CH2S), 132.1 (2C, 2×C-1′′), 132.0 (4C, 2×C-2′′ and 2×C-6′′), 129.3 (4C, 2×C-3′′ and 2×C-5′′), 128.7 (2C, 2×C-4′′); EIMS m/z 837.3104 [M+H]+ (calcd for C45H53N6O6S2, 837.3468).
Methyl(11,12-O-(4-(((1-phenylthio)methyl)-1H-1,2,3-triazol-4-yl)-butanoyloxy)-abieta-8,11,13-triene)-20-oate (10). Pale yellow resin; +19 (c 0.204, CHCl3); IR νmax (film) 3148, 2959, 2870, 1769, 1746, 1489, 1112, 760 cm−1; 1H-NMR (CDCl3): δ 7.46, 7.38 (each 1H, s, H-6′), 7.26–7.31 (10H, m, 2×SPh), 6.94 (1H, s, H-14), 5.57 (4H, brs, 2×CH2S), 3.46 (3H, s, COOMe), 3.23 (1H, brd, J = 12.1 Hz, H-1), 2.87–2.95 (2H, m, H-7), 2.73–2.85 (5H, m, H-15 and 2×H-4′), 2.45–2.63 (4H, m, 2×H-2′), 2.28 (1H, m, H-6), 1.98–2.09 (5H, m, H-2 and 2×H-3′), 1.84 (1H, m, H-6), 1.44–1.53 (3H, m, H-2; H-3 and H-5), 1.20–1.29 (2H, m, H-1 and H-3), 1.18 (3H, d, J = 6.9 Hz, H-16), 1.10 (3H, d, J = 6.9 Hz, H-17), 0.95 (3H, s, H-18), 0.72 (3H, s, H-19); 13C-NMR (CDCl3): δ 34.6 (C-1), 19.8 (C-2), 41.0 (C-3), 33.8 (C-4), 53.4 (C-5), 18.2 (C-6), 31.8 (C-7), 136.6 (C-8), 131.6 (C-9), 47.6 (C-10), 141.2 (C-11), 138.4 (C-12), 139.5 (C-13), 124.9 (C-14), 27.2 (C-15), 22.6 (C-16), 22.9 (C-17), 32.4 (C-18), 19.6 (C-19), 175.2 (C-20), 51.6 (OMe), 170.7, 170.4 (C-1′), 32.9, 32.8 (C-2′), 24.4, 23.9 (C-3′), 24.7 (2C, 2×C-4′), 147.7, 147.3 (C-5′), 120.7, 120.6 (C-6′), 53.6, 53.5 (CH2S), 132.1 (2C, 2×C-1′′), 132.1 (4C, 2×C-2′′ and 2×C-6′′), 129.3 (4C, 2×C-3′′ and 2×C-5′′), 128.1 (2C, 2×C-4′′); EIMS m/z 865.3250 [M+H]+ (calcd for C47H57N6O6S2, 865.3781).
Methyl(11,12-O-(3-(1-benzyl-1H-1,2,3-triazol-4-yl)-propanoyloxy)-abieta-8,11,13-triene)-20-oate (11). White resin; +21 (c 0.192, CHCl3); IR νmax (film) 3134, 2959, 2867, 1769, 1749, 1437, 1109, 752 cm−1; 1H-NMR (CDCl3): δ 7.43 (2H, brs, 2×H-5′), 7.31–7.34 (6H, m, 2×H-2′′; 2×H-4′′ and 2×H-6′′), 7.22–7.26 (4H, m, 2×H-3′′ and 2×H-5′′), 6.93 (1H, s, H-14), 5.45, 5.42 (each 2H, s, CH2Ph), 3.44 (3H, s, COOMe), 3.22 (1H, brd, J = 12.5 Hz, H-1), 2.97–3.04 (4H, m, 2×H-3′), 2.89–2.95 (2H, m, H-7), 2.65–2.88 (5H, m, H-15 and 2×H-2′), 2.31 (1H, m, H-6), 2.08 (1H, m, H-2), 1.85 (1H, m, H-6), 1.45–1.53 (3H, m, H-2; H-3 and H-5), 1.21–1.30 (2H, m, H-1 and H-3), 1.15 (3H, d, J = 6.9 Hz, H-16), 1.03 (3H, d, J = 6.9 Hz, H-17), 0.97 (3H, s, H-18), 0.73 (3H, s, H-19); 13C-NMR (CDCl3): δ 35.1 (C-1), 20.3 (C-2), 41.5 (C-3), 34.3 (C-4), 54.0 (C-5), 18.7 (C-6), 32.3 (C-7), 135.3 (C-8), 129.4 (C-9), 48.1 (C-10), 141.7 (C-11), 137.1 (C-12), 140.9 (C-13), 125.5 (C-14), 27.6 (C-15), 22.9 (C-16), 23.4 (C-17), 32.8 (C-18), 20.1 (C-19), 175.6 (C-20), 52.1 (OMe), 170.8, 170.5 (C-1′), 33.5, 33.3 (C-2′), 21.2, 21.0 (C-3′), 147.1, 146.7 (C-4′), 122.2 (2C, 2×C-5′), 54.4 (2C, 2×CH2Ph), 135.3 (2C, 2×C-1′′), 129.4 (4C, 2×C-2′′ and 2×C-6′′), 128.5 (4C, 2×C-3′′ and 2×C-5′′), 129.0 (2C, 2×C-4′′); EIMS m/z 773.3461 [M+H]+ (calcd for C45H53N6O6, 773.4027).
