Abstract
We have built a new isonucleoside derivative on a 2,6-dioxobicyclo[3.2.0]heptane skeleton as a potential anti-HIV agent. To synthesize the target compound, an acetal-protected dihydroxyacetone was first converted to a 2,3-epoxy-tetrahydrofuran derivative. Introduction of an azide group, followed by the formation of an oxetane ring, gave a pseudosugar derivative with a 2,6-dioxobicyclo[3.2.0]heptane skeleton. The desired isonucleoside was obtained by constructing a purine base moiety on the scaffold, followed by amination.
1. Introduction
Since the discovery of 3'-azidothymidine (AZT), much attention has been paid to the development of effective chemotherapeutic agents against the human immunodeficiency virus (HIV), a causative agent for AIDS [1,2]. More than 20 anti-HIV drugs have now been approved and are clinically used for the treatment of AIDS. Among them, nucleoside reverse transcriptase inhibitors (NRTIs) play a critical role in the treatment of AIDS patients. In the most successful regimen for AIDS referred to as ART (Anti-Retroviral Therapy), a cocktail of anti-HIV drugs, including NRTIs, non-nucleoside reverse transcriptase inhibitors (NNRTIs), and protease inhibitors (PIs) [3], is used. Although ART greatly contributes to increasing the lifespan of patients, drug-resistant strains of the virus are still a serious problem [4,5]. Therefore, new drugs that are effective against the resistant virus strains are constantly needed.
Most NRTIs belong to a category of dideoxynucleosides, e.g., zalcitabine (ddC) [6] and didanosine (ddI) [6]. AZT [7] and lamivudine [8] are 3'-substituted dideoxynucleoside derivatives, and abacavir is a carbocyclic analogue of dideoxynucleoside. Only tenofovir [9], which is a nucleoside phosphonate (Figure 1 and Figure 2), is different. From the viewpoint of designing new anti-HIV agents, nucleosides constructed on a novel scaffold are expected to have antiviral activity against the resistant virus strains and may avoid cross-resistance to the known NRTIs.
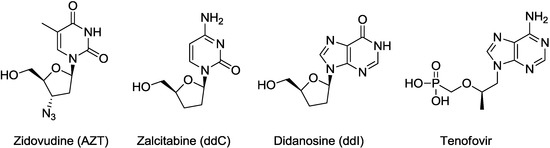
Figure 1.
Approved NRTIs.
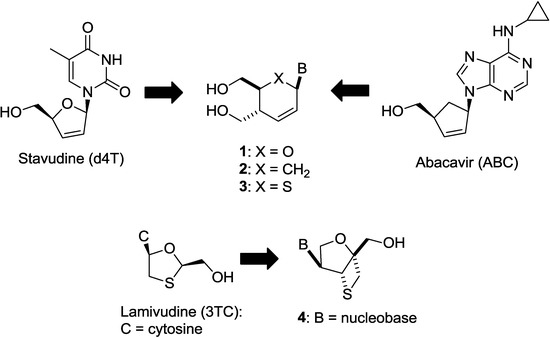
Figure 2.
Our previous works searching for anti-HIV nucleosides built on a new scaffold.
Thus, we have been focusing on the design and synthesis of nucleoside derivatives attached to a pseudosugar scaffold [10,11,12,13,14,15,16,17]. Among them, nucleosides with cyclohexenyl [13], dihydrothiophenyl [15], and dihydropyranyl [17] groups in place of a furanose ring have been synthesized as “ring-expanded” analogues of stavudine and abacavir. Dihydropyranyl derivative 1 did not show any activity, whereas cyclohexenyl derivative 2 showed weak anti-HIV activity [13,17]. On the other hand, dihydrothiophenyl derivative 3 showed significant anti-HIV activity [15]. In addition, we have applied the “ring-expanding” concept to lamivudine and synthesized isonucleosides 4 constructed on 2-oxa-6-thiabicyclo[3.2.0]heptane [14]. The isonucleoside 4 was also considered as conformationally-restricted analogue of lamivudine by introducing a fused thietane ring (vide infra). However, 4 showed no anti-HIV activity (Figure 2). In this study, we planned to build isonucleoside 6 on a 2,6-dioxobicyclo[3.2.0]heptane skeleton, an analogue of dioxolane nucleoside 5 which exhibited potent anti-HIV activity [18,19,20]. The similar conformationally-restricted analogue of d4T was known: cyclopropane-fused carbocyclic d4T (N-MCd4T), fixed in north conformation, was originally reported by Marquez and his colleagues and had significant anti-HIV activity with lesser cytotoxicity [21]. In addition, D-enantiomer of 5 was known to have potent cytotoxicity [18,19,20]. Thus, isonucleoside 6 should be promising although the thietane-fused derivative 4 was inactive (Figure 3).
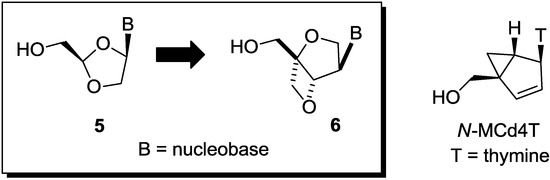
Figure 3.
Design of nucleoside derivative built on a 2,6-bicyclo[3.2.0]heptane skeleton.
