Employing Response Surface Methodology for the Optimization of Ultrasound Assisted Extraction of Lutein and β-Carotene from Spinach
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chromatographic Separation and Image Analysis Software
2.2. Fitting the Models
2.3. Effect of Ultrasonic Parameters on Lutein and β-Carotene Contents of Spinach and Analysis of Response Surfaces
| Run | Factor X1: Temperature (°C) | Factor X2: Power (%) | Factor X3: Time (min) | Lutein μg/g | β-Carotene μg/g | ||
|---|---|---|---|---|---|---|---|
| Actual | Predicted | Actual | Predicted | ||||
| 1 | 40 | 30 | 10 | 2.08 | 2.05 | 3.10 | 3.07 |
| 2 | 50 | 50 | 10 | 1.92 | 1.91 | 2.90 | 2.88 |
| 3 | 40 | 70 | 10 | 1.82 | 1.85 | 2.80 | 2.82 |
| 4 | 30 | 50 | 30 | 1.88 | 1.89 | 2.86 | 2.88 |
| 5 | 40 | 50 | 20 | 2.04 | 2.06 | 3.08 | 3.10 |
| 6 | 40 | 70 | 30 | 1.97 | 1.98 | 2.93 | 2.96 |
| 7 | 50 | 50 | 30 | 1.84 | 1.85 | 2.81 | 2.78 |
| 8 | 30 | 30 | 20 | 1.87 | 1.89 | 2.90 | 2.90 |
| 9 | 40 | 50 | 20 | 2.07 | 2.06 | 3.11 | 3.10 |
| 10 | 50 | 70 | 20 | 1.83 | 1.81 | 2.80 | 2.80 |
| 11 | 30 | 50 | 10 | 1.88 | 1.87 | 2.82 | 2.85 |
| 12 | 30 | 70 | 20 | 1.85 | 1.83 | 2.91 | 2.86 |
| 13 | 40 | 50 | 20 | 2.09 | 2.06 | 3.12 | 3.10 |
| 14 | 50 | 30 | 20 | 1.87 | 1.89 | 2.84 | 2.89 |
| 15 | 40 | 50 | 20 | 2.03 | 2.06 | 3.06 | 3.10 |
| 16 | 40 | 30 | 30 | 1.92 | 1.89 | 2.88 | 2.86 |
| 17 | 40 | 50 | 20 | 2.09 | 2.06 | 3.12 | 3.10 |
| Source | Degree of Freedom | Sum of Square | Mean Square | f-Value | p-Value |