Methyl(11,12-O-(4-(1-benzyl-1H-1,2,3-triazol-4-yl)-butanoyloxy)-abieta-8,11,13-triene)-20-oate (12). White resin; +24 (c 0.187, CHCl3); IR νmax (film) 3142, 2959, 2867, 1772, 1746, 1460, 1112, 757 cm−1; 1H-NMR (CDCl3): δ 7.41, 7.31 (each 1H, s, H-6′), 7.31–7.34 (6H, m, 2×H-2′′; 2×H-4′′′ and 2×H-6′′), 7.25–7.28 (4H, m, 2×H-3′′ and 2×H-5′′), 6.95 (1H, s, H-14), 5.48 (4H, brs, 2×CH2Ph), 3.46 (3H, s, COOMe), 3.23 (1H, brd, J = 12.2 Hz, H-1), 2.88–2.96 (2H, m, H-7), 2.75–2.87 (5H, m, H-15 and 2×H-4′), 2.47–2.66 (4H, m, 2×H-2′), 2.29 (1H, m, H-6), 1.99–2.11 (5H, m, H-2 and 2×H-3′), 1.85 (1H, m, H-6), 1.45–1.54 (3H, m, H-2; H-3 and H-5), 1.20–1.31 (2H, m, H-1 and H-3), 1.20 (3H, d, J = 6.9 Hz, H-16), 1.11 (3H, d, J = 6.9 Hz, H-17), 0.97 (3H, s, H-18), 0.74 (3H, s, H-19); 13C-NMR (CDCl3): δ 35.1 (C-1), 20.2 (C-2), 41.5 (C-3), 34.3 (C-4), 53.9 (C-5), 18.7 (C-6), 32.3 (C-7), 135.4 (C-8), 132.1 (C-9), 48.1 (C-10), 141.8 (C-11), 137.0 (C-12), 140.1 (C-13), 125.4 (C-14), 27.7 (C-15), 23.1 (C-16), 23.4 (C-17), 32.9 (C-18), 20.1 (C-19), 175.6 (C-20), 52.1 (OMe), 171.2, 171.0 (C-1′), 33.5, 33.4 (C-2′), 24.9, 24.4 (C-3′), 25.3, 25.2 (C-4′), 148.1, 147.7 (C-5′), 121.6, 121.5 (C-6′), 54.4 (2C, 2×CH2Ph), 135.4 (2C, 2×C-1′′), 129.4 (4C, 2×C-2” and 2×C-6′′), 128.4 (4C, 2×C-3′′ and 2×C-5′′), 129.0 (2×C-4′′); EIMS m/z 801.3727 [M+H]+ (calcd for C47H57N6O6, 801.4340).
Methyl(11,12-O-(3-(1-(4-bromobenzyl)-1H-1,2,3-triazol-4-yl)-propanoyloxy)-abieta-8,11,13-triene)-20-oate (13). White resin; +72 (c 0.133, CHCl3); IR νmax (film) 3145, 2956, 2867, 1801, 1760, 1443, 1131, 757 cm−1; 1H-NMR (CDCl3): δ 7.46 (4H, brd, J = 7.7 Hz, 2×H-3′′ and 2×H-5′′), 7.45 (2H, brs, 2×H-5′), 7.13, 7.10 (each 2H, d, J = 8.9 Hz, H-2′′ and H-6′′), 6.94 (1H, s, H-14), 5.41, 5.38 (each 2H, brs, CH2PhBr), 3.45 (3H, s, COOMe), 3.20 (1H, brd, J = 12.3 Hz, H-1), 2.97–3.04 (4H, m, 2×H-3′), 2.88–2.95 (2H, m, H-7), 2.64–2.87 (5H, m, H-15 and 2×H-2′), 2.30 (1H, m, H-6), 2.07 (1H, m, H-2), 1.85 (1H, m, H-6), 1.45–1.53 (3H, m, H-2; H-3 and H-5), 1.20–1.29 (2H, m, H-1 and H-3), 1.15 (3H, d, J = 6.9 Hz, H-16), 1.02 (3H, d, J = 6.9 Hz, H-17), 0.97 (3H, s, H-18), 0.73 (3H, s, H-19); 13C-NMR (CDCl3): δ 35.1 (C-1), 20.3 (C-2), 41.5 (C-3), 34.3 (C-4), 54.0 (C-5), 18.7 (C-6), 32.3 (C-7), 136.3 (C-8), 131.3 (C-9), 48.1 (C-10), 141.6 (C-11), 137.2 (C-12), 140.1 (C-13), 125.5 (C-14), 27.6 (C-15), 22.9 (C-16), 23.4 (C-17), 32.8 (C-18), 20.1 (C-19), 175.6 (C-20), 52.1 (OMe), 170.7, 170.5 (C-1′), 33.5, 33.3 (C-2′), 21.2, 20.9 (C-3′), 146.9, 146.5 (C-4′), 122.2 (2C, 2×C-5′), 53.7 (2C, 2×CH2PhBr), 134.3 (2C, 2×C-1′′), 130.1 (4C, 2×C-2′′ and 2×C-6′′), 132.6 (4C, 2×C-3′′ and 2×C-5′′), 123.1 (2C, 2×C-4′′); EIMS m/z 929.2422 [M+H]+ (calcd for C45H51Br2N6O6, 929.2237).
