Interference of Phenylethanoid Glycosides from Cistanche tubulosa with the MTT Assay
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of Compounds

| Position | Echinacoside | Acteoside | ||
|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | 130.1 | 130.1 | ||
| 2 | 6.69 (d, 2.0) | 115.8 | 6.69 (d, 2.0) | 115.8 |
| 3 | 144.7 | 144.7 | ||
| 4 | 143.3 | 143.3 | ||
| 5 | 6.67 (d, 8.0), | 115.0 | 6.67 (d, 8.0) | 115.0 |
| 6 | 6.57 (dd, 1.9, 8.0) | 119.9 | 6.56 (dd, 1.9, 8.0) | 119.9 |
| 7 | 2.79 (m, 2H) | 35.2 | 2.79 (m, 2H) | 35.2 |
| 8 | 3.75 (m) | 70.1 | 3.73 (m) | 70.7 |
| 4.03 (m) | 4.04 (m) | |||
| 8-O-Glc | ||||
| 1' | 4.38 (d, 7.9) | 102.8 | 4.37 (d, 7.9) | 102.8 |
| 2' | 3.39 (dd, 8.2, 9.2) | 74.8 | 3.39 (dd 7.9, 9.2) | 74.8 |
| 3' | 3.81 (t, 9.2) | 80.3 | 3.81 (t, 9.2) | 80.3 |
| 4' | 5.00 (dd, 9.7, 9.7) | 69.2 | 4.91 (t, 9.7, 9.8) | 69.2 |
| 5' | 3.71 (m) | 73.4 | 3.59 (m) | 74.6 |
| 6' | 3.64 (m) | 68.0 | 3.52 (m) | 60.9 |
| 3.93 (m) | 3.61 (m) | |||
| 3'-O-Rha | ||||
| 1'' | 5.18 (d, 1.8) | 101.7 | 5.19 (d, 1.8) | 101.6 |
| 2'' | 3.91 (dd, 1.8, 3.0) | 71.0 | 3.92 (dd, 1.8, 3.0) | 71.0 |
| 3'' | 3.55 (m) | 70.7 | 3.57 (m) | 70.8 |
| 4'' | 3.28 (m) | 72.4 | 3.30 (m) | 72.4 |
| 5'' | 3.53 (m) | 69.1 | 3.55 (m) | 69.1 |
| 6'' | 1.08 (3H, d, 6.2) | 17.1 | 1.09 (3H, d, 6.2) | 17.1 |
| 6'-O-Glc | ||||
| 1''' | 4.29 (d, 7.7) | 103.3 | - | - |
| 2''' | 3.19 (m) | 73.7 | - | - |
| 3''' | 3.54 (m) | 76.4 | - | - |