2. Results and Discussion
Following our previous reports [11,14], epoxide 7 was synthesized. We first attempted to introduce an adenine onto 7 by treating it with DBU [22]. However, the reaction did not give the desired product 9 (Scheme 1).
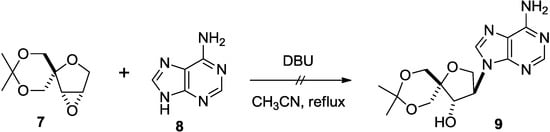
Scheme 1.
Attempt to introduce adenine moiety.
In addition, Lewis acid-catalyzed reactions did not afford 9 either (data not shown). Since the low reactivity of 7 might be due to its rigid structure, we next tried nucleophilic substitution using a more reactive cyclic sulfate derivative [23]. Cis-allyl alcohol 10, a precursor of epoxide 7 [11,14], was cyclized under Mitsunobu conditions, as in the case of epoxide 7 [11,14], to give dihydrofuran 11 in 71% yield. Treatment of dihydrofuran 11 with potassium osmate in the presence of N-methylmorpholine N-oxide afforded cis-diol 12. The desired cyclic sulfate 13 was obtained by treatment of 12 with thionyl chloride, followed by oxidation. However, the nucleophilic substitution of 13 with adenine did not afford the desired isonucleoside 14 (Scheme 2).
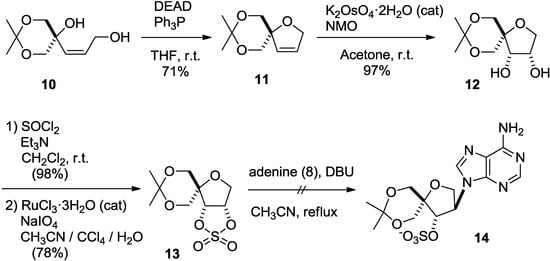
Scheme 2.
Second attempt to introduce adenine using a cyclic sulfate.
Therefore, we revised our plan to synthesize an isoadenosine constructed on a 2,6-dioxobicyclo[3.2.0]heptane scaffold, and the revised scheme is shown in Scheme 3 in a retrosynthetic manner. Instead of the direct introduction of adenine, we decided to build the adenine ring on the 2,6-dioxobicyclo[3.2.0]heptane pseudosugar skeleton in a stepwise manner. According to this plan, compound 16 was thought to be a suitable intermediate for preparing 15 since it can be transformed to 6 by the formation of an imidazole ring, followed by amination. Fused oxetane derivative 16 can be obtained from dimesylate 17. Finally, epoxide 7, described above, was selected as the starting compound because it can be converted to 17 by the selective cleavage of the oxirane ring with an azide anion (Scheme 2).
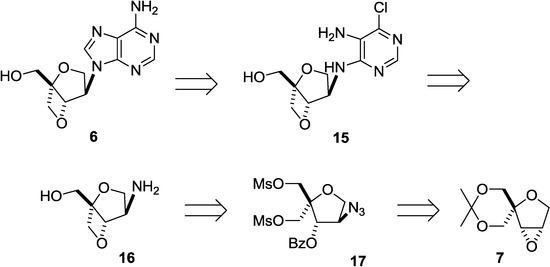
Scheme 3.
Revised retrosynthesis of isoadenosine 6 built on a 2,6-dioxobicyclo[3.2.0]heptane skeleton.
First, regioselective cleavage of the oxirane ring of 7 with sodium azide in 2-methoxyethanol under reflux conditions gave the desired azide-alcohol 18 as a single regioisomer in 66% yield. It is obvious that the nucleophilic azide anion attacked from the less hindered side since similar regioselective epoxide opening was observed in our previous report [11,14]. After benzoylation of the hydroxyl group, the acetal group of 19 was removed by using acidic hydrolysis, and the resulting diol was mesylated to give dimesylate 20 in good yield. Deprotection of the benzoyl group and the subsequent formation of an oxetane ring were achieved by treating 20 with sodium methoxide under reflux conditions to give mesylate 21 in 72% yield. The structure of 21 was unambiguously determined by comparison of 1D NMR spectrum with that of 2-oxa-6-thiabicyclo[3.2.0]heptane skeleton [14] after converting it to benzoate 22 by treatment with benzoic acid in the presence of cesium fluoride. In 1H-NMR spectra of 22, the peaks corresponding to the methyl groups of the dimesylate were absent, and only the peaks corresponding to the benzoyl group in the range of 8.1–7.4 ppm were present. In addition, one of the methylene protons at the 2-position was observed as a doublet at 4.42 ppm, meaning that the coupling with H-3 disappeared. This indicates that the conformation around the tetrahydrofuran ring changes and becomes fixed, which causes a loss of coupling between one pair of H-2 and H-3 protons. A similar correlation between conformation and couplings in 1H-NMR spectra has been reported for the 2-oxa-6-thiabicyclo[3.2.0]heptane skeleton [14]. Moreover, in the mass spectrum of the compound, a molecular ion peak was observed at m/z = 276, further supporting the assignment of the structure. Finally, 22 was deprotected to afford azido-alcohol 23 in 88% yield (Scheme 4).