Methyl(11,12-O-(4-(1-(4-bromobenzyl)-1H-1,2,3-triazol-4-yl)-butanoyloxy)-abieta-8,11,13-triene)-20-oate (14). White resin; +77 (c 0.121, CHCl3); IR νmax (film) 3142, 2956, 2870, 1801, 1760, 1440, 1128, 757 cm−1; 1H-NMR (CDCl3): δ 7.46 (4H, brd, J = 8.3 Hz, 2×H-3′′ and 2×H-5′′), 7.43, 7.34 (each 1H, s, H-6′), 7.13, 7.12 (each 2H, d, J = 8.3 Hz, H-2′′ and H-6′′), 6.94 (1H, s, H-14), 5.43 (4H, brs, 2×CH2PhBr), 3.45 (3H, s, COOMe), 3.21 (1H, brd, J = 12.1 Hz, H-1), 2.85–2.95 (2H, m, H-7), 2.75–2.83 (5H, m, H-15 and 2×H-4′), 2.49–2.65 (4H, m, 2×H-2′), 2.28 (1H, m, H-6), 2.00–2.08 (5H, m, H-2 and 2×H-3′), 1.85 (1H, m, H-6), 1.44–1.53 (3H, m, H-2; H-3 and H-5), 1.20–1.31 (2H, m, H-1 and H-3), 1.17 (3H, d, J = 6.9 Hz, H-16), 1.10 (3H, d, J = 6.9 Hz, H-17), 0.97 (3H, s, H-18), 0.73 (3H, s, H-19); 13C-NMR (CDCl3): δ 35.1 (C-1), 20.2 (C-2), 41.4 (C-3), 34.3 (C-4), 53.9 (C-5), 18.7 (C-6), 32.3 (C-7), 137.1 (C-8), 131.4 (C-9), 48.1 (C-10), 141.2 (C-11), 139.0 (C-12), 140.1 (C-13), 125.4 (C-14), 27.7 (C-15), 23.1 (C-16), 23.4 (C-17), 32.9 (C-18), 20.1 (C-19), 175.7 (C-20), 52.1 (OMe), 171.2, 170.9 (C-1′), 33.5, 33.4 (C-2′), 24.9, 24.4 (C-3′), 25.3, 25.2 (C-4′), 148.2, 147.9 (C-5′), 121.6, 121.5 (C-6′), 53.7 (2C, 2×CH2PhBr), 134.4 (2C, 2×C-1′′), 130.1 (4C, 2×C-2′′ and 2×C-6′′), 132.6 (4C, 2×C-3′′ and 2×C-5′′), 123.1 (2C, 2×C-4′′); EIMS m/z 957.2837 [M+H]+ (calcd for C47H55Br2N6O6, 957.2550).
Methyl(11,12-O-(3-(1-(4-nitrobenzyl)-1H-1,2,3-triazol-4-yl)-propanoyloxy)-abieta-8,11,13-triene)-20-oate (15). Colorless resin; +89 (c 0.167, CHCl3); IR νmax (film) 3142, 2956, 2867, 1801, 1760, 1437, 1131, 754 cm−1; 1H-NMR (CDCl3): δ 8.20, 8.19 (each 2H, d, J = 8.3 Hz, H-3′′ and H-5′′), 7.52, 7.50 (each 1H, s, H-5′), 7.40 (4H, brd, J = 8.5 Hz, 2×H-2′′ and 2×H-6′′), 6.94 (1H, s, H-14), 5.60 (4H, brs, 2×CH2PhNO2), 3.46 (3H, s, COOMe), 3.21 (1H, brd, J = 12.7 Hz, H-1), 3.00–3.07 (4H, m, 2×H-3′), 2.91–2.98 (2H, m, H-7), 2.70–2.90 (5H, m, H-15 and 2×H-2′), 2.31 (1H, m, H-6), 2.07 (1H, m, H-2), 1.85 (1H, m, H-6), 1.46–1.53 (3H, m, H-2; H-3 and H-5), 1.21–1.29 (2H, m, H-1 and H-3), 1.15 (3H, d, J = 6.9 Hz, H-16), 1.03 (3H, d , J = 6.9 Hz, H-17), 0.98 (3H, s, H-18), 0.74 (3H, s, H-19); 13C-NMR (CDCl3): δ 35.2 (C-1), 20.3 (C-2), 41.5 (C-3), 34.4 (C-4), 54.0 (C-5), 18.7 (C-6), 32.2 (C-7), 137.4 (C-8), 132.1 (C-9), 48.1 (C-10), 141.5 (C-11), 138.8 (C-12), 140.1 (C-13), 125.6 (C-14), 27.7 (C-15), 22.9 (C-16), 23.4 (C-17), 32.8 (C-18), 20.1 (C-19), 175.7 (C-20), 52.1 (OMe), 170.7, 170.5 (C-1′), 33.5, 33.3 (C-2′), 21.2, 20.9 (C-3′), 147.6, 147.1 (C-4′), 122.6, 122.5 (C-5′), 53.4 (2C, 2×CH2PhNO2), 142.3, 142.2 (C-1′′), 129.0 (4C, 2×C-2′′ and 2×C-6′′), 124.6 (4C, 2×C-3′′ and 2×C-5′′), 148.4 (2C, 2×C-4′′); EIMS m/z 863.4050 [M+H]+ (calcd for C45H51N8O10, 863.3728).