| 4''' | 3.26 (m) | 70.8 | - | - |
| 5''' | 3.23 (m) | 76.5 | - | - |
| 6''' | 3.62 (m) | 61.2 | - | - |
| 3.82 (m) | ||||
| 4'-O-Caf | ||||
| 1 | 126.3 | 126.3 | ||
| 2 | 7.06 (d, 2.0) | 113.9 | 7.05 (d, 1.9) | 113.9 |
| 3 | 145.4 | 145.4 | ||
| 4 | 148.4 | 148.4 | ||
| 5 | 6.78 (d, 8.2) | 115.2 | 6.78 (d, 8.0) | 115.2 |
| 6 | 6.96 (dd, 1.9, 8.2) | 121.9 | 6.94 (dd, 1.9, 8.0) | 121.8 |
| 7 | 7.60 (d, 15.9) | 146.8 | 7.59 (d, 15.9) | 146.6 |
| 8 | 6.27 (d, 15.9) | 113.3 | 6.26 (d, 15.9) | 113.4 |
| 9 | 167.1 | 166.9 | ||
2.2. Cell Viability Assays
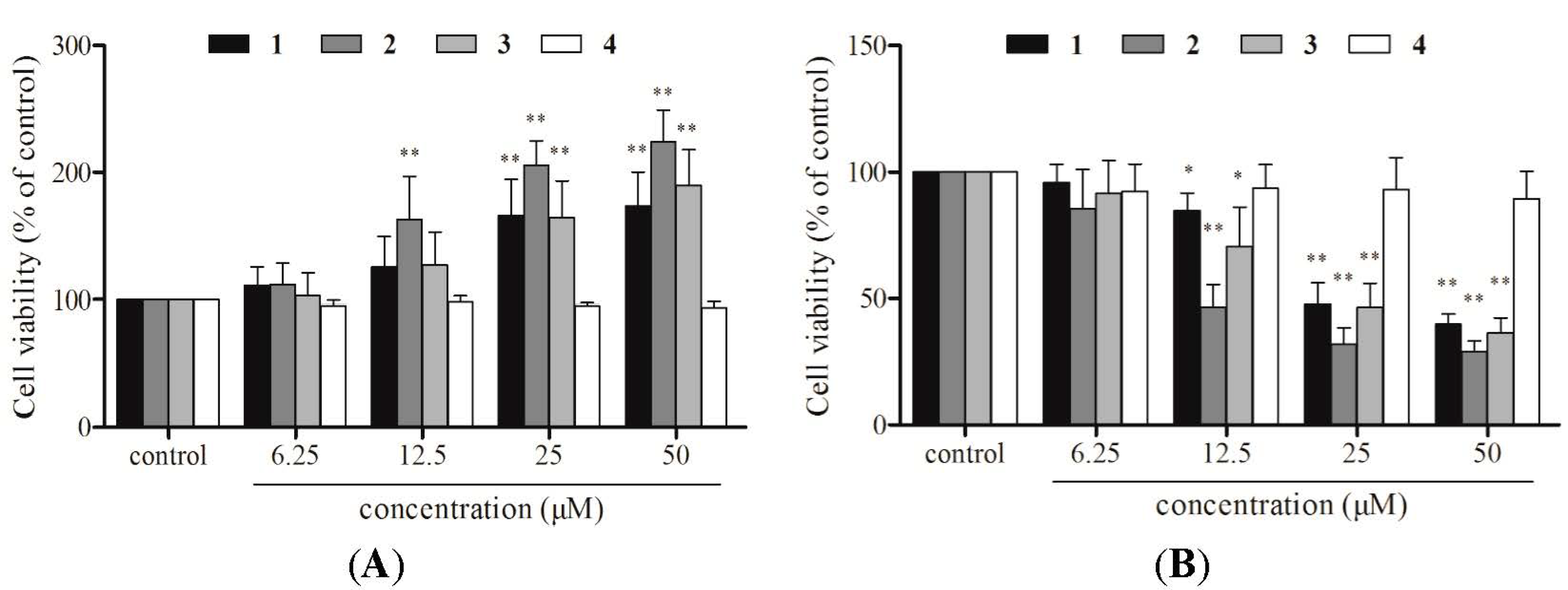
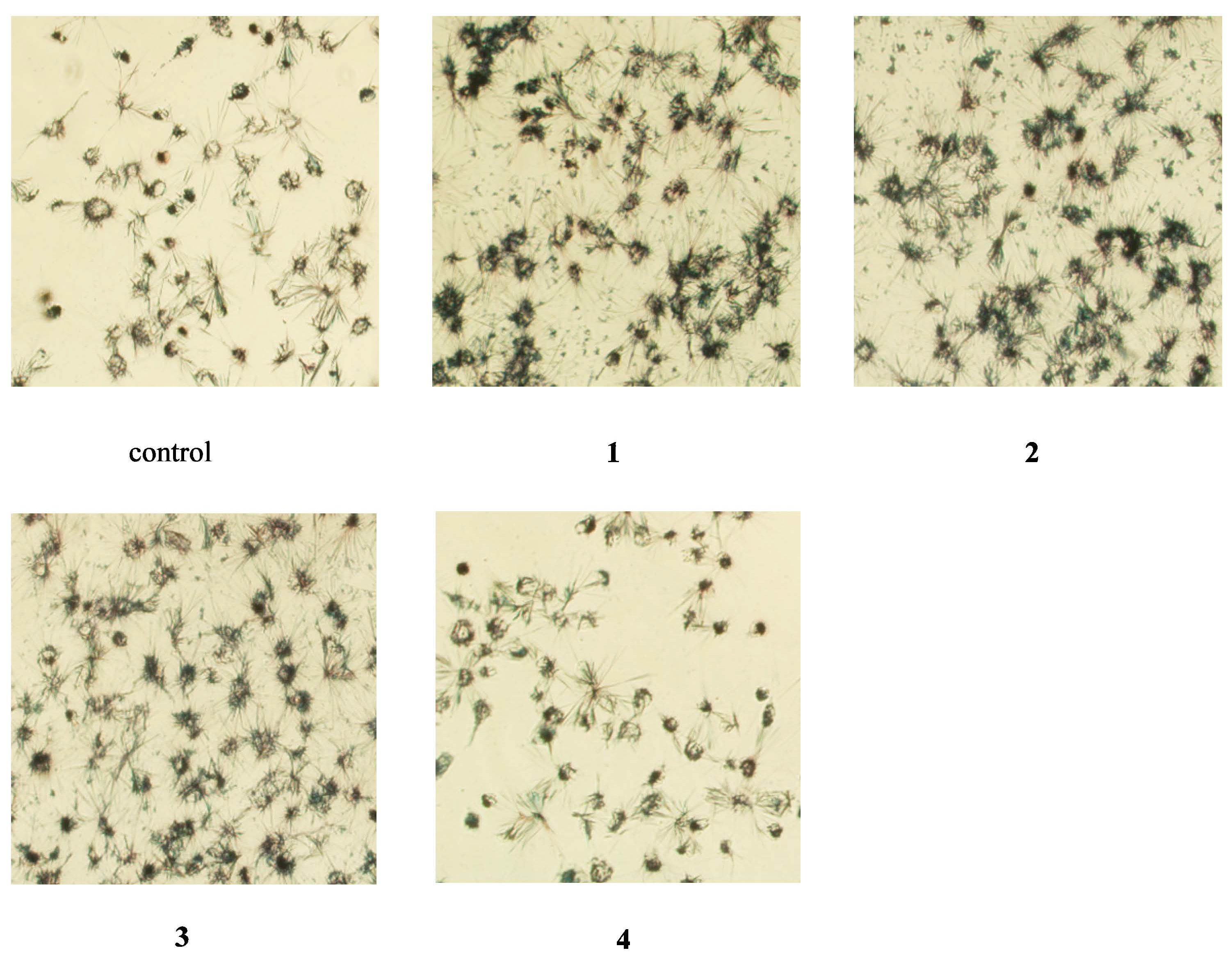
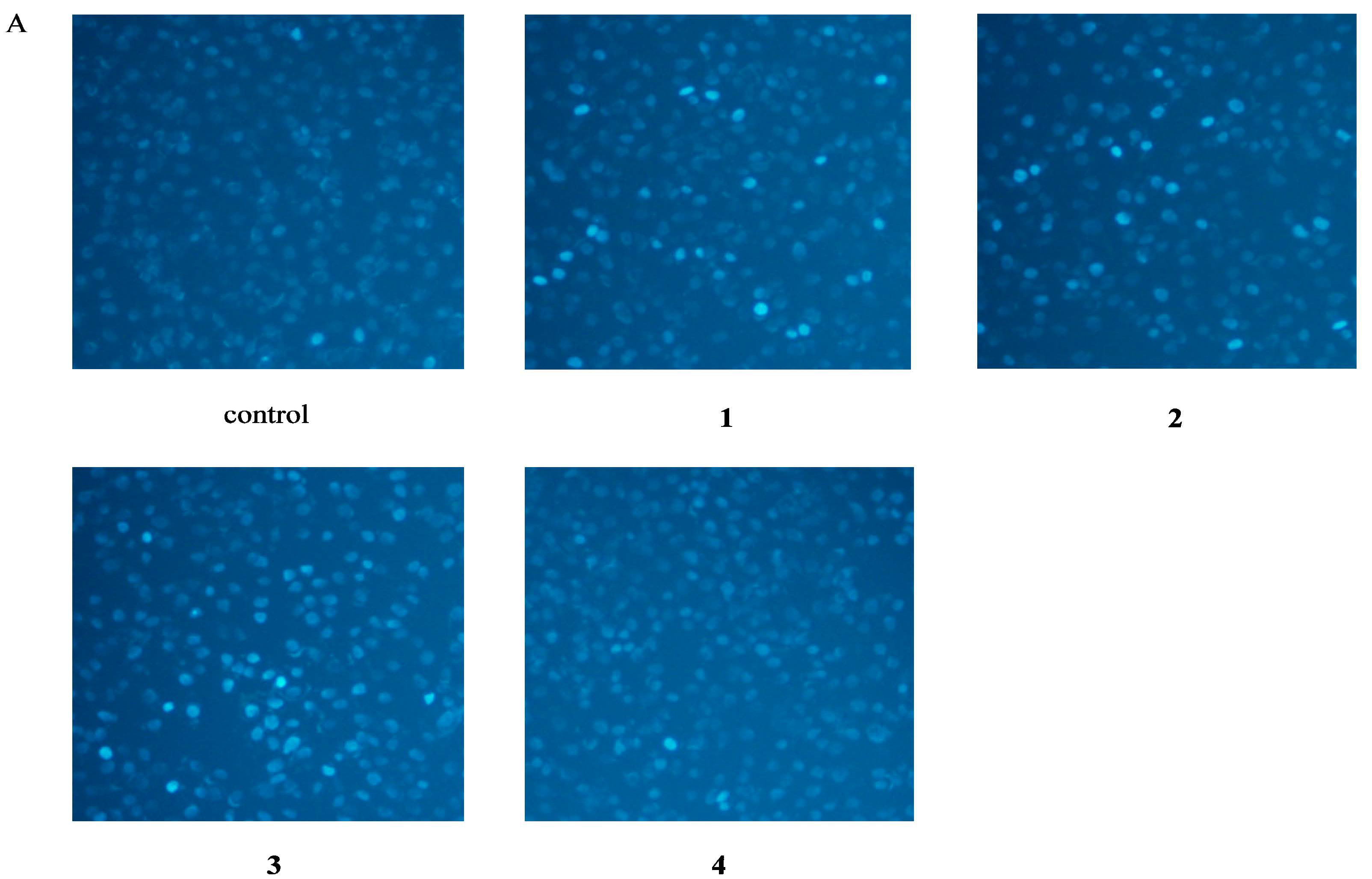
2.3. Apoptosis Assessment by Hoechst Staining
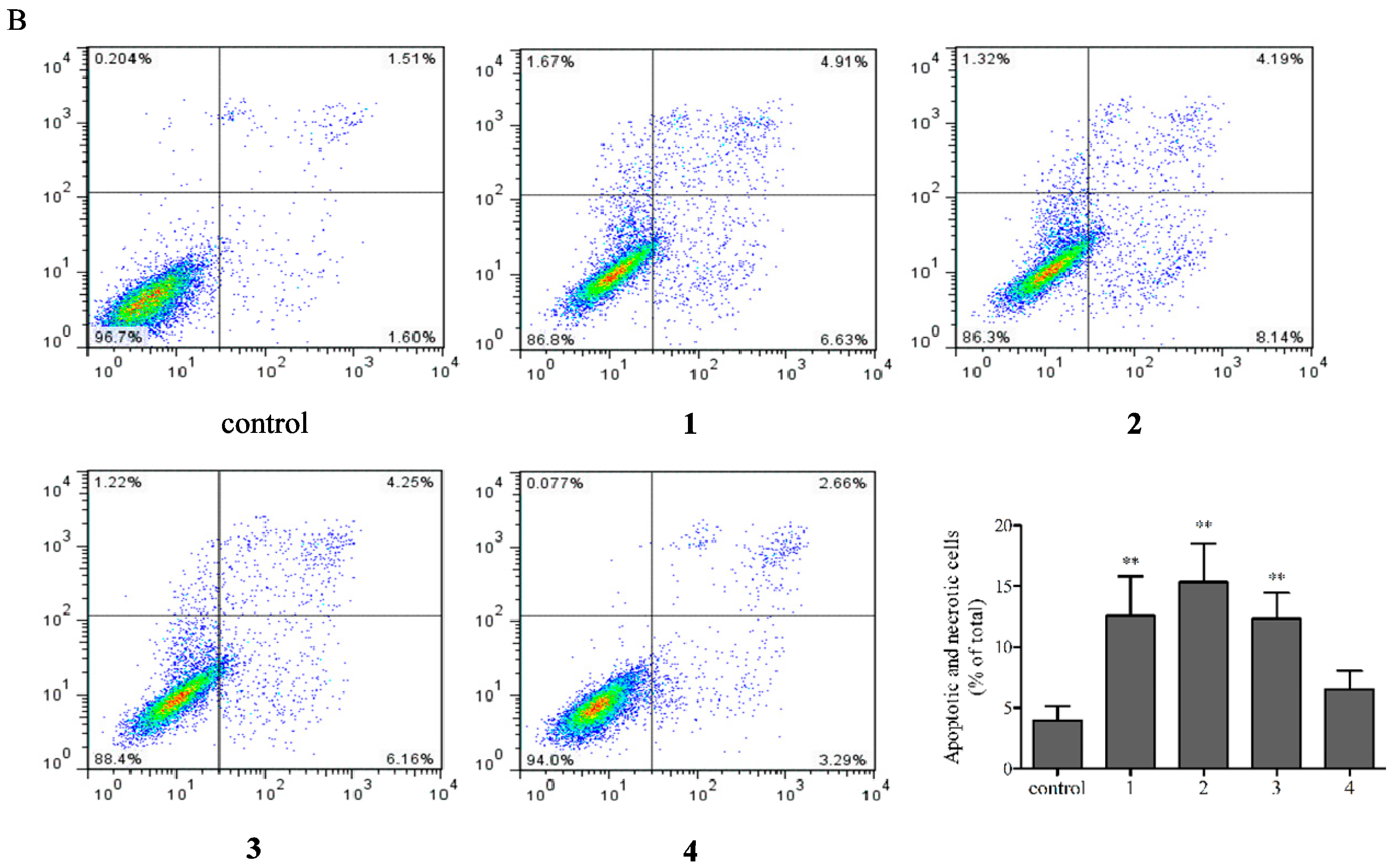
2.4. Flow Cytometric Analysis of Apoptotic Cells
2.5. Discussion
3. Experimental Section
3.1. Reagents and Chemicals
3.2. Plant Material
3.3. Extraction and Isolation of Plant Material
3.4. Cell Culture
3.5. Cell Viability
3.6. Apoptosis Assessment by Hoechst 33342 Staining
3.7. Apoptosis Analysis by Flow Cytometry
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kobayashi, H.; Oguchi, H.; Takizawa, N.; Miyase, T.; Ueno, A.; Usmanghani, K.; Ahmad, M. New phenylethanoid glycosides from Cistanche tubulosa (SCHRENK) HOOK. f. I. Chem. Pharm. Bull. 1987, 35, 3309–3314. [Google Scholar] [CrossRef]
- Editorial Committee of Chinese Pharmacopoeia. Chinese Pharmacopoeia. Medical Science and Technology Press: Beijing, China, 2010; Volume 1, p. 126. [Google Scholar]