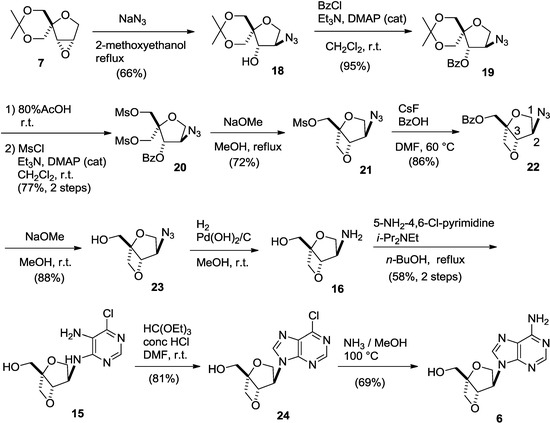
Scheme 4.
Synthesis of isoadenosine 6.
Azido-alcohol 23 was reduced by catalytic hydrogenation to give key intermediate 16, which was treated with 5-amino-4,6-dichloropyrimidine and diisopropylethylamine in refluxing n-butanol [23] to give diaminopyrimidine derivative 15 in 58% yield from 23. Formation of the imidazole ring of 15 was accomplished by treatment with orthoethyl formate under acidic conditions [24] to give 6-chloropurine nucleoside 24. Finally, the isoadenosine was built on the 2,6-dioxobicyclo[3.2.0]heptane scaffold 6 by heating 24 with methanolic ammonia in a sealed tube in 69% yield (Scheme 4). Isoadenosine 6 did not show any significant activity against HIV even at a concentration of 100 µM.
3. Experimental Section
General Information
Melting points are uncorrected. NMR spectra were recorded at 400 MHz (1H), 100 MHz (13C) using CDCl3 as a solvent. As an internal standard, tetramethylsilane was used for CDCl3. Mass spectra were obtained by EI or FAB mode. Silica gel for chromatography was Silica Gel 60N (spherical, neutral, 100–210 µm, Kanto Chemical Co. Inc., Tokyo, Japan). When the reagents sensitive to moisture were used, the reaction was performed under argon atmosphere.
8,8-Dimethyl-1,7,9-trioxaspiro[4,5]dec-3-ene (11). To a solution of PPh3 (2.49 g, 9.48 mmol) in THF (10 mL) was added DEAD (4.31 mL, 9.48 mmol) and the mixture was stirred at room temperature for 5 min. To this mixture, a solution of 10 [11,14] (1.04 g, 5.58 mmol) in THF (10 mL) was added. The mixture was stirred at room temperature for 1 h. After the solvent was removed under reduced pressure, the residue was purified by silica gel column chromatography (hexane–ethyl acetate = 19:1) to give 11 (677 mg, 71%) as a white crystal. mp 47–49 °C; 1H-NMR (CDCl3) δ 1.45 (3H, s), 1.48 (3H, s), 3.76 (2H, d, J = 11.6 Hz), 3.81 (2H, d, J = 11.6 Hz), 4.71 (2H, t, J = 1.9 Hz), 5.84-5.87 (1H, m), 6.02 (1H, d, J = 6.3 Hz); 13C-NMR (CDCl3) δ 22.6, 24.6, 66.7, 74.9, 98.0, 128.1, 129.1; IR (neat) 2924.2, 2853.1, 1724.1, 1215.9, 758.3 cm−1; FAB-MS (m/z) 155 [M−15]+; Anal. Calcd for C9H14O3; C, 62.84; H, 8.32. Found; C, 62.78; H, 8.44.
(3S*,4S*)-8,8-Dimethyl-1,7,9,-trioxaspiro[4.5]decane-3,4-diol (12). To a solution of 11 (73 mg, 0.43 mmol) and NMO (0.22 mL) in acetone (4 mL), was added a solution of K2OsO4·2H2O (1 mg, 0.004 mmol) in H2O (0.4mL) at 0 °C. After stirred at room temperature for 60 h, Na2S2O3·5H2O (125 mg) was added and the mixture was stirred at room temperature for 30 min. After the whole mixture was dried over Na2SO4, the solid materials were removed by suction and washed with ethyl acetate. The combined filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (CHCl3–MeOH = 19:1) to give 12 (84 mg, 97%). 1H-NMR (CDCl3) δ 1.43 (3H, s), 1.49 (3H, s), 3.59 (1H, dd, J = 11.6, 1.9 Hz), 3.80 (1H, d, J = 9.7 Hz), 3.82–3.87 (2H, m), 3.94 (1H, dd, J = 9.7, 4.9 Hz), 4.14 (1H, dd, J = 11.6, 1.9 Hz), 4.22 (1H, d, J = 5.3 Hz) 4.34 (1H, q, J = 5.0 Hz); 13C-NMR (CDCl3) δ 21.0, 25.8, 63.3, 66.5, 71.0, 71.4, 74.9, 76.7, 98.4; IR (KBr) 3306.4, 2953.6, 2741.6, 1452.2, 524.19 cm−1; EI-MS (m/z): 204 [M+1]+; HRMS Calcd for C9H15N3O4: 204.0998, Found: 204.0992.