Methyl(11,12-O-(4-(1-(4-nitrobenzyl)-1H-1,2,3-triazol-4-yl)-butanoyloxy)-abieta-8,11,13-triene)-20-oate (16). Colorless resin; +64 (c 0.131, CHCl3); IR νmax (film) 3139, 2956, 2867, 1772, 1718, 1454, 1120, 754 cm−1; 1H-NMR (CDCl3): δ 8.18, 8.17 (each 2H, d, J = 8.5 Hz, H-3′′ and H-5′′), 7.52, 7.43 (each 1H, s, H-6′), 7.40, 7.39 (each 2H, d, J = 8.5 Hz, H-2′′ and H-6′′), 6.94 (1H, s, H-14), 5.62 (4H, brs, 2×CH2PhNO2), 3.44 (3H, s, COOMe), 3.20 (1H, brd, J = 12.2 Hz, H-1), 2.85–2.95 (2H, m, H-7), 2.78–2.84 (5H, m, H-15 and 2×H-4′), 2.49–2.66 (4H, m, 2×H-2′), 2.27 (1H, m, H-6), 2.00–2.10 (5H, m, H-2 and 2×H-3′), 1.85 (1H, m, H-6), 1.42–1.53 (3H, m, H-2; H-3 and H-5), 1.19–1.30 (2H, m, H-1 and H-3), 1.16 (3H, d, J = 6.9 Hz, H-17), 1.09 (3H, d, J = 6.9 Hz, H-16), 0.96 (3H, s, H-18), 0.71 (3H, s, H-19); 13C-NMR (CDCl3): δ 35.1 (C-1), 20.2 (C-2), 41.4 (C-3), 34.3 (C-4), 53.9 (C-5), 18.7 (C-6), 32.2 (C-7), 137.2 (C-8), 132.1 (C-9), 48.1 (C-10), 141.7 (C-11), 138.9 (C-12), 140.1 (C-13), 125.5 (C-14), 27.7 (C-15), 23.1 (C-16), 23.4 (C-17), 32.8 (C-18), 20.1 (C-19), 175.7 (C-20), 52.1 (OMe), 171.2, 170.1 (C-1′), 33.5, 33.4 (C-2′), 24.9, 24.3 (C-3′), 25.3, 25.2 (C-4′), 148.1, 147.8 (C-5′), 122.0, 121.9 (C-6′), 53.3 (2C, 2×CH2PhNO2), 142.5 (2C, 2×C-1′′), 129.0 (4C, 2×C-2′′ and 2×C-6′′), 124.6 (4C, 2×C-3′′ and 2×C-5′′), 148.4 (2C, 2×C-4′′); EIMS m/z 891.4382 [M+H]+ (calcd for C47H55N8O10, 891.4041).