|---|---|---|---|---|---|
| lutein | |||||
| Model | 9 | 0.1560 | 0.017 | 18.40 | 0.0004 |
| X1 | 1 | 4.9999 | 4.9999 | 0.053 | 0.8244 |
| X2 | 1 | 0.0091 | 0.0091 | 9.67 | 0.0171 |
| X3 | 1 | 0.0010 | 0.0010 | 1.07 | 0.3344 |
| X1X2 | 1 | 0.0001 | 0.0001 | 0.11 | 0.7541 |
| X1X3 | 1 | 0.0016 | 0.0016 | 1.70 | 0.2337 |
| X2X3 | 1 | 0.0240 | 0.0240 | 25.50 | 0.0015 |
| 1 | 0.0804 | 0.0804 | 85.42 | <0.0001 | |
| 1 | 0.0210 | 0.0210 | 22.37 | 0.0021 | |
| 1 | 0.0088 | 0.0088 | 9.35 | 0.0184 | |
| Lack of fit | 3 | 3.475 | 0.0011 | 1.49 | 0.3461 |
| β-carotene | |||||
| Model | 9 | 0.2336 | 0.026 | 12.86 | 0.0014 |
| X1 | 1 | 0.00245 | 0.00245 | 1.21 | 0.3070 |
| X2 | 1 | 0.0097 | 0.0097 | 4.85 | 0.0434 |
| X3 | 1 | 0.00245 | 0.00245 | 1.21 | 0.3070 |
| X1X2 | 1 | 0.0006 | 0.0006 | 0.31 | 0.5952 |
| X1X3 | 1 | 0.004225 | 0.004225 | 2.09 | 0.1912 |
| X2X3 | 1 | 0.030625 | 0.030625 | 15.17 | 0.0059 |
| 1 | 0.1047 | 0.1047 | 51.91 | 0.0002 | |
| 1 | 0.0254 | 0.0254 | 12.61 | 0.0093 | |
| 1 | 0.0362 | 0.0362 | 17.94 | 0.0039 | |
| Lack of fit | 3 | 0.011 | 3.750 | 5.21 | 0.0724 |
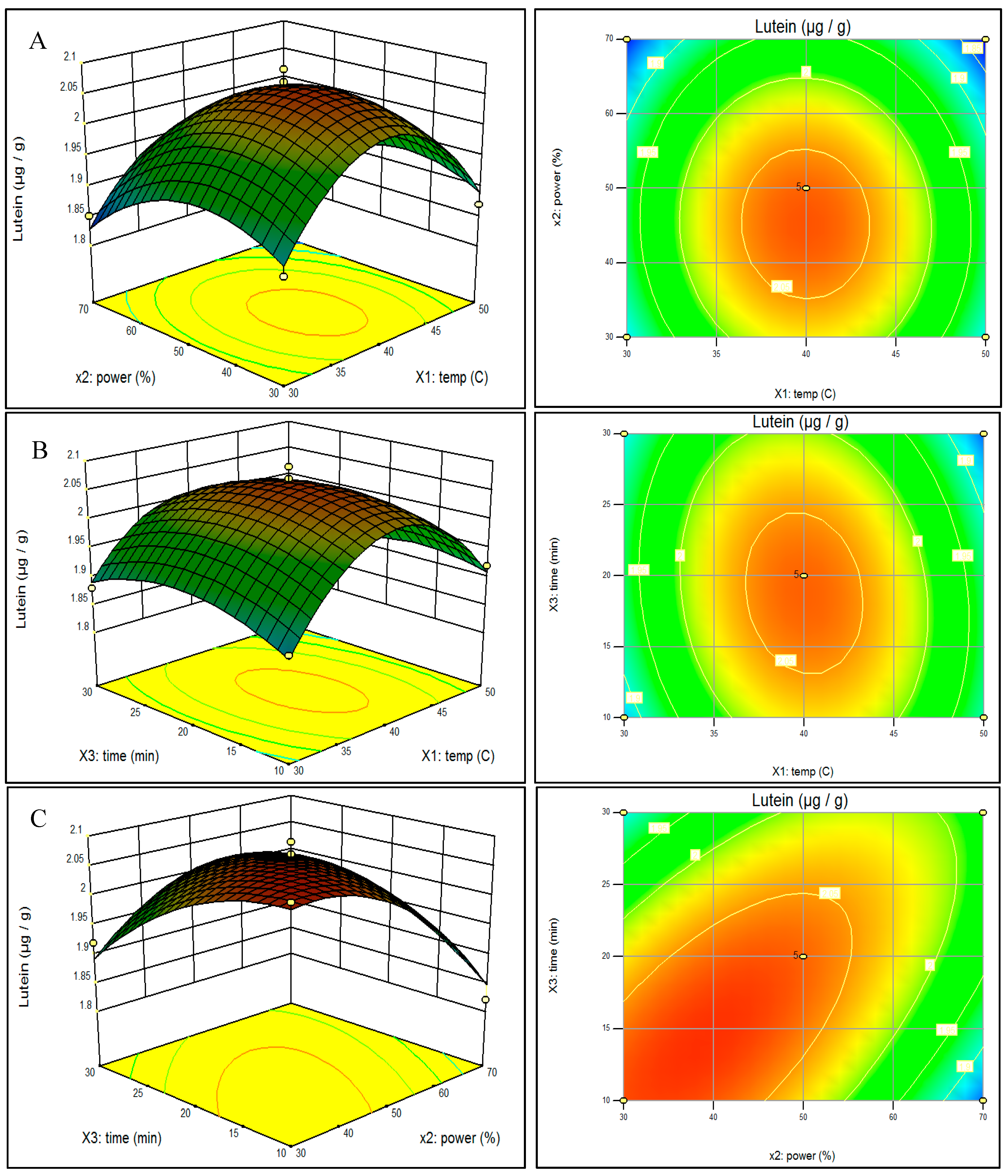
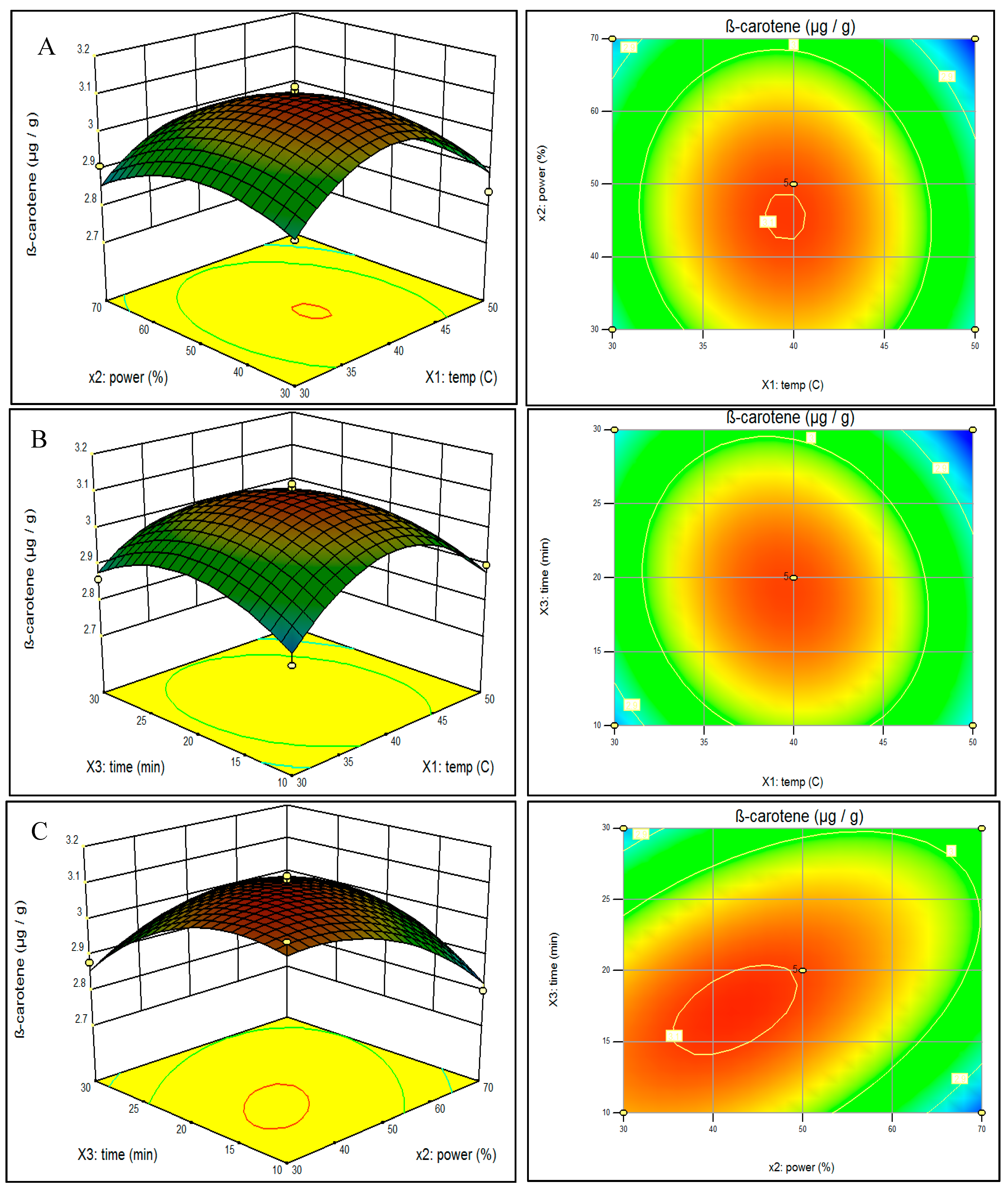
2.4. Optimization and Verification of the Model for Ultrasonic Parameters
| Name | Extraction Variables | Lutein (LS) | β-Carotene (BS) | ||
|---|---|---|---|---|---|
| X1 (°C) | X2 (%) | X3 (min) | |||
| Optimum conditions( predicted) | 40.14 | 41.13 | 16.21 | 2.07 | 3.10 |