(3S*,4S*)-8,8-Dimethyl-1,7,9,-trioxaspiro[4.5]decane-3,4-cyclicsulfate (13). To a solution of 12 (410 mg, 2.01 mmol) and Et3N (672 µL, 4.82 mmol) in CH2Cl2 (10 mL), was added dropwise a solution of SOCl2 (113 μL, 1.55 mmoL) in CH2Cl2 (10 mL) at 0 °C. After stirred at room temperature for 15 min, the mixture was washed with water. The water layer was extracted with CHCl3 twice and the combined organic layer was washed with brine, then dried over Na2SO4. After filtration, the residue was passed through a short silica gel column (eluate: hexane–ethyl acetate = 1:1). After the solvents were removed under reduced pressure, the residue was dissolved in CCl4–CH3CN–H2O (2:2:3, 3 mL). To this solution, were added RuCl3·3H2O (2.7 mg) and NaIO4 (73 mg, 0.34 mmol) at 0 °C. The mixture was stirred at the same temperature for 1.5 h. After diluted with ether, the mixture was washed with water, sat.NaHCO3 and brine, then dried over Na2SO4. After filtration, the solvents were removed under reduce pressure, the residue was purified by silica gel column chromatography (hexane-ethyl acetate = 6:1) to give 13 (82 mg, 77%). 1H-NMR (CDCl3) δ1.43 (3H, s), 1.50 (3H, s), 3.56 (1H, dd, J = 12.1, 2.4 Hz), 3.82 (1H, d, J = 12.1 Hz), 3.91 (1H, d, J = 12.1 Hz), 3.97 (1H, dd, J = 12.6, 4.4 Hz), 4.06 (1H, dd, J = 12.1, 2.4 Hz), 4.28 (1H, d, J = 12.6 Hz), 5.42 (1H, d, J = 6.3 Hz), 5.48 (1H, t, J = 5.1 Hz); 13C-NMR (CDCl3) δ 19.6, 27.1, 61.0, 62.4, 69.7, 79.0, 83.3, 84.5, 99.2; IR (KBr) 3000.8, 2892.3, 1699.7, 1380.9, 1089.9 cm−1; EI-MS (m/z): 267 [M+1]+; HRMS Calcd for C9H15N3O4: 266.0460, Found: 266.0467.
(3R*,4S*)-3-Azido-8,8-dimethyl-1,7,9,-trioxaspiro[4.5]decan-4-ol (18). A mixture of 7 [11,14] (433 mg, 2.33 mmol) and NaN3 (752 mg 11.6 mmol) in 2-methoxyethanol (26 mL) was kept at 100 °C for 5 h. After the solvent was removed under reduced pressure, the residue was dissolved in ethyl acetate. After washed with water and brine, the organic layer was dried over Na2SO4. After filtration, the solvents were removed under reduce pressure, the residue was purified by silica gel column chromatography (hexane–ethyl acetate = 5:1) to give 18 (351 mg, 66%). 1H-NMR (CDCl3) δ 1.40 (3H, s), 1.49 (3H, s), 3.69-3.80 (3H, m), 3.84 (1H, d, J = 11.6 Hz), 4.01–4.08 (3H, m), 4.38 (1H, t, J = 1.9, 2.4 Hz); 13C-NMR (CDCl3) δ 19.5, 27.4, 62.3, 66.0, 67.6, 69.4, 78.2, 79.1, 98.5; IR (neat) 3419.0, 2104.8, 1086.6, 831.7 cm−1; EI-MS (m/z): 229 [M+1]+; HRMS Calcd for C9H15N3O4: 229.1063, Found: 229.1062.
(3R*,4S*)-3-Azido-8,8-dimethyl-1,7,9,-trioxaspiro[4.5]decan-4-yl benzoate (19). To a solution of 18 (432 mg, 1.88 mmol), Et3N (0.59 mL, 4.24 mmol), and DMAP (23 mg, 0.19 mmol) in CH2Cl2 (15 mL) was added benzoyl chloride (0.40 mL, 3.39 mmol) and the mixture was stirred at room temperature for 6.5 h. The reaction was quenched by addition of MeOH, and the whole was stirred at room temperature for 10 min. The mixture was diluted with CH2Cl2 and washed with water and brine, then dried over Na2SO4. After filtration, the solvents were removed under reduce pressure, the residue was purified by silica gel column chromatography (hexane–ethyl acetate = 4:1) to give 19 (598 mg, 95%). 1H-NMR (CDCl3) δ 1.37 (3H, s), 1.47 (3H, s), 3.84-3.91 (3H, m), 3.99 (1H, dd, J = 1.4, 10.6 Hz), 4.07 (1H, dd, J = 1.4, 10.6 Hz), 4.18-4.25 (2H, m) 5.47 (1H, d, J = 1.0 Hz), 7.48 (2H, t, J = 7.2 Hz), 7.62 (1H, J = 7.2 Hz), 8.03 (2H, J = 7.2 Hz); 13C-NMR (CDCl3) δ 22.4, 24.2, 62.1, 65.0, 66.2, 69.9, 78.9, 79.2, 98.4, 128.6, 129.0, 129.6, 133.6, 165.3; IR (neat) 2993.1, 2107.2, 1725.4, 1267.4, 1091.3, 711.5 cm−1; EI-MS (m/z): 333 [M]+; HRMS Calcd for C16H19N3O5: 333.1325, Found: 333.1336.
