1. Introduction
Cancer is the leading cause of death in economically developed countries and the second leading cause of death in developing countries. In recent years, the increase in the number of cancer cases has motivated the growth of cancer research [
1]. A large number of natural products and dietary components have been evaluated as potential chemopreventive agents, and herbal remedies used in traditional folk medicine provide a largely unexplored source of potential novel drugs [
2]. The traditional Chinese medicine have a long time clinical practice, so it is a very safe and effective cure [
3].
Sauromatum giganteum (Engl.)
Cusimano & Hett is a herbal plant, a synonym is
Typhonium giganteum Engl. or
Typhonium giraldii (Baroni) Engl. or
Typhonium stoliczkae Engl., the dried root tuber of which is named Baifuzi in Chinese and recorded in Chinese pharmacopoeia as a traditional Chinese medicine [
4,
5]. It has the effect of “dispelling wind-phelgm”, and been used for the folklore treatment of cancer for a long time in Northeast of China.
There are a few research on
Sauromatum giganteum (Engl.)
Cusimano & Hett, Chen XS
et al. reported the chemical components of
Typhonium giganteum Engl. tuber [
6] and synthesis method of a new cerebroside isolated from
Typhonium giganteum Engl [
7,
8], and Chi S reported that Baifuzi reduces transient ischemic brain damage through an interaction with the STREX domain of BKCa channels [
4], and the cerebrosides [
9] from baifuzi, a novel potential blocker of calcium-activated chloride channels in rat pulmonary artery smooth muscle cells [
5], is the active compounds of activation of BKCa channels [
10].
As concerns experimental research on the anti-tumor activity, only Li Q
et al. reported that SFE-CO
2 extract from
Typhonium giganteum Engl. Tubers induced apoptosis in human hepatoma SMMC-7721 cells [
11]. Ma YL reported the up-regulation of TRAIL/TRAIL-R1 and TRAIL-R2 by Lignans of Rhizoma Typhonii could be involved in the induction of apoptosis in SGC-7901 cells [
12]. Shan BE found Rhizoma typhonii extract has immunoenhancing activity to human T cell and macrophage, through stimulating the killer cell and phagocytosis of tumor cell and allo-antigen, which could be used clinically for modulating immune responses and for treating tumor and other diseases [
13]. Wang SQ reported the effect of aqueous extract of
Typhonium giganteum on genes expression in hepatocellular carcinoma SMMC-7721 cells [
14].
Study of traditional Chinese medicine is very difficult because the chemical components are very complex. “Serum pharmacology” was first presented by Tashino [
15], a Japanese scholar, in 1984. The theory stated that only chemical components of a Chinese herb absorbed in blood could exert their activity on diseases [
16]; therefore, the serum pharmacology method provides a good research approach for traditional Chinese medicine [
17,
18,
19,
20], which could avoid the interference of the chemical components not absorbed in blood [
21,
22]. This method not only reveals the mechanism of the active ingredient’s action due to its excellent controllability of experimental conditions and detection convenience, but also prevents the interference from crude herbal, so it is more scientific and real. Until now, serum-pharmacology has been widely utilized to explore herbal or traditional Chinese formulations because of its advantage. In addition, it has been widely applied in the research of natural medicine in China [
23,
24,
25,
26] and East Asian countries in recent years [
27].
The antitumor bioactive solvent fraction of Sauromatum giganteum (Engl.) Cusimano & Hett Tuber and cytotoxicity on different tumor cell lines has not been revealed. In this paper, the serum pharmacology method and MTT assay in vitro and tumor transplantation method in vivo were adopted to determine the antitumor bioactive fraction of Sauromatum giganteum (Engl.) Cusimano & Hett tuber, sensitive cell lines and antitumor activity in vivo. The results might provide a scientific explanation for the folk or traditional application of Sauromatum giganteum (Engl.) Cusimano & Hett in cancer therapy.