- Wuliya, Y.; Wang, X.W.; Zaoranmu, N. The protective effect of the glycosides of Cistanche on the cerebral hypoxia in mice. J. Xinjiang. Med. Univ. 2003, 26, 561–562. [Google Scholar]
- Morikawa, T.; Pan, Y.N.; Ninomiya, K.; Imura, K.; Matsuda, H.; Yoshikawa, M.; Yuan, D.; Muraoka, O. Acylated phenylethanoid oligoglycosides with hepatoprotective activity from the desert plant Cistanche tubulosa. Bioorg. Med. Chem. 2010, 18, 1882–1890. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tsao, R.; Yang, R.; Liu, C.M.; Young, J.C.; Zhu, H.H. Isolation and purification of phenylethanoid glycosides from Cistanche deserticola by high-speed counter-current chromatography. Food Chem. 2010, 108, 702–710. [Google Scholar] [CrossRef]
- Gong, Y.; Wu, J.; Huang, Y.F.; Shen, S.N.; Han, X.D. Nonylphenol induces apoptosis in rat testicular Sertoli cells via endoplasmic reticulum stress. Toxicol. Lett. 2009, 186, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Jaruga, E.; Salvioli, S.; Dobrucki, J.; Chrul, S.; Bandorowicz-Pikula, J.; Sikora, E.; Franceschi, C.; Cossarizza, A.; Bartosz, G. Apoptosis-like, reversible changes in plasma membrane asymmetry and permeability, and transient modifications in mitochondrial membrane potential induced by curcumin in rat thymocytes. FEBS Lett. 1998, 433, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. Biotechnol. Annu. Rev. 2005, 11, 127–152. [Google Scholar] [PubMed]