(3S*,4R*)-4-Azido-2,2-bis((methylsulfonyloxy)methyl)tetrahydrohuran-3-yl benzoate (20). A mixture of 19 (1.01 g, 3.04 mmol) in 80% AcOH (80 mL) was stirred at room temperature for 5 h. After the solvent was removed under reduced pressure, the residue was co-evaporated with EtOH five times to remove residual AcOH. The resulting crude product was dissolved in CH2Cl2 (40 mL). To this mixture, were added, MsCl (1.19 mL, 15.18 mmol), Et3N (2.14 mL, 15.18 mmol), and DMAP (38 mg, 0.30 mmol). After stirred at room temperature for 1 h, the mixture was diluted with CH2Cl2 washed with 5%HCl, sat.NaHCO3 and brine. The separated organic layer was dried over Na2SO4. After filtration, the solvents were removed under reduce pressure, the residue was purified by silica gel column chromatography (hexane–ethyl acetate = 2:1) to give 20 (1.05 g, 77%). 1H-NMR (CDCl3) δ 3.00 (3H, s), 3.12 (3H, s), 3.94 (1H, dd, J = 5.80, 4.37 Hz), 4.33~4.48 (6H, m), 5.48 (1H, d, J = 3.4 Hz), 7.50 (2H, t, J = 7.7 Hz), 7.64 (1H, t, J = 8.0 Hz), 8.04 (2H, d, J = 7.3 Hz); 13C-NMR (CDCl3) δ 37.7, 65.5, 65.8, 67.4, 70.3, 77.2, 78.9, 82.8, 128.1, 128.8, 129.9, 134.2, 165.2; IR (near) 2110.7, 1728.8, 1360.0, 1267.0 cm−1; FAB-MS (m/z): 450 [M+1]+; HRMS Calcd for C15H20N3O9S2: 450.0641, Found: 450.0631.
((1R*,4R*,5S*)-4-Azido-2,6-dioxabicyclo[3.2.0]heptan-1-yl)methyl methanesulfonate (21). A mixture of 20 (36 mg, 0.08 mmol) and NaOCH3 (4.6 mg, 0.08 mmol) in MeOH (2 mL) was kept at 75 °C overnight. After the solvent was removed under reduced pressure, the residue was dissolved in CHCl3 and washed with water and brine, then dried over Na2SO4. After filtration, the solvents were removed under reduce pressure, the residue was purified by silica gel column chromatography (hexane–ethyl acetate = 2:1) to give 21 (14 mg, 72%). 1H-NMR (CDCl3) δ 3.09 (3H, s), 4.05 (1H, d, J = 3.4 Hz), 3.40 (1H, d, J = 11.1 Hz), 4.29~4.77 (4H, m), 4.76 (1H, d, J = 8.2 Hz), 5.06 (1H, s); 13C-NMR (CDCl3) δ 37.8, 63.6, 67.4, 71.9, 77.1, 84.4, 89.9; IR (neat) 2098.2, 1360.4, 1175.0, 960.2, 815.5 cm−1; FAB-MS (m/z): 250 [M+1]+; HRMS Calcd for C7H12N3O5S: 250.0498, Found: 250.0490.
((1R*,4R*,5S*)-4-Azido-2,6-dioxabicyclo[3.2.0]heptan-1-yl)methyl benzoate (22). A mixture of CsF (332 mg, 2.19 mmol) and PhCOOH (267 mg, 2.19 mmol) in DMF (40 mL) was stirred at room temperature for 20 min. To this mixture was added a solution of 21 (182 mg, 0.73 mmol) in DMF (20 mL). After stirred at 60 °C overnight, the mixture was partitioned between EtOAc and H2O. The separated water layer was extracted with EtOAc, and the organic layer was washed with sat.NaHCO3, brine, then dried over Na2SO4. After filtration, the filtrated was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (hexane-ethyl acetate = 5:1) to give 22 (170 mg, 86%). 1H-NMR (CDCl3) δ 4.08 (1H, d, J = 3.4 Hz), 4.42 (1H, d, J = 10.6 Hz), 4.52 (2H, dd, J = 10.6, 3.4 Hz), 4.60 (2H, s), 4.83 (1H, d, J = 7.7 Hz), 5.15 (1H, s), 7.61 (2H, t, J = 15.5 Hz) 7.59 (1H, t, J = 17.4 Hz), 8.10 (2H, d, J = 9.7 Hz); 13C-NMR (CDCl3) δ 63.1, 63.8, 71.5, 77.8, 85.1, 90.4, 128.4, 129.4, 129.7, 133.3, 166.1; IR (neat) 2099.7, 1723.1, 1284.3, 713.4 cm−1; FAB-MS (m/z); 276 [M+1]+; HRMS Calcd for C13H14N3O4: 276.0984, Found: 276.0977.
