A Trinuclear Oxo-Chromium(III) Complex Containing the Natural Flavonoid Primuletin: Synthesis, Characterization, and Antiradical Properties
Abstract
:1. Introduction
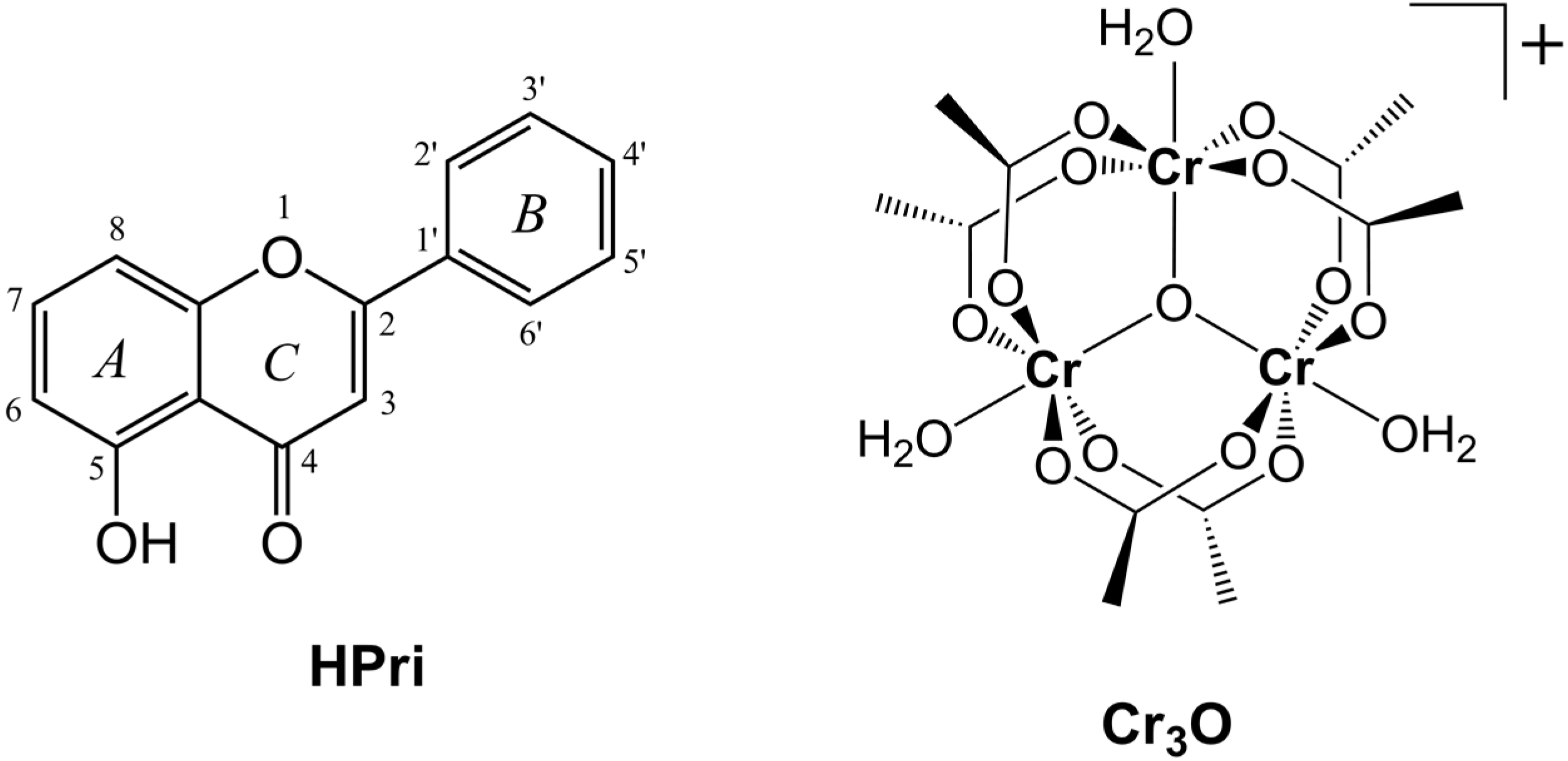
2. Results and Discussion
| Compound | Dehydration Process | Decomposition Process | ||||
|---|---|---|---|---|---|---|
| ΔT (°C) | Δmexp (%) | Δmcalc (%) | ΔT (°C) | Δmexp (%) | Δmcalc (%) | |
| Cr3O | 25–158 | 14.5 | 14.4 | 158–600 | 39.5 | 38.3 (4Ac−) |
| HPri | – | – | – | 190–232 | 100 | 100 |
| Cr3O-Pri | 25–97 | 4.5 | 4.6 | 97–272 | 7.4 | 7.4 (1Ac−) |
| 272–585 | 28.9 | 29.7 (Pri) | ||||
| 585–860 | 22.2 | 22.1 (3Ac−) | ||||

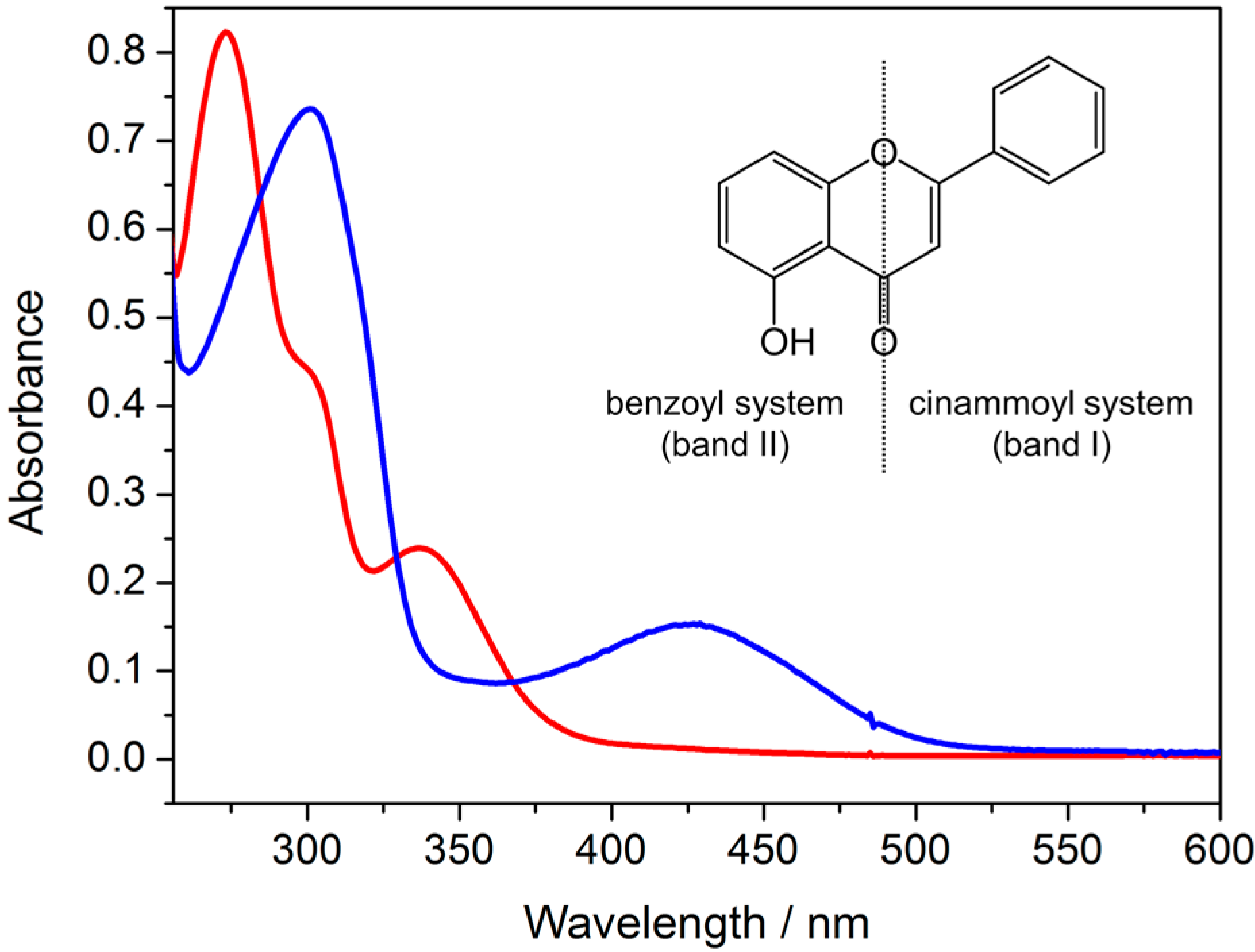
| Compound | Band II (π→π *) | Band I (π→π *) | Ligand Field (d→d) |
|---|---|---|---|
| HPri | 273 (2.8 × 104) | 337 (8.0 × 103) | – |
| Cr3O-Pri | 301 (2.9 × 104) | 426 (8.1 × 103) | – (a) |
| Cr3O | – | – | 442, 588 (<100) |
| Compound | Flavonoid | Acetate | Cr-O | |||
|---|---|---|---|---|---|---|
| ν(C=O) | ν(C2=C3) | δ(C-OH) | νs(COO) | νas(COO) | ||
| Cr3O | – | – | – | 1450 vs | 1611 vs | 665 s 442 m |
| primuletin | 1655 vs | 1587 w | 1319 vw | – | – | – |
| Cr3O-Pri | 1626 vs | 1576 w | – | 1445 vs | 1593 m | 627 w |
| 525 w | ||||||
| Compound | ΔAbs | [DPPHseq] | [flav] | n |
|---|---|---|---|---|
| HPri | 0.017 | 1.50 × 10−6 | 1.01 × 10−5 | 0.155 |
| Cr3O-Pri | 0.020 | 1.77 × 10−6 | 2.50 × 10−6 | 0.734 |
3. Experimental Section
3.1. Synthesis of [Cr3O(CH3CO2)6(Pri)(H2O)2]
3.2. Computational Details
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Andersen, Ø.M.; Markham, K.R. Flavonoids: Chemistry, Biochemistry, and Applications; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2006. [Google Scholar]