(7β)-11,12-O-(3-(((1-phenylthio)methyl)-1H-1,2,3-triazol-4-yl)-propanoyloxy)-7,20-epoxyabieta-8,11,13-trien-20-one (17). Pale yellow resin; +61 (c 0.162, CHCl3); IR νmax (film) 3133, 2959, 2870, 1769, 1715, 1460, 1120, 757 cm−1; 1H-NMR (CDCl3): δ 7.49, 7.45 (each 1H, s, H-5′), 7.25-7.33 (10H, m, 2×SPh), 7.08 (1H, s, H-14), 5.63, 5.57 (each 2H, brs, CH2S), 5.47 (1H, d, J = 2.7 Hz, H-7α), 3.04 (4H, m, 2×H-3′), 2.88–2.96 (4H, m, 2×H-2′), 2.80–2.87 (1H, m, H-15), 2.22 (1H, m, H-6β), 1.88–2.00 (1H, m, H-1α), 184–190 (2H, m, H-2α and H-6α), 1.72–1.82 (1H, m, H-1β), 1.60 (1H, dd, J = 10.5, 5.8 Hz, H-5), 1.44–1.48 (2H, m, H-2β and H-3α), 1.09–1.15 (1H, m, H-3β), 1.13 (3H, d, J = 7.5 Hz, H-16), 1.11 (3H, d, J = 7.5 Hz, H-17), 0.86 (3H, s, H-18), 0.81 (3H, s, H-19); 13C-NMR (CDCl3): δ 28.4 (C-1), 19.1 (C-2), 41.0 (C-3), 34.9 (C-4), 44.9 (C-5), 29.5 (C-6), 77.5 (C-7), 129.6 (C-8), 138.5 (C-9), 48.4 (C-10), 141.2 (C-11), 141.7 (C-12), 139.4 (C-13), 119.0 (C-14), 27.9 (C-15), 23.2 (C-16), 23.3 (C-17), 20.1 (C-18), 32.0 (C-19), 174.6 (C-20), 170.8, 170.7 (C-1′), 33.8, 33.1 (C-2′), 21.1, 20.9 (C-3′), 146.6, 146.5 (C-4′), 121.8 (2C, 2×C-5′), 54.2, 54.1 (CH2S), 132.5 (2C, 2×C-1′′), 132.6 (4C, 2×C-2′′ and 2×C-6′′), 129.9, 129.8 (each 2C, C-3′′ and C-5′′), 129.1, 128.9 (C-4′′); EIMS m/z 821.3427 [M+H]+ (calcd for C44H49N6O6S2, 821.3155).
(7β)-11,12-O-(4-(((1-phenylthio)methyl)-1H-1,2,3-triazol-4-yl)-butanoyloxy)-7,20-epoxyabieta-8,11,13-trien-20-one (18). Pale yellow resin; +56 (c 0.153, CHCl3); IR νmax (film) 3132, 2957, 2869, 1771, 1718, 1456, 1120, 756 cm−1; 1H-NMR (CDCl3): δ 7.37, 7.36 (each 1H, s, H-6′), 7.25–7.30 (10H, m, 2×SPh), 7.07 (1H, s, H-14), 5.57, 5.56 (each 2H, brs, CH2S), 5.46 (1H, brs, H-7α), 2.85 (1H, m, H-15), 2.74 (4H, m, 2×H-4′), 2.55 (4H, m, 2×H-2′), 2.22 (1H, m, H-6β), 1.93–2.07 (5H, m, H-1α and 2×H-3′), 185–191 (2H, m, H-2α and H-6α), 1.71–1.76 (1H, m, H-1β), 1.67 (1H, dd, J = 10.1, 5.5 Hz, H-5), 1.46–1.54 (2H, m, H-2β and H-3α), 1.13–1.24 (1H, m, H-3β), 1.13 (6H, brd, J = 6.6 Hz, H-16 and H-17), 0.86 (3H, s, H-18), 0.82 (3H, s, H-19); 13C-NMR (CDCl3): δ 28.0 (C-1), 18.6 (C-2), 40.5 (C-3), 34.4 (C-4), 44.4 (C-5), 29.0 (C-6), 77.0 (C-7), 130.8 (C-8), 137.9 (C-9), 48.3 (C-10), 140.7 (C-11), 141.1 (C-12), 139.0 (C-13), 118.4 (C-14), 27.4 (C-15), 23.9 (C-16), 24.3 (C-17), 20.1 (C-18), 31.5 (C-19), 174.1 (C-20), 170.7, 170.6 (C-1′), 32.9, 32.8 (C-2′), 22.8, 22.7 (C-3′), 24.6 (2C, 2×C-4′), 147.1 (2C, 2×C-5′), 120.6 (2C, 2×C-6′), 53.5 (2C, 2×CH2S), 131.9 (2C, 2×C-1′′), 132.0 (4C, 2×C-2′′ and 2×C-6′′), 129.3 (4C, 2×C-3′′ and 2×C-5′′), 128.5 (2C, 2×C-4′′); EIMS m/z 849.3661 [M+H]+ (calcd for C46H53N6O6S2, 849.3468).