((1S*,4R*,5S*)-4-Azido-2,6-dioxa-bicyclo[3.2.0]heptan-1-yl)methanol (23). A mixture of 22 (184 mg, 0.67 mmol) and NaOCH3 (19 mg, 0.33 mmol) in MeOH (15 mL) was stirred at room temperature. After the mixture was neutralized with AcOH (19 µL), the solvents were removed under reduced pressure and the residue was purified by silica gel column chromatography (hexane–ethyl acetate = 4:1) to give 23 (90 mg, 79%). 1H-NMR (CDCl3) δ 3.91 (2H, d, J = 6.3 Hz), 4.04 (1H, d, J = 3.9 Hz), 4.36 (1H, d, J = 10.6 Hz), 4.48 (2H, dd, J = 10.6, 3.4 Hz), 4.48 (1H, d, J = 8.2 Hz), 4.68 (1H, d, J = 8.2 Hz), 5.05 (1H, s); 13C-NMR (CDCl3) δ 62.3, 64.0, 71.5, 77.6, 86.9, 90.2; IR (neat) 3431.5, 2101.6, 1248.2, 870.88 cm−1; EI-MS (m/z): 171 [M]+; HRMS Calcd for C6H9N3O3: 171.0644, Found: 171.0643.
((1S*,4R*,5S*)-4-(5-Amino-6-chloropyrimidin-4-ylamino)-2,6-dioxa-bicyclo[3.2.0]heptan-1-yl)methanol (15). A mixture of 23 (69 mg, 0.40 mmol) and Pd(OH)2 (6.2 mg, 0.04 mmol) in MeOH (5 mL) was stirred at room temperature overnight under H2 atmosphere. After insoluble materials were removed by filtration, the solvents were removed under reduced pressure. The resulting crude product was dissolved in n-BuOH (3 mL). To this mixture, were added 5-amino-4,6-dichloropyrimidine (140.1 mg, 0.86 mmol) and i-Pr2NEt (298 µL, 1.71 mmol). The mixture was kept under reflux overnight. After the solvents were removed under reduced pressure, the residue was purified by silica gel column chromatography (chloroform–methanol = 19:1) to give 15 (64 mg, 58%). 1H-NMR (CD3OD) δ3.67 (2H, q, J = 12.6 Hz), 4.24 (1H, d, J = 10.1 Hz), 4.38 (1H, d, J = 7.7 Hz), 4.45 (1H, d, J = 4.4 Hz), 4.50 (1H, d, J = 10.1, 4.4 Hz), 4.63 (1H, d, J = 7.2 Hz) 4.90 (1H, s), 7.72 (1H, s); 13C NMR (CD3OD) δ 58.1, 58.3, 62.7, 73.1, 88.3, 92.1, 125.5, 138.9, 147.3, 153.3; IR (KBr) 3381.3, 2926.7, 1578.6, 1056.3 cm−1; EI-MS (m/z): 272 [M]+; HRMS Calcd for C10H13ClN4O3: 272.0676, Found: 272.0673.
((1S*,4R*,5S*)-4-(6-Chloro-9H-purin-9-yl)-2,6-dioxa-bicyclo[3.2.0]heptan-1-yl)methanol (24). To a solution of 15 (18 mg, 0.07 mmol) in DMF (0.5mL), were added orthoethyl formate (0.7 mL, 4.21 mmol) and conc HCl (2 µL, 0.024 mmol) at 0 °C. After the mixture was stirred at room temperature, the solvents were removed under reduced pressure. The residue was dissolved in 0.5 M aqHCl (1 mL) and the mixture was stirred at room temperature for 1 h. The mixture was neutralized with 1M aqNaOH (0.5 mL) and concentrated under reduced pressure. The residue was extracted with a solution of chloroform–methanol = 1:1. After the insoluble materials were removed by filtration, the solvents were removed under reduced pressure. The residue was purified by pTLC (developed by chloroform-methanol = 5:1) to give 24 (14.9 mg, 81%). 1H-NMR (CDCl3) δ 4.12 (2H, q, J = 12.6 Hz), 4.59 (1H, d, J = 11.6 Hz), 4.66 (1H, d, J = 9.7 Hz), 4.68 (1H, d, J = 9.7 Hz), 4.66 (1H, d, J = 9.7 Hz), 4.90 (1H, dd, J = 11.1, 4.8 Hz), 5.24 (1H, s), 5.38 (1H, d, J = 4.4 Hz), 8.68 (1H, s), 8.76 (1H, s); 13C-NMR (CDCl3-CD3OD = 19 : 1) δ 58.6, 61.2, 71.9, 77.2, 87.8, 90.9, 130.7, 144.6, 150.7, 151.2, 151.8; IR (KBr) 3401.4, 2931.1, 1597.4, 1567.4, 1056.6 cm−1; EI-MS (m/z): 282 [M]+; HRMS Calcd for C11H11ClN4O3: 282.0520, Found: 282.0506.