| Modified optimal condition (experimental values) * | 40 | 40 | 16 | 2.01 ± 0.040 | 3.07 ± 0.02 |
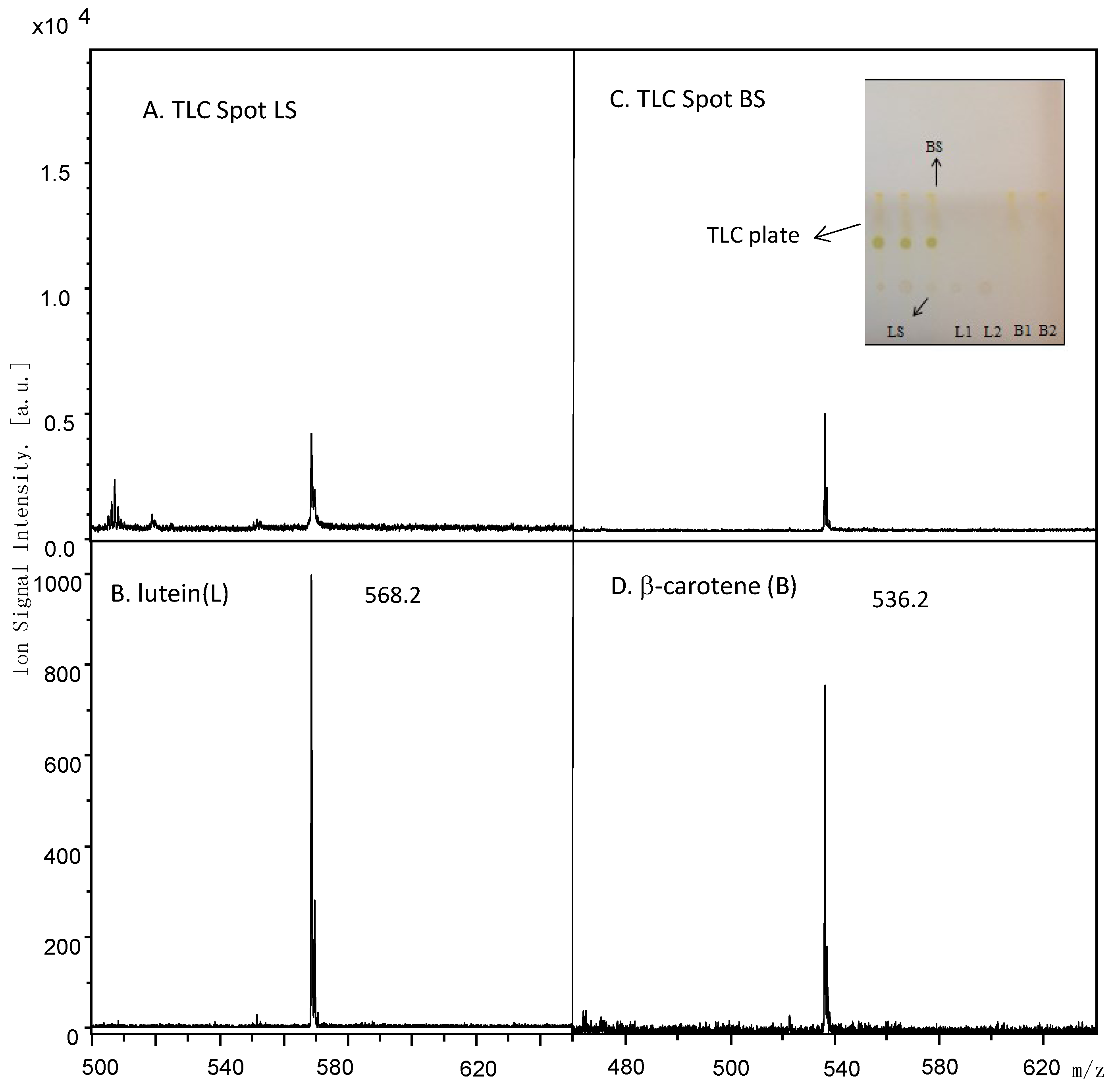
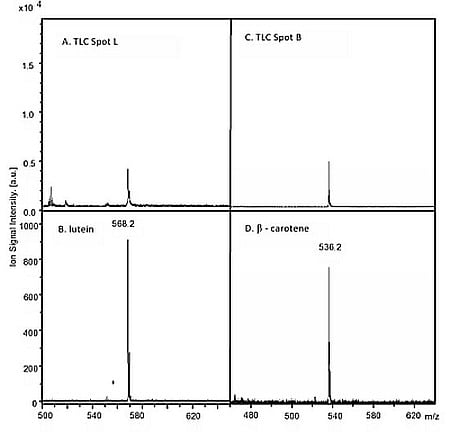
2.5. MALDI Identification
3. Experimental Section
3.1. Spinach Material
3.2. Ultrasonic–Assisted Extraction (UAE)
3.3. Experimental Design
| Symbols | Independent Variables | −1 | 0 | 1 |
|---|---|---|---|---|
| X1 | Temperature (°C) | 30 | 40 | 50 |
| X2 | Power (%) | 30 | 50 | 70 |
| X3 | Time (min) | 10 | 20 | 30 |
3.4. Thin Layer Chromatography Chemical Screening
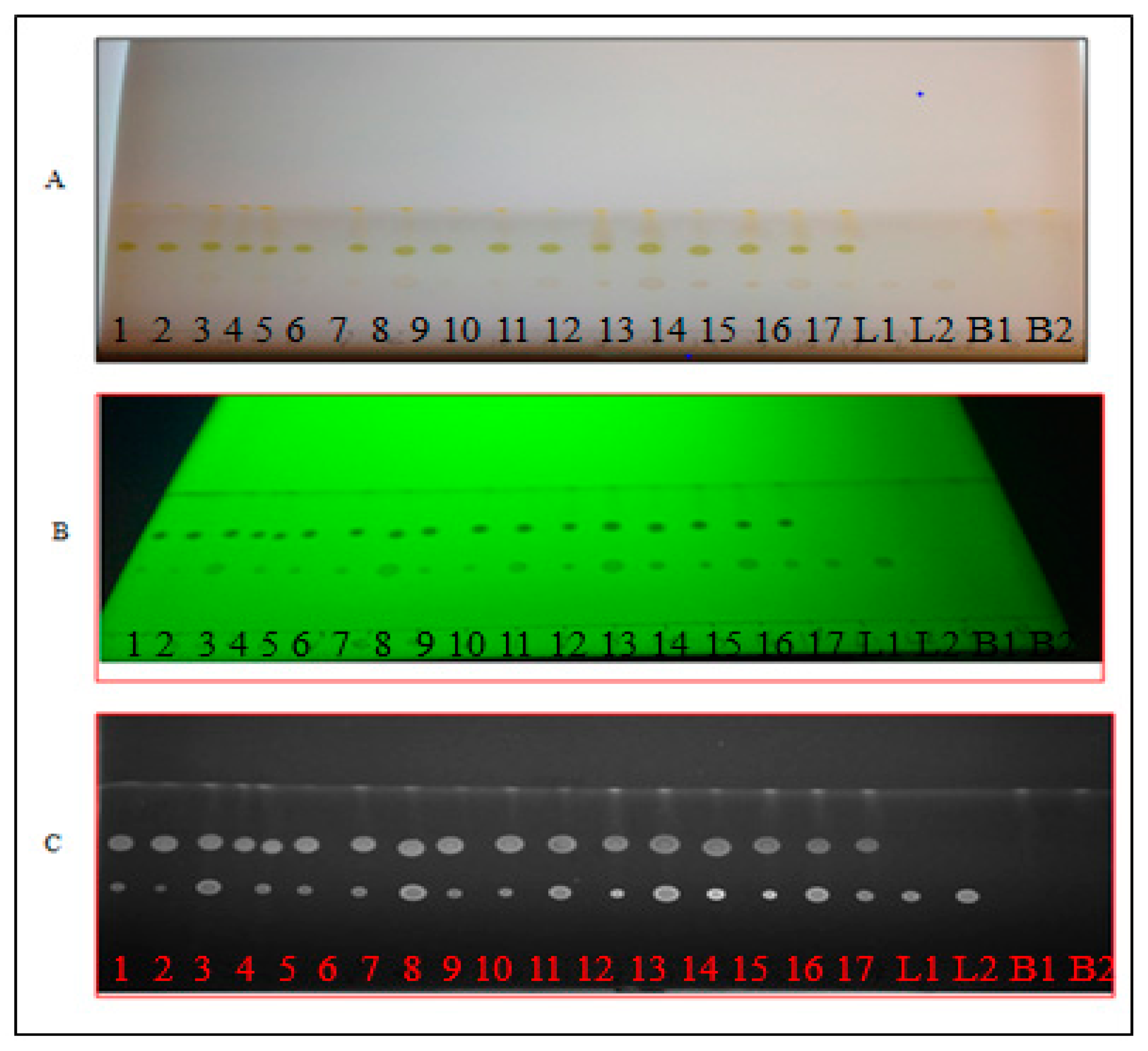
3.5. Preparation of Calibration Curves for Lutein and β-Carotene
3.6. Simultaneous Quantification of Lutein and β-Carotene in the Spinach Extracts
3.7. RSM Model and Validity Testing
3.8. Mass Spectrometric Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gurib-Fakim, A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Asp. Med. 2006, 27, 1–93. [Google Scholar] [CrossRef]
- Nassr-Allah, A.A.; Aboul-Enein, A.M.; Aboul-Enein, K.M.; Lightfoot, D.A.; Cocchetto, A.; El-Shemy, H.A. Anti-cancer and anti-oxidant activity of some Egyptian medicinal plants. J. Med. Plants Res. 2009, 3, 799–808. [Google Scholar]
- El-Shemy, H.A.; Aboul-Enein, K.M.; Lightfoot, D.A. Predicting In Silico Which Mixtures of the Natural Products of Plants Might Most Effectively Kill Human Leukemia Cells? Evid. Based Complement. Altern. Med. 2013, 2013, 801501. [Google Scholar] [CrossRef]