(7β)-11,12-O-(3-(1-benzyl-1H-1,2,3-triazol-4-yl)-propanoyloxy)-7,20-epoxyabieta-8,11,13-trien-20-one (19). Colorless resin; +67 (c 0.039, CHCl3); IR νmax (film) 3140, 2959, 2871, 1769, 1716, 1460, 1121, 756 cm−1; 1H-NMR (CDCl3): δ 7.68, 7.50 (each 1H, s, H-5′), 7.27–7.34 (6H, m, 2×H-2′′; 2×H-4′′ and 2×H-6′′), 7.19–7.27 (4H, m, 2×H-3′′ and 2×H-5′′), 7.06 (1H, s, H-14), 5.44 (5H, brs, H-7α and 2×CH2S), 2.96–3.14 (4H, m, 2×H-3′), 2.84–2.96 (4H, m, 2×H-2′), 2.75–2.84 (1H, m, H-15), 2.21 (1H, m, H-6β), 1.82–1.93 (3H, m, H-1α; H-2α and H-6α), 1.68–1.76 (1H, m, H-1β), 1.63 (1H, dd, J = 10.5, 5.8 Hz, H-5), 1.45–1.50 (2H, m, H-2β and H-3α), 1.07–1.13 (1H, m, H-3β), 1.09 (6H, brd, J = 6.6 Hz, H-16 and H-17), 0.86 (3H, s, H-18), 0.82 (3H, s, H-19); 13C-NMR (CDCl3): δ 28.4 (C-1), 19.1 (C-2), 41.1 (C-3), 35.0 (C-4), 44.9 (C-5), 29.6 (C-6), 77.6 (C-7), 129.2 (C-8), 138.5 (C-9), 48.7 (C-10), 141.2 (C-11), 141.7 (C-12), 139.4 (C-13), 119.1 (C-14), 27.9 (C-15), 23.2 (C-16), 23.3 (C-17), 20.1 (C-18), 32.0 (C-19), 174.6 (C-20), 170.9, 170.8 (C-1′), 33.6, 33.4 (C-2′), 21.4, 21.0 (C-3′), 147.1, 147.9 (C-4′), 121.9 (2C, 2×C-5′), 54.9 (2C, 2×CH2Ph), 135.3, 135.1 (C-1′′), 129.5, 129.4 (each 2C, C-2′′ and C-6′′), 128.7, 128.6 (each 2C, C-3′′ and C-5′′), 129.1, 129.0 (C-4′′); EIMS m/z 757.4006 [M+H]+ (calcd for C44H49N6O6, 757.3741).
(7β)-11,12-O-(4-(1-benzyl-1H-1,2,3-triazol-4-yl)-butanoyloxy)-7,20-epoxyabieta-8,11,13-trien-20-one (20). Colorless resin; +49 (c 0.039, CHCl3); IR νmax (film) 3142, 2959, 2870, 1767, 1718, 1460, 1120, 754 cm−1; 1H-NMR (CDCl3): δ 7.32–7.36 (8H, m, 2×H-6′; 2×H-2′′; 2×H-4′′ and 2×H-6′′), 7.25–7.28 (4H, m, 2×H-3′′ and 2×H-5′′), 7.09 (1H, s, H-14), 5.48 (5H, brs, H-7α and 2×CH2S), 2.88 (1H, m, H-15), 2.78 (4H, m, 2×H-4′), 2.60 (4H, m, 2×H-2′), 2.23 (1H, m, H-6β), 2.03–2.12 (5H, m, H-1α and 2×H-3′), 188–194 (2H, m, H-2α and H-6α), 1.72–1.78 (1H, m, H-1β), 1.69 (1H, dd, J = 10.5, 5.5 Hz, H-5), 1.49–1.55 (2H, m, H-2β and H-3α), 1.14–1.25 (1H, m, H-3β), 1.15 (6H, brd, J = 6.7 Hz, H-16 and H-17), 0.90 (3H, s, H-18), 0.85 (3H, s, H-19); 13C-NMR (CDCl3): δ 28.4 (C-1), 19.1 (C-2), 41.1 (C-3), 35.0 (C-4), 45.0 (C-5), 29.6 (C-6), 77.6 (C-7), 129.5 (C-8), 138.4 (C-9), 48.1 (C-10), 141.2 (C-11), 141.7 (C-12), 139.4 (C-13), 118.9 (C-14), 28.0 (C-15), 23.2 (C-16), 23.3 (C-17), 20.1 (C-18), 32.0 (C-19), 174.6 (C-20), 170.9, 170.8 (C-1′), 33.6, 33.4 (C-2′), 24.9, 24.5 (C-3′), 25.2 (2C, 2×C-4′), 147.6, 147.4 (C-5′), 121.5 (2C, 2×C-6′), 54.4 (2C, 2×CH2Ph), 135.3 (2C, 2×C-1′′), 129.5 (4C, 2×C-2′′ and 2×C-6′′), 128.4 (4C, 2×C-3′′ and 2×C-5′′), 129.0 (2C, 2×C-4′′); EIMS m/z 785.4452 [M+H]+ (calcd for C46H53N6O6, 785.4027).