3. Experimental Section
3.1. Materials
The tuber of Sauromatum giganteum (Engl.) Cusimano & Hett Tuber were purchased from the Harbin Pharmaceutical Group (Harbin, China). The field studies did not involve endangered or protected species. The study protocol was approved by Animal Ethics Committee, Harbin Commerce University. RPMI 1640 culture medium was purchased from GIBCO BRL (Gaithersburg, MD, USA). Fetal bovine serum (FBS) was purchased from Hyclone company (Logan, UT, USA). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) and fluorouracil (5-FU) were purchased from Sigma Chemical, Co. Ltd. (St. Louis, MO, USA). Adriamycin (ADR) were purchased from Pfizer Inc. (New York, NY, USA).
3.2. Preparation of Fractions from Sauromatum giganteum (Engl.) Cusimano & Hett
Sauromatum giganteum (Engl.) Cusimano & Hett Tuber was purchased from Harbin Pharmaceutical Group (Harbin, China) and identified as the tuber part of Sauromatum giganteum (Engl.) Cusimano & Hett by D.-L. Zhang (The School of Pharmacy, Harbin Commerce University, Harbin, China).
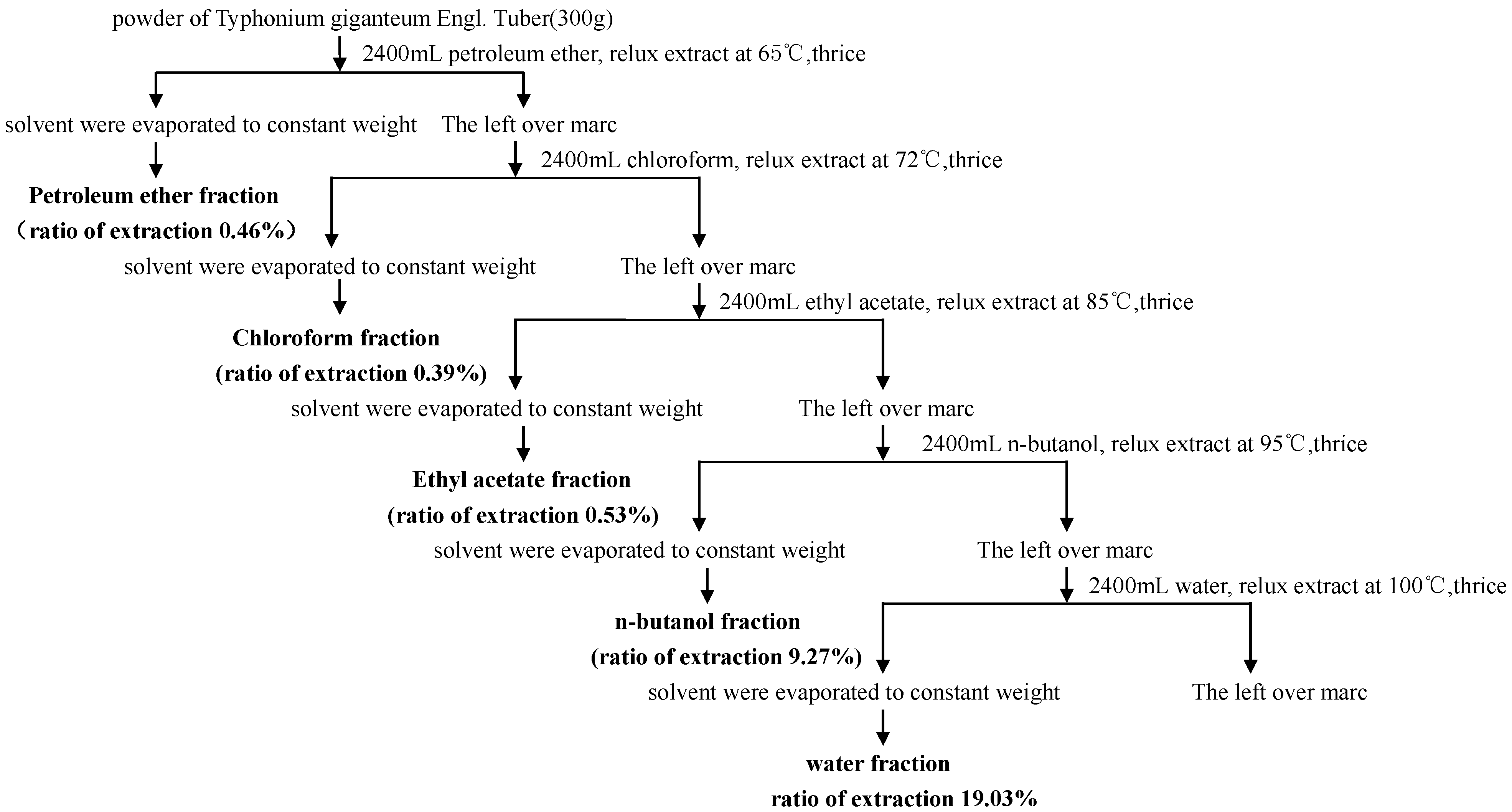
A separate extraction was performed on the dried plant material to obtain various solvent fractions following the method described by Tadiwos Feyissa
et al. [
28], with slight modifications. Thus, powdered air-dried tuber (300 g) of