- Oliver, J.; Palou, A. Chromatographic determination of carotenoids in foods. J. Chromatogr. A. 2000, 881, 543–555. [Google Scholar] [CrossRef] [PubMed]
- West, B.J.; Deng, S. Thin layer chromatography methods for rapid identity testing of Morinda citrifolia L. (Noni) fruit and leaf. Adv. J. Food Sci. Technol. 2010, 2, 298–302. [Google Scholar]
- Karnjanawipagul, P.; Nittayanuntawech, W.; Rojsanga, P.; Suntornsuk, L. Analysis of β-carotene in carrot by spectrophotometry. Mahidol Univ. J. Pharm. Sci. 2010, 37, 8–16. [Google Scholar]
- Bunea, A.; Andjelkovic, M.; Socaciu, C.; Bobis, O.; Neacsu, M.; Verhe, R. Total and individual carotenoids and phenolic acids content in fresh, refrigerated and processed spinach (Spinacia oleracea L.). Food Chem. 2008, 108, 649–656. [Google Scholar] [CrossRef]
- Lakshminarayana, R.; Aruna, G.; Sangeetha, R.K.; Bhaskar, N.; Divakar, S.; Baskaran, V. Possible degradation/biotransformation of lutein in vitro and in vivo: Isolation and structural elucidation of lutein metabolites by HPLC and LC-MS (atmospheric pressure chemical ionization). Free Radic. Biol. Med. 2008, 45, 982–993. [Google Scholar] [CrossRef] [PubMed]
- Olmedilla, B.; Granado, F.; Blanco, I.; Vaquero, M. Lutein, but notalpha-tocopherol, supplementation improves visual function in patients with age-related cataracts: A 2-year double blind, placebo-controlled pilot study. Nutrition 2003, 19, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Seddon, J.M.; Ajani, U.A.; Sperduto, R.D.; Hiller, R.; Blair, N.; Burton, T.C.; Farber, M.D.; Gragoudas, E.S.; Haller, J.; Miller, D.T.; et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. JAMA 1994, 272, 1413–1420. [Google Scholar]
- Pratt, D.E.; Watts, B.M. The antioxidant activity of vegetable extracts I. flavone aglycones. J. Food Sci. 1964, 29, 27–33. [Google Scholar] [CrossRef]
- Zeb, A.; Murkovic, M. Thin-layer chromatographic analysis of carotenoids in plant and animal samples. JPC-J. Planar Chromat. 2011, 23, 94–103. [Google Scholar] [CrossRef]
- Chaowuttikul, C.; Thitikornpong, W.; Palanuvej, C. Quantitative determination of usnic acid content in usnea siamensis by TLC-densitometry and TLC image analysis. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 118–125. [Google Scholar]
- Berkov, S.; Pavlov, A. A rapid densitometric method for the analysis of hyoscyamine and scopolamine in solanaceous plants and their transformed root cultures. Phytochem. Anal. 2004, 15, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Cieśla, Ł.; Staszek, D.; Kowalska, T.; Waksmundzka-Hajnos, M. The use of TLC-DPPH test with image processing to study direct antioxidant activity of phenolic acid fractions of selected Lamiaceae family species. J. AOAC Int. 2013, 96, 1228–1232. [Google Scholar] [CrossRef] [PubMed]
- Tokusoglu, Ö.; Ünal, M.K.; Yildirim, Z. HPLC-UV and GC-MS charachterization of the flavonol aglycons quercetin, kaempferol, and myricetin in tomato pastes and other tomato-based products. Acta Chromatogr. 2003, 13, 196–207. [Google Scholar]