- Perron, N.R.; Hodges, J.N.; Jenkins, M.; Brumaghim, J.L. Predicting How Polyphenol Antioxidants Prevent DNA Damage by Binding to Iron. Inorg. Chem. 2008, 47, 6153–6161. [Google Scholar] [CrossRef] [PubMed]
- Afanas’eva, I.B.; Ostrakhovitch, E.A.; Mikhal’chik, E.V.; Ibragimova, G.A.; Korkina, L.G. Enhancement of Antioxidant and Anti-inflammatory Activities of Bioflavonoid Rutin by Complexation with Transition Metals. Biochem. Pharmacol. 2001, 61, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.F.V.; Giovani, W.F. Antioxidant Properties of Complexes of Flavonoids with Metal Ions. Redox Rep. 2004, 9, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Z.; Li, T. Synthesis, Characterization, Antioxidative Activity and DNA Binding Properties of the Copper(II), Zinc(II), Nickel(II) Complexes with 1,2-Di(4'-iminonaringenin)ethane. Chem. Pharm. Bull. 2004, 56, 1528–1534. [Google Scholar] [CrossRef]
- Nguyen, A.; Mulyani, I.; Levina, A.; Lay, P.A. Reactivity of Chromium(III) Nutritional Supplements in Biological Media: An X-Ray Absorption Spectroscopic Study. Inorg. Chem. 2008, 47, 4299–4309. [Google Scholar] [CrossRef] [PubMed]
- Uemura, S.; Spencer, A.; Wilkinson, G. µ3-Oxotrimetal Acetato-Complexes of Chromium, Manganese, Iron, Cobalt, Rhodium, and Iridium. J. Chem. Soc. Dalton Trans. 1973, 2565–2571. [Google Scholar] [CrossRef]
- Geary, W.J. The Use of Conductivity Measurements in Organic Solvents for the Characterization of Coordination Compounds. Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
- Park, Y.; Moon, B.-H.; Lee, E.; Lee, Y.; Yoon, Y.; Ahn, J.-H.; Lim, Y. 1H and 13C-NMR Data of Hydroxyflavone Derivatives. Magn. Reson. Chem. 2007, 45, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Mabry, T.; Markham, K.R.; Thomas, M.B. The Systematic Investigation of Flavonoids; Springer: New York, NY, USA, 1970. [Google Scholar]