Sauromatum giganteum (Engl.)
Cusimano & Hett were successively extracted with petroleum ether. The leftover marc was then extracted with chloroform, ethyl acetate,
n-butanol and distilled water by turning the maceration under reflux to obtain the respective fractions (show in
Figure 2). The petroleum ether fraction, chloroform fraction, ethyl acetate fraction,
n-butanol fraction and water fraction solvent were evaporated in a rotary vacuum evaporator at 40 °C to a constant weight of dry extract. The extraction ratio of petroleum ether fraction, chloroform fraction, ethyl acetate fraction,
n-butanol fraction and water fraction was 0.46%, 0.39%, 0.53%, 9.27% and 19.03%, respectively. The obtained extracts were kept in air-tight containers wrapped with aluminum foil and stored in a refrigerator until use.
Figure 2.
Preparation of fractions from Sauromatum giganteum (Engl.) Cusimano & Hett.
Figure 2.
Preparation of fractions from Sauromatum giganteum (Engl.) Cusimano & Hett.
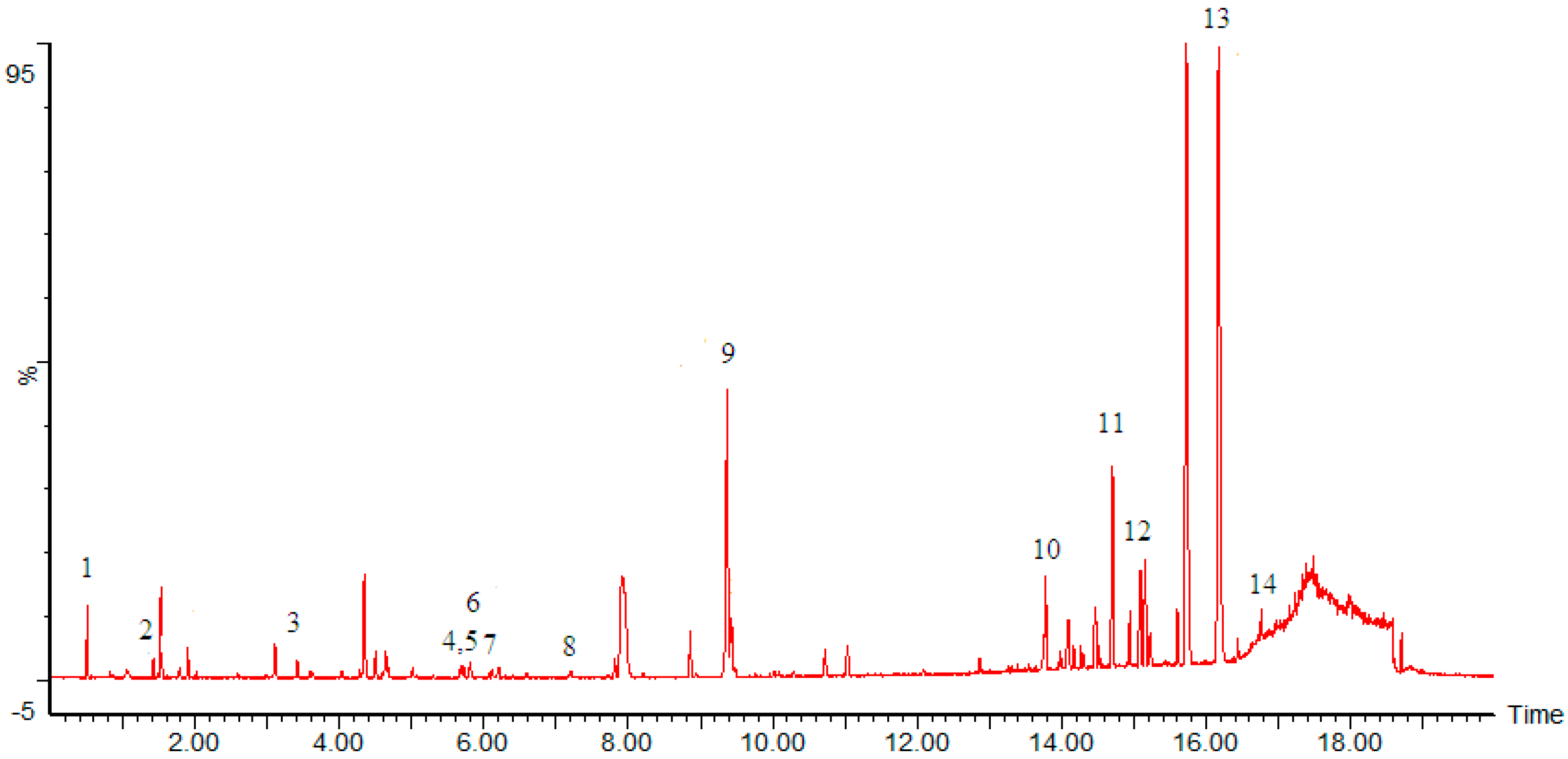
Ai FW and Ma YL
et al. [
12,
29] reports 16 compounds were isolated and identified by ESI-MS and NMR from
Sauromatum giganteum (Engl.)
Cusimano & Hett Tuber. coniferin (1), 5-hydroxy methyl-2-furaldehyde (2), pinoresinol-4-
O-β-
d-glucopyranoside (3), pinoresinol (4), neoolivil (5), lariciresinol (6), methylconiferin (7), β-sitosterol (8), tianshic acid (9), palmitic acid (10), cinnamic acid (11), and daucosterol (12) were from the ethyl acetate fraction; uridine (13), and adenosine (14) were from the
n-butanol fraction; 1-decanoyl-rac-glycerol (15), and 3-Glyceryl monooleate (16) were from the petroleum ether fraction.
3.3. Animals
Kunming mice of both sexes were used in this study, which were purchased from Changchun Gao-Xin Experimental Animal Center (Changchun, China) with an initial body weight of 18–22 g.
The animals were given water and food ad libitum. The temperature of the animal laboratory was controlled within 20 ± 2 °C, the humidity was 50%–60% with a natural day-night cycle. All animal care and treatments were carried out in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals published by the National Institute of Health, U.S.A.
After 3 days of acclimation. Eighty mice were randomly divided into eight groups with ten mice per group (each group with five male mice and five female mice): water control group, oil control group, positive control group (5-FU), petroleum ether fraction group, chloroform fraction group, ethyl acetate fraction group, n-butanol fraction group, and water fraction group.