(7β)-11,12-O-(3-(1-(4-bromobenzyl)-1H-1,2,3-triazol-4-yl)-propanoyloxy)-7,20-epoxyabieta-8,11,13-trien-20-one (21). White resin; +53 (c 0.140, CHCl3); IR νmax (film) 3136, 2956, 2867, 1763, 1715, 1454, 1123, 757 cm−1; 1H-NMR (CDCl3): δ 7.47, 7.46 (each 2H, d, J = 8.3 Hz, H-3′′ and H-5′′), 7.42, 7.40 (each 1H, s, H-5′), 7.14, 7.13 (each 2H, d, J = 8.1 Hz, H-2′′ and H-6′′), 7.08 (1H, s, H-14), 5.48 (1H, d, J = 2.7 Hz, H-7α), 5.46, 5.42 (each 2H, brs, CH2PhBr), 3.05 (4H, m, 2×H-3′), 2.85–2.90 (4H, m, 2×H-2′), 2.78–2.85 (1H, m, H-15), 2.23 (1H, m, H-6β), 1.88–2.00 (1H, m, H-1α), 185–191 (2H, m, H-2α and H-6α), 1.74–1.80 (1H, m, H-1β), 1.65 (1H, dd, J = 10.6, 5.8 Hz, H-5), 1.46–1.50 (2H, m, H-2β and H-3α), 1.09–1.15 (1H, m, H-3β), 1.13 (3H, d, J = 6.8 Hz, H-16), 1.09 (3H, d, J = 6.8 Hz, H-17), 0.88 (3H, s, H-18), 0.84 (3H, s, H-19); 13C-NMR (CDCl3): δ 28.3 (C-1), 19.1 (C-2), 41.0 (C-3), 35.0 (C-4), 44.9 (C-5), 29.5 (C-6), 77.5 (C-7), 129.6 (C-8), 138.5 (C-9), 48.7 (C-10), 141.1 (C-11), 141.7 (C-12), 139.4 (C-13), 119.1 (C-14), 27.9 (C-15), 23.2 (2C, C-16 and C-17), 20.1 (C-18), 32.0 (C-19), 174.6 (C-20), 170.9, 170.7 (C-1′), 33.1 (2C, 2×C-2′), 21.1, 20.9 (C-3′), 147.0, 146.8 (C-4′), 122.2 (2C, 2×C-5′), 53.7 (2C, 2×CH2PhBr), 134.3, 134.2 (C-1′′), 130.2, 130.1 (each 2C, C-2′′ and C-6′′), 132.6, 132.6 (each 2C, C-3′′ and C-5′′), 123.2, 123.1 (C-4′′); EIMS m/z 913.1844 [M+H]+ (calcd for C44H47Br2N6O6, 913.1924).
(7β)-11,12-O-(4-(1-(4-bromobenzyl)-1H-1,2,3-triazol-4-yl)-butanoyloxy)-7,20-epoxyabieta-8,11,13-trien-20-one (22). White resin; +55 (c 0.132, CHCl3); IR νmax (film) 3133, 2962, 2873, 1769, 1752, 1454, 1109, 754 cm−1; 1H-NMR (CDCl3): δ 7.45 (4H, d, J = 8.3 Hz, H-3′′ and H-5′′), 7.35 (2H, s, 2×H-6′), 7.13 (4H, brd, J = 8.3 Hz, H-2′′ and H-6′′), 7.09 (1H, s, H-14), 5.48 (1H, d, J = 2.4 Hz, H-7α), 5.43 (4H, brs, 2×CH2PhBr), 2.87 (1H, m, H-15), 2.78 (4H, m, 2×H-4′), 2.60 (4H, m, 2×H-2′), 2.22 (1H, m, H-6β), 2.03–2.10 (5H, m, H-1α and 2×H-3′), 188–194 (2H, m, H-2α and H-6α), 1.72–1.76 (1H, m, H-1β), 1.67 (1H, dd, J = 10.4, 5.8 Hz, H-5), 1.48–1.53 (2H, m, H-2β and H-3α), 1.14–1.24 (1H, m, H-3β), 1.14 (6H, brd, J = 6.8 Hz, H-16 and H-17), 0.88 (3H, s, H-18), 0.84 (3H, s, H-19); 13C-NMR (CDCl3): δ 28.5 (C-1), 19.1 (C-2), 41.1 (C-3), 35.0 (C-4), 45.0 (C-5), 29.5 (C-6), 77.5 (C-7), 129.4 (C-8), 138.5 (C-9), 48.1 (C-10), 141.1 (C-11), 141.7 (C-12), 139.5 (C-13), 19.0 (C-14), 28.0 (C-15), 23.2 (C-16), 23.3 (C-17), 20.1 (C-18), 32.0 (C-19), 174.6 (C-20), 171.2, 170.9 (C-1′), 33.4 (2C, 2×C-2′), 24.8, 24.5 (C-3′), 25.2 (2C, 2×C-4′), 147.7 (2C, 2×C-5′), 121.5 (2C, 2×C-6′), 53.7 (2C, 2×CH2PhBr), 134.4 (2C, 2×C-1′′), 130.1 (4C, 2×C-2′′ and 2×C-6′′), 132.6 (4C, 2×C-3′′ and 2×C-5′′), 123.1 (2C, 2×C-4′′); EIMS m/z 941.2499 [M+H]+ (calcd for C46H51Br2N6O6, 941.2237).