- Cannon, R.D.; White, R.P. Chemical and Physical Properties of Triangular Bridged Metal Complexes. Prog. Inorg. Chem. 1988, 36, 195–298. [Google Scholar]
- Anson, C.E.; Bourke, J.P.; Cannon, R.D.; Jayasooriya, U.A.; Molinier, M.; Powell, A.K. Crystal Structures of the Isomorphous Prototypic Oxo-Centered Trinuclear Complexes [Cr3O(OOCCH3)6(H2O)3]Cl·6H2O and [Fe3O(OOCCH3)6(H2O)3]Cl·6H2O. Inorg. Chem. 1997, 36, 1265–1267. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; van Horn, J.D. Breakdown Kinetics of the Tri-chromium(III) Oxo Acetate Cluster ([Cr3O(OAc)6]+) with Some Ligands of Biological Interest. J. Inorg. Biochem. 2007, 101, 329–335. [Google Scholar] [CrossRef]
- Markham, K.R. Techniques of Flavonoid Identification; Academic Press: London, UK, 1982. [Google Scholar]
- Teslova, T.; Corredor, C.; Livingstone, R.; Spataru, T.; Birke, R.L.; Lombardi, J.R.; Canãmares, M.V.; Leona, M. Raman and Surface-enhanced Raman Spectra of Flavone and Several Hydroxy Derivatives. J. Raman Spectrosc. 2007, 38, 802–818. [Google Scholar] [CrossRef]
- Hiraki, K.; Onishi, M.; Ikeda, T.; Tomioka, K.; Obayashi, Y. Syntheses of 5-Hydroxyflavone-Transition Metal Complexes. Bull. Chem. Soc. Jpn. 1978, 51, 2425–2426. [Google Scholar] [CrossRef]
- Uivarosi, V.; Badea, M.; Olar, R.; Draghici, C.; Barbuceanu, S.F. Synthesis and Characterization of Some New Complexes of Magnesium (II) and Zinc (II) with the Natural Flavonoid Primuletin. Molecules 2013, 18, 7631–7645. [Google Scholar] [CrossRef] [PubMed]
- Eckert, C.R. Determination of the Antiradical Activity of Flavonoids and Extracts of the Species Baccharis by the Chemiluminescent Luminol Reaction. Master of Science Dissertation, University of São Paulo, São Paulo, SP, Brazil, 2006. [Google Scholar]