3.4. Administration of Fractions from Sauromatum giganteum (Engl.) Cusimano & Hett
The petroleum ether fraction, chloroform fraction, and ethyl acetate fraction were respectively dissolved in oil, but the n-butanol fraction and water fraction were respectively dissolved in distilled water before administration at saturation.
The animals were fasted for 12 h, but given water ad libitum before administration. The treatment groups of animals (n = 10) were orally administered different fractions, twice a day. The animals the in water control group and oil control group were orally administered distilled water and olive oil respectively, The animals in the positive control group were orally administered 100 mg/kg·d 5-FU, and they were run concurrently with different fraction-treated groups. The animals in the petroleum ether fraction group, chloroform fraction group, ethyl acetate fraction group, n-butanol fraction group, and water fraction group were orally administered at saturation concentrations of 1.150, 1.014, 0.954, 4.635 and 4.7575 g/kg·d in a final volume 0.5 mL. The animals were fasted for 12 h but given water ad libitum before collection of serum.
3.5. Preparation of Fraction-Containing Serum
Sixty minutes after the seventh treatment, the blood of the animals was extracted by eyeball extirpating one by one under aseptic condition, and let stand at 4 °C for twelve hours, then centrifuged at 3000×
g for 15 min. Both fraction-containing serum and control serum were filtered through a 0.22 μm micropore film (Millipore, Danvers, MA, USA), termed as PEF-S (petroleum ether fraction-containing mice serum), CF-S (chloroform fraction-containing mice serum), EAF-S (ethyl acetate fraction-containing mice serum), BF-S (
n-butanol fraction-containing mice serum), WF-S (water fraction-containing mice serum), 5-FU-S (5-FU-containing mice serum), water-control-S (Negative control of serum containing water) and oil-control-S (Negative control of mice serum containing olive oil) and control-FB-S (Negative control of fetal bovine mice serum), respectively, and stored at −80 °C until use [
30].
3.6. Cell Culture
The human SMMC-7721 hepatocellular carcinoma, human SGC-7901 gastric carcinoma, human MCF-7 breast adenocarcinoma, human HeLa cervix adenocarcinoma, human A549 lung carcinoma epithelial, human HT-29 colon adenocarcinoma, and human MDA-MB-231 breast adenocarcinoma cell lines were obtained from Harbin Medicine University (Harbin, China). Cells were cultured in RPMI 1640 medium (Gibco, 31800-022) supplemented with 10% (v/v) fetal bovine serum (Gibco, 10099-141), 100 U/mL penicillin, 100 μg/mL streptomycin and 1 mM L-glutamine at 37 °C in an atmosphere of 5% CO2. The medium was renewed two or three times/week. Cells in the logarithmic growth phase were used for further experiments.
3.7. Cytotoxicity Activity of Fraction-Containing Serum on the Human Tumor Cell Lines
The cytotoxicity of 5 different fraction-containing serum on the human tumor cell lines were evaluated using MTT assay. The cells treated with fractions-containing serum were incubated for 72 h after which the MTT [3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide] assay was carried out as described by Mahmoud,
et al. [
31,
32], but with slight modifications. Cells were plated in 96-well plates (4 × 10
3 cells/well for SMMC-7721 cells, 8 × 10
3 cells/well for SGC-7901 cells, 6 × 10
3 cells/well for MCF-7 cells, 8 × 10
3 cells/well for HeLa cells, 1 × 10
4 cells/well for A549 cells, 8 × 10
3 cells/well for HT-29 cells, 8 × 10
3 cells/well for MDA-MB-231 cells) in 100 µL of RPMI 1640 medium containing 10% (v/v) fetal bovine serum for 24 h incubation. After 24 h, the cells were cultured for 72 h in RPMI 1640 medium containing 10% serum of rats treated with different fractions of
Sauromatum giganteum (Engl.)