(7β)-11,12-O-(3-(1-(4-nitrobenzyl)-1H-1,2,3-triazol-4-yl)-propanoyloxy)-7,20-epoxyabieta-8,11,13-trien-20-one (23). White resin; +18 (c 0.193, CHCl3); IR νmax (film) 3136, 2957, 2868, 1765, 1744, 1458, 1111, 757 cm−1; 1H-NMR (CDCl3): δ 8.17, 8.16 (each 2H, d, J = 8.6 Hz, H-3′′ and H-5′′), 7.49, 7.46 (each 1H, s, H-5′), 7.41, 7.38 (each 2H, d, J = 8.6 Hz, 2×H-2′′ and 2×H-6′′), 7.09 (1H, s, H-14), 5.66, 5.61 (each 2H, s, CH2PhNO2), 5.49 (1H, d, J = 2.7 Hz, H-7α), 3.06 (4H, m, 2×H-3′), 2.89–2.97 (4H, m, 2×H-2′), 2.79–2.89 (1H, m, H-15), 2.23 (1H, m, H-6β), 1.99–2.10 (1H, m, H-1α), 187–194 (2H, m, H-2α and H-6α), 1.74–1.80 (1H, m, H-1β), 1.64 (1H, dd, J = 10.6, 5.8 Hz, H-5), 1.44–1.49 (2H, m, H-2β and H-3α), 1.07–1.17 (1H, m, H-3β), 1.12 (3H, d, J = 6.9 Hz, H-16), 1.08 (3H, d, J = 6.9 Hz, H-17), 0.86 (3H, s, H-18), 0.83 (3H, s, H-19); 13C-NMR (CDCl3): δ 28.4 (C-1), 19.1 (C-2), 40.9 (C-3), 34.9 (C-4), 44.9 (C-5), 29.5 (C-6), 77.5 (C-7), 129.6 (C-8), 138.6 (C-9), 48.7 (C-10), 141.1 (C-11), 141.8 (C-12), 139.3 (C-13), 119.2 (C-14), 27.9 (C-15), 23.2 (2C, C-16 and C-17), 20.1 (C-18), 32.0 (C-19), 174.7 (C-20), 171.0, 170.7 (C-1′), 33.9, 33.1 (C-2′), 21.0, 20.9 (C-3′), 146.9, 146.8 (C-4′), 122.5 (2C, 2×C-5′), 53.4 (2C, 2×CH2PhNO2), 142.4, 142.3 (C-1′′), 129.2, 129.0 (each 2C, C-2′′ and C-6′′), 124.6 (4C, 2×C-3′′ and 2×C-5′′), 148.4, 148.3 (C-4′′); EIMS m/z 847.3828 [M+H]+ (calcd for C44H47N8O10, 847.3415).
(7β)-11,12-O-(4-(1-(4-nitrobenzyl)-1H-1,2,3-triazol-4-yl)-butanoyloxy)-7,20-epoxyabieta-8,11,13-trien-20-one (24). White resin; +26(c 0.039, CHCl3); IR νmax (film) 3139, 2959, 2870, 1763, 1746, 1457, 1111, 757 cm−1; 1H-NMR (CDCl3): δ 8.16 (4H, brd, J = 8.4 Hz, H-3′′ and H-5′′), 7.45, 7.44 (each 1H, s, H-6′), 7.39 (4H, brd, J = 7.9 Hz, 2×H-2′′ and 2×H-6′′), 7.10 (1H, s, H-14), 5.62, 5.61 (each 2H, s, CH2PhNO2), 5.49 (1H, d, J = 2.4 Hz, H-7α), 2.87 (1H, m, H-15), 2.81 (4H, m, 2×H-4′), 2.62 (4H, m, 2×H-2′), 2.23 (1H, m, H-6β), 2.03–2.11 (5H, m, H-1α and 2×H-3′), 188–194 (2H, m, H-2α and H-6α), 1.73–1.77 (1H, m, H-1β), 1.67 (1H, dd, J = 10.5, 5.7 Hz, H-5), 1.48–1.54 (2H, m, H-2β and H-3α), 1.14–1.23 (1H, m, H-3β), 1.14 (6H, brd, J = 6.8 Hz, H-16 and H-17), 0.87 (3H, s, H-18), 0.84 (3H, s, H-19); 13C-NMR (CDCl3): δ 28.6 (C-1), 19.2 (C-2), 41.1 (C-3), 35.0 (C-4), 45.1 (C-5), 29.6 (C-6), 77.6 (C-7), 129.5 (C-8), 138.5 (C-9), 48.9 (C-10), 141.2 (C-11), 141.8 (C-12), 139.6 (C-13), 119.1 (C-14), 28.1 (C-15), 23.3 (2C, C-16 and C-17), 20.2 (C-18), 32.0 (C-19), 174.8 (C-20), 171.3, 171.2 (C-1′), 33.6, 33.4 (C-2′), 24.9, 24.5 (C-3′), 25.3, 25.2 (C-4′), 147.6 (2C, 2×C-5′), 122.0 (2C, 2×C-6′), 53.4 (2C, 2×CH2PhNO2), 142.5 (2C, 2×C-1′′), 129.1 (4C, 2×C-2′′ and 2×C-6′′), 124.6 (4C, 2×C-3′′ and 2×C-5′′), 148.4, 148.1 (C-4′′); EIMS m/z 875.3885 [M+H]+ (calcd for C46H51N8O10, 875.3728).