- Abdel-gawad, F.M.; Issa, Y.M.; Hussien, E.M.; Ibrahim, M.M.; Barakat, S. Simple and accurate RP-HPLC and TLC-Densitometric methods for determination of carvedilol in pharmaceutical formulations. Int. J. Res. Pharm. Chem. 2012, 2, 741–748. [Google Scholar]
- Chemat, F.; Zill-e-Huma; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Nikolova, M.; Berkov, S.; Ivancheva, S. A rapid TLC method for analysis of external flavonoid aglycones in plant exudates. Acta Chromatogr. 2004, 14, 110–114. [Google Scholar]
- Lee, L.-S.; Lee, N.; Kim, Y.H.; Lee, C.-H.; Hong, S.P.; Jeon, Y.-W.; Kim, Y.-E. Optimization of ultrasonic extraction of phenolic antioxidants from green tea using response surface methodology. Molecules 2013, 18, 13530–13545. [Google Scholar] [CrossRef] [PubMed]
- Trinh, T.K.; Kang, L. Application of response surface method as an experimental design to optimize coagulation tests. Environ. Eng. Res. 2010, 15, 63–70. [Google Scholar] [CrossRef]
- Zhao, Y.; Hou, Y.; Tang, G.; Cai, E.; Liu, S.; Yang, H.; Zhang, L.; Wang, S. Optimization of ultrasonic extraction of phenolic compounds from Epimedium brevicornum maxim using response surface methodology and evaluation of its antioxidant activities in vitro. J. Anal. Methods Chem. 2014, 2014, 864654. [Google Scholar] [PubMed]