- Liu, Y.B.; Peterson, D.A.; Kimura, H.; Schubert, D. Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J. Neurochem. 1997, 69, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.W.; Henning, S.M.; Heber, D. Limitations of MTT and MTS-based assays for measurement of antiproliferative activity of green tea polyphenols. PLoS ONE 2010, 5, e10202. [Google Scholar] [CrossRef] [PubMed]
- Ulukaya, E.; Colakogullari, M.; Wood, E.J. Interference by anti-cancer chemotherapeutic agents in the MTT-tumor chemosensitivity assay. Chemotherapy 2004, 50, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.T.; Plattner, R. MTT assays cannot be utilized to study the effects of STI571/Gleevec on the viability of solid tumor cell lines. Cancer Chemother Pharmacol. 2009, 64, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Bruggisser, R.; Daeniken, K.V.; Jundt, G.; Schaffner, W.; Tullberg-Reinert, H. Interference of plant extracts, phytoestrogens and antioxidants with the MTT tetrazolium assay. Planta Med. 2002, 68, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Paull, K.D.; Shoemaker, R.H.; Boyd, M.R.; Parsons, J.L.; Risbood, P.A.; Barbera, W.A.; Sharma, M.N.; Baker, D.C.; Hand, E.; Scudiero, D.A.; et al. The synthesis of XTT: A new tetrazolium reagent that is bioreducible to a water-soluble formazan. J. Heter. Chem. 1988, 25, 911–914. [Google Scholar] [CrossRef]