((1S*,4R*,5S*)-4-(6-Amino-9H-purin-9-yl)-2,6-dioxa-bicyclo[3.2.0]heptan-1-yl)methanol (6). Compound 24 (29.4 mg, 0.10 mmol) was dissolved in sat. methanolic ammonia (7 mL) and the mixture was kept at 100 °C for 21 h in a glass sealed tube. After the solvents were removed under reduced pressure, the residue was purified by pTLC (developed by chloroform–methanol = 5:1) to give 6 (18.8 mg, 69%). 1H-NMR (CDCl3-CD3OD = 17:3) δ 3.91 (1H, d, J = 12.6 Hz), 4.00 (1H, d, J = 12.1 Hz), 4.57 (1H, d, J = 10.6 Hz), 4.64 (1H, d, J = 7.7 Hz), 4.70 (1H, d, J = 7.7 Hz), 4.87 (1H, dd, J = 11.1, 4.8 Hz), 5.19 (1H, s), 5.25 (1H, d, J = 4.8 Hz), 8.25 (1H, s), 8.28 (1H, s); 13C-NMR (CDCl3–CD3OD = 17:3) δ 29.5, 58.3, 61.3, 72.1, 87.6, 91.0, 118.2, 139.1, 148.9, 152.6, 155.3; IR (KBr) 3192.2, 2409.9, 1660.5, 1615.0, 1054.9 cm−1; EI-MS (m/z): 263 [M]+; HRMS Calcd for C11H13N5O3: 263.1018, Found: 263.1021.
4. Conclusions
We constructed an isoadenosine derivative on a 2,6-dioxobicyclo[3.2.0]heptane scaffold. Since our initial attempt to synthesize 6 by directly introducing the adenine moiety was not successful, we synthesized it by the de novo synthesis of an adenine ring on a pseudosugar moiety. However, this unique adenosine analogue showed no activity against HIV. Previously, we have reported that neither thymine nor adenine analogues 4 built on a 2-oxa-6-thiabicyclo[3.2.0]heptane skeleton inhibit HIV [14]. The structural rigidities of these analogues and isoadenosine 6 due to the introduction of fused thietane and oxetane rings, respectively, appear to inhibit anti-HIV activity. In particular, phosphorylation at the 5'-hydroxyl group would be inhibited since deoxynucleoside kinase recognizes the puckering of sugars [25]. Thus, we are currently preparing new substituted nucleoside derivatives based on 4 and 6, and the results will be reported elsewhere.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research (No. 24590143, Y.Y.) from JSPS and by a grant of Strategic Research Foundation Grant-aided Project for Private Universities from Ministry of Education, Culture, Sport, Science, and Technology, Japan (MEXT), 2010–2014.
Author Contributions
YY and TI designed research; SK, HK and TS performed the synthesis of compounds and TI assayed anti-HIV activity. YY wrote the paper. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cihlar, T.; Ray, A.S. Nucleoside and nucleotide HIV reverse transcriptase inhibitors: 25 years after zidovudine. Antivir. Res. 2010, 85, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Mehellou, Y.; de Clercq, E. Twenty-six years of anti-HIV drug discovery: Where do we stand and where do we go? J. Med. Chem. 2010, 53, 521–538. [Google Scholar] [CrossRef] [PubMed]
- WHO. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Available online: http://www.who.int/hiv/pub/guidelines/arv2013/download/en/ (accessed on 30 June 2013).
- Meadows, D.C.; Gervey-Hague, J. Current developments in HIV chemotherapy. ChemMedChem 2006, 1, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Imamichi, T. Action of anti-HIV drugs and resistance: Reverse transcriptase inhibitors and protease inhibitors. Curr. Pharm. Des. 2004, 10, 4039–4053. [Google Scholar] [CrossRef] [PubMed]
- Mitsuya, H.; Broder, S. Inhibition of the in vitro infectivity and cytopathic effect of human T-lymphotrophic virus type III/lymphadenopathy-associated virus (HTLV-III/LAV) by 2',3'-dideoxynucleosides. Proc. Natl. Acad. Sci. USA 1986, 83, 1911–1915. [Google Scholar] [CrossRef] [PubMed]
- Mitsuya, H.; Weinhold, K.J.; Furman, P.A.; St Clair, M.H.; Lehrman, S.N.; Gallo, R.C.; Bolognesi, D.; Barry, D.W.; Broder, S. 3'-Azido-3'-deoxythymidine (BW A509U): An antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc. Natl. Acad. Sci. USA 1985, 82, 7096–7100. [Google Scholar] [CrossRef] [PubMed]
- Schinazi, R.F.; Chu, C.K.; Peck, A.; McMillan, A.; Mathis, R.; Cannon, D.; Jeong, L.S.; Beach, J.W.; Choi, W.B.; Yeola, S.; et al. Activities of the four optical isomers of 2',3'-dideoxy-3'-thiacytidine (BCH-189) against human immunodeficiency virus type 1 in human lymphocytes. Antimicrob. Agents Chemother. 1992, 36, 672–676. [Google Scholar] [CrossRef]