- Palma, M.; Taylor, L.T. Extraction of polyphenolic compounds from grape seeds with near critical carbon dioxide. J. Chromatogr. A 1999, 849, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Karabegović, I.T.; Stojičević, S.S.; Veličković, D.T.; Nikolić, N.Č.; Lazić, M.L. Optimization of microwave-assisted extraction of cherry laurel fruit. Sep. Sci. Technol. 2014, 49, 416–423. [Google Scholar]
- Rebecca, L.J.; Sharmila, S.; Paul Das, M.; Seshiah, C. Extraction and purification of carotenoids from vegetables. J. Chem. Pharm. Res. 2014, 6, 594–598. [Google Scholar]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.; Choudhary, R.; Watson, D.G.; Lightfoot, D.A. Effects of ultrasonic treatments on the polyphenol and antioxidant content of spinach extracts. Ultrason. Sonochem. 2015, 24, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Harbone, J. Phytochemical Method, 2nd ed.; Chapman Hall: New York, NY, USA, 1986. [Google Scholar]
- Jaime, L.; Mendiola, J.A.; Herrero, M.; Soler-Rivas, C.; Santoyo, S.; Señorans, F.J.; Ibáñez, E. Separation and characterization of antioxidants from Spirulina platensis microalga combining pressurized liquid extraction, TLC, and HPLC-DAD. J. Sep. Sci. 2005, 28, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Khachik, F.; Beltsville, M. Process for Extraction, Purification of Lutein, Zeaxanthin and Rare Carotenoids from Marigold Flowers and Plants. US Patent 6,262,284, 17 July 2001. [Google Scholar]
- Alisa, P.; Helen, R.; Elizabeth, J.J. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J. Food Compos. Anal. 2009, 22, 9–15. [Google Scholar] [CrossRef]