- Barltrop, J.A.; Owen, T.C; Cory, A.H.; Cory, J.G. 5-(3-Carboxymethoxyphenyl)-2-(4,5-Dimethylthiazolyl)-3-(4-sulfophenyl)tetrazolium, inner salt (MTS) and related analogs of 3-(4,5-dimethylthiazolyl)-2,5-diphenyltetrazolium bromide (MTT) reducing to purple water-soluble formazans as cell-viability indicators. Bioorg. Med. Chem. Lett. 1991, 1, 611–614. [Google Scholar] [CrossRef]
- Marshall, N.A.; Goodwin, C.J.; Holt, S.J. A critical assessment of the use of microculture tetrazolium assays to measure cell growth and function. Growth Regul. 1995, 5, 69–84. [Google Scholar] [PubMed]
- Ishiyama, M.; Shiga, M.; Sasamoto, K.; Mizoguchi, M.; He, P.G. A new sulfonated tetrazolium salt that produces a highly water-soluble formazan dye. Chem. Pharm. Bull. 1993, 41, 1118–1122. [Google Scholar] [CrossRef]
- Tominaga, H.; Ishiyama, M.; Ohseto, F.; Sasamoto, K.; Hamamoto, T.; Suzuki, K.; Watanabe, M. A water-soluble tetrazolium salt useful for colorimetric cell viability. Anal. Commun. 1999, 36, 47–50. [Google Scholar] [CrossRef]
- Tan, A.S.; Berridge, M.V. Tetrazolium dye reduction discriminates between mitochondrial and glycolytic metabolism. Redox Rep. 2004, 9, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, C.; Fortino, V.; Capurro, E.; Valacchi, G.; Pacini, A.; Muscettola, M.; Soucek, K.; Maioli, E. Rottlerin inhibits the nuclear factor kappaB/cyclin-D1 cascade in MCF-7 breast cancer cells. Life Sci. 2008, 82, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Maioli, E.; Torricelli, C.; Fortino, V.; Carlucci, F.; Tommassini, V.; Pacini, A. Critical appraisal of the MTT assay in the presence of rottlerin and uncouplers. Biol. Proced. Online 2009, 11, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Heng, B.C.; Zhao, X.X.; Xiong, S.J.; Ng, K.W.; Boey, F.Y.; Loo, J.S. Toxicity of zinc oxide (ZnO) nanoparticles on human bronchial epithelial cells (BEAS-2B) is accentuated by oxidative stress. Food Chem. Toxicol. 2010, 48, 1762–1766. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Sample of the compounds 1 and 2 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-J.; Zhou, S.-M.; Xu, G.; Gao, Y.-Q. Interference of Phenylethanoid Glycosides from Cistanche tubulosa with the MTT Assay. Molecules 2015, 20, 8060-8071. https://doi.org/10.3390/molecules20058060
Wang Y-J, Zhou S-M, Xu G, Gao Y-Q. Interference of Phenylethanoid Glycosides from Cistanche tubulosa with the MTT Assay. Molecules. 2015; 20(5):8060-8071. https://doi.org/10.3390/molecules20058060
Chicago/Turabian StyleWang, Yu-Jie, Si-Min Zhou, Gang Xu, and Yu-Qi Gao. 2015. "Interference of Phenylethanoid Glycosides from Cistanche tubulosa with the MTT Assay" Molecules 20, no. 5: 8060-8071. https://doi.org/10.3390/molecules20058060
APA StyleWang, Y.-J., Zhou, S.-M., Xu, G., & Gao, Y.-Q. (2015). Interference of Phenylethanoid Glycosides from Cistanche tubulosa with the MTT Assay. Molecules, 20(5), 8060-8071. https://doi.org/10.3390/molecules20058060