- Liu, Z.-Q. Chemical Methods to Evaluate Antioxidant Ability. Chem. Rev. 2010, 110, 5675–5691. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Sun, S.; Cao, W.; Liang, Y.; Song, J. Antioxidant Property of Quercetin-Cr(III) Complex: The Role of Cr(III) Ion. J. Mol. Struct. 2009, 918, 194–197. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Sample Availability: Samples of the compounds reported herein are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexiou, A.D.P.; Decandio, C.C.; Almeida, S.D.N.; Ferreira, M.J.P.; Romoff, P.; Rocha, R.C. A Trinuclear Oxo-Chromium(III) Complex Containing the Natural Flavonoid Primuletin: Synthesis, Characterization, and Antiradical Properties. Molecules 2015, 20, 6310-6318. https://doi.org/10.3390/molecules20046310
Alexiou ADP, Decandio CC, Almeida SDN, Ferreira MJP, Romoff P, Rocha RC. A Trinuclear Oxo-Chromium(III) Complex Containing the Natural Flavonoid Primuletin: Synthesis, Characterization, and Antiradical Properties. Molecules. 2015; 20(4):6310-6318. https://doi.org/10.3390/molecules20046310
Chicago/Turabian StyleAlexiou, Anamaria D. P., Carla C. Decandio, Sabrina Da N. Almeida, Marcelo J. P. Ferreira, Paulete Romoff, and Reginaldo C. Rocha. 2015. "A Trinuclear Oxo-Chromium(III) Complex Containing the Natural Flavonoid Primuletin: Synthesis, Characterization, and Antiradical Properties" Molecules 20, no. 4: 6310-6318. https://doi.org/10.3390/molecules20046310
APA StyleAlexiou, A. D. P., Decandio, C. C., Almeida, S. D. N., Ferreira, M. J. P., Romoff, P., & Rocha, R. C. (2015). A Trinuclear Oxo-Chromium(III) Complex Containing the Natural Flavonoid Primuletin: Synthesis, Characterization, and Antiradical Properties. Molecules, 20(4), 6310-6318. https://doi.org/10.3390/molecules20046310