- Balzarini, J.; Holy, A.; Jindrich, J.; Naesens, L.; Snoeck, R.; Schols, D.; de Clercq, E. Differential antiherpesvirus and antiretrovirus effects of the (S) and (R) enantiomers of acyclic nucleoside phosphonates: Potent and selective in vitro and in vivo antiretrovirus activities of (R)-9-(2-phosphonomethoxypropyl)-2,6-diaminopurine. Antimicrob. Agents Chemother. 1993, 37, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Yamazaki, Y.; Kawahata, M.; Yamaguchi, K.; Takahata, H. Design and synthesis of a novel ring-expanded 4'-Thio-apio-nucleoside derivatives. Tetrahedron Lett. 2007, 48, 4519–4522. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Asami, K.; Matsui, H.; Tanaka, H.; Takahata, H. New synthesis of (±)-isonucleosides. Org. Lett. 2006, 8, 6015–6018. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Yamazaki, Y.; Saito, Y.; Takahata, H. Synthesis of 1-(5,6-dihydro-2H-thiopyran-2-yl)uracil by a Pummerer-type thioglycosylation reaction: The regioselectivity of allylic substitution. Tetrahedron 2009, 65, 9091–9102. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Ohta, M.; Imahori, T.; Imamichi, T.; Takahata, H. A new entry to carbocyclic nucleosides: Oxidative coupling reaction of cycloalkenylsilanes with a nucleobase mediated by hypervalent iodine reagent. Org. Lett. 2008, 10, 3449–3452. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Asami, K.; Imamichi, T.; Okuda, T.; Shiraki, K.; Takahata, H. Design and synthesis of isonucleosides constructed on a 2-oxa-6-thiabicyclo[3.2.0]heptane scaffold. J. Org. Chem. 2010, 75, 4161–4171. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Yamazaki, Y.; Saito, Y.; Natori, Y.; Imamichi, T.; Takahata, H. Synthesis of 5-thiodidehydropyranylcytosine derivatives as potential anti-HIV agents. Bioorg. Med. Chem. Lett. 2011, 21, 3313–3316. [Google Scholar] [CrossRef] [PubMed]
- Kiran, Y.B.; Wakamatsu, H.; Natori, Y.; Takahata, H.; Yoshimura, Y. Design and synthesis of a nucleoside and a phosphonate analogue constructed on a branched-threo-tetrofuranose skeleton. Tetrahedron Lett. 2013, 54, 3949–3952. [Google Scholar] [CrossRef]
- Kan-no, H.; Saito, Y.; Omoto, S.; Minato, S.; Wakamatsu, H.; Natori, Y.; Imamichi, T.; Takahata, H.; Yoshimura, Y. Synthesis of a dihydropyranonucleoside using an oxidative glycosylation reaction mediated by hypervalent iodine. Synthesis 2014, 46, 879–886. [Google Scholar] [CrossRef]
- Chu, C.K.; Ahn, S.K.; Kim, H.O.; Beach, J.W.; Alves, A.J.; Jeong, L.S.; Islam, Q.; van Roey, P.; Schinazi, R.F. Asymmetric synthesis of enantiomerically pure (−)-(1'R,4'R)-dioxolane-thymine and its anti-HIV activity. Tetrahedron Lett. 1991, 32, 3791–3794. [Google Scholar] [CrossRef]
- Kim, H.O.; Schinazi, R.F.; Nampalli, S.; Shanmuganathan, K.; Cannon, D.L.; Alves, A.J.; Jeong, L.S.; Beach, J.W.; Chu, C.K. 1,3-dioxolanylpurine nucleosides (2R,4R) and (2R,4S) with selective anti-HIV-1 activity in human lymphocytes. J. Med. Chem. 1993, 36, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.O.; Schinazi, R.F.; Shanmuganathan, K.; Jeong, L.S.; Beach, J.W.; Nampalli, S.; Cannon, D.L.; Chu, C.K. l-beta-(2S,4S)- and l-alpha-(2S,4R)-dioxolanyl nucleosides as potential anti-HIV agents: Asymmetric synthesis and structure-activity relationships. J. Med. Chem. 1993, 36, 519–528. [Google Scholar] [CrossRef]
- Choi, Y.; George, C.; Comin, M.J.; Brachi, J.J.; Kim, H.S.; Jacobsen, K.A.; Balzarini, J.; Mitsuya, H.; Boyer, P.L.; Hughes, S.H.; et al. A conformationally locked analogue of the anti-HIV agent stavudine. An important correlation between pseudorotation and maximum amplitude. J. Med. Chem. 2003, 46, 3292–3299. [Google Scholar] [CrossRef] [PubMed]
- D’Alonzo, D.; van Aerschot, A.; Guaragna, A.; Palumbo, G.; Schepers, G.; Capone, S.; Rozenski, J.; Herdewijn, P. Synthesis and base pairing properties of 1',5'-anhydro-l-hexitol nucleic acids (l-HNA). Chem. Eur. J. 2009, 15, 10121–10131. [Google Scholar] [CrossRef] [PubMed]
- Bera, S.; Nair, V. A new general synthesis of isomeric nucleosides. Tetrahedron Lett. 2001, 42, 5813–5815. [Google Scholar] [CrossRef]
- Quadrelli, P.; Piccanello, A.; Mella, M.; Corsaro, A.; Pistarà, V. From cyclopentadiene to isoxazoline-carbocyclic nucleosides: A rapid access to biological molecules through aza-Dielse Alder reactions. Tetrahedron 2008, 64, 3541–3547. [Google Scholar] [CrossRef]
- Comin, M.J.; Vu, B.C.; Boyer, P.L.; Liao, C.; Hughes, S.H.; Marquez, V.E. d-(+)-iso-methanocarbathymidine: A high-affinity substrate for herpes simplex virus 1 thymidine kinase. ChemMedChem 2008, 3, 1129–1134. [Google Scholar] [CrossRef]
- Sample Availability: Sample of the final compound is available from the authors. About the other compounds, please contact the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).