Cusimano & Hett Tuber. Adriamycin (ADR), which is widely used for the treatment of several human cancers, was used as the positive reference as well as 5-FU-S in this study. At the end of 72 h incubation, the medium were discarded and 100 μL of MTT stock solution (1 mg/mL) were added to each well and the plates were further incubated. Four hours later, DMSO (150 μL) was added to each well to solubilize the water-insoluble purple formazan crystals. The amount of MTT-formazan is directly proportional to the number of living cells and was determined by measuring the optical density (OD) at 570 nm using microplate reader (Bio-Rad). The percentage of cytotoxic activity compared to the untreated cells was determined using the following equation [
33,
34]:
3.8. Cytotoxicity Activity of Ethyl Acetate Fraction-Containing Serum on 7 Human Tumor Cell Lines
The assay and operation was same with 3.7, ethyl acetate fraction is an effective anti-tumor bioactive solvent fraction according to our experimental data in 3.7 (
Table 8). However, the mice serum containing ethyl acetate fraction were from different batches of mice, likely to cause errors in results. Therefore, using the same batch of mice, we prepared the mice serum containing ethyl acetate fraction again and observed the cytotoxicity on SMMC-7721, SGC-7901, MCF-7, HT-29, HeLa, A549, MDA-MB-231 cell by MTT assay (
Table 9).
3.9. Acute Toxicity Test
Fifty normal kunming mice (20 ± 2 g) of either sex, fed with pellet diets were randomly divided into five groups with ten mice per group (each group with five male mice and five female mice) and administrated with 1.150 g/kg of the petroleum ether fraction, 1.014 g/kg of the chloroform fraction, 0.954 g/kg of the ethyl acetate fraction, 4.635 g/kg of the
n-butanol fraction, 4.7575 g/kg of the water fraction orally by gastric gavage. The animals were observed for general behavioral changes, signs of toxicity and mortality continuously for 1 h after treatment, then intermittently for 4 h, and thereafter over a period of 24 h. Further, the mice were observed for up to 14 days following the treatment for any lethality and death [
35].
3.10. In Vivo Antitumor Effect of Ethyl Acetate Fraction on Mice Bearing-S180 or H22 Cell Line
S180 or H22 tumor cells were harvested and washed three times with normal saline (NS). The cells were pelleted by brief centrifugation at 300× g. The supernatant was aspirated, and the cells were resuspended in NS at a density of 1 × 107 cells/mL. The mice were subcutaneously implanted with 2 × 106 cells/mouse on the right oxter for S180-tumor-model or peritoneum for H22-tumor-model (day 0). Twenty-four hours after inoculation, fifty mice with S180 cells or fifty mice with H22 cells were randomly divided into 5 groups, respectively.
After tumor implantation for 24 h, mice were administered orally with various doses of ethyl acetate fraction of Sauromatum giganteum (Engl.) Cusimano & Hett Tuber (dissolved in oil, twice a day, 106, 318, 954 mg/kg·d Ethyl acetate fraction dry extract) for 7 days. 5-fluorouracil (25 mg/kg·d) was served as a positive drug.
24 h after the last administration, the mice bearing S180 tumor lines were sacrificed and the tumors were excised and weighted. The inhibition rate (IR) of tumor growth was calculated by the following formula: IR (%) = [(average tumor weight of the control group − average tumor weight of the treatment group)/average tumor weight of the control group] × 100.
The mice bearing H22 tumor lines were recorded the survival time, the life span were determined using the following equations: increase in life span (%) = [(median survival time of treated mice − median survival time of untreated mice)/median survival time of untreated mice] × 100.
All experimental procedures were performed in accordance with the Guide lines of Animal Experiments from the Committee of Medical Ethics, National Health Department of China (1998).
3.11. Analytical Conditions of Ethyl Acetate Fraction of Sauromatum giganteum (Engl.) Cusimano & Hett by UPLC- TOF-MS
The chromatographic separation was achieved on Waters Acquity UPLC HSS T3 (2.1 mm × 100 mm, 1.8 μm) column by employing the Waters Acquity UPLC system (Waters Corp., Milford, MA, USA) consisting of a binary solvent manager