- Vechpanich, J.; Shotipruk, A. Recovery of free lutein from Tagetes erecta: Determination of suitable saponification and crystallization conditions. Sep. Sci. Technol. 2011, 46, 265–271. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the extracts are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altemimi, A.; Lightfoot, D.A.; Kinsel, M.; Watson, D.G. Employing Response Surface Methodology for the Optimization of Ultrasound Assisted Extraction of Lutein and β-Carotene from Spinach. Molecules 2015, 20, 6611-6625. https://doi.org/10.3390/molecules20046611
Altemimi A, Lightfoot DA, Kinsel M, Watson DG. Employing Response Surface Methodology for the Optimization of Ultrasound Assisted Extraction of Lutein and β-Carotene from Spinach. Molecules. 2015; 20(4):6611-6625. https://doi.org/10.3390/molecules20046611
Chicago/Turabian StyleAltemimi, Ammar, David A. Lightfoot, Mary Kinsel, and Dennis G. Watson. 2015. "Employing Response Surface Methodology for the Optimization of Ultrasound Assisted Extraction of Lutein and β-Carotene from Spinach" Molecules 20, no. 4: 6611-6625. https://doi.org/10.3390/molecules20046611
APA StyleAltemimi, A., Lightfoot, D. A., Kinsel, M., & Watson, D. G. (2015). Employing Response Surface Methodology for the Optimization of Ultrasound Assisted Extraction of Lutein and β-Carotene from Spinach. Molecules, 20(4), 6611-6625. https://doi.org/10.3390/molecules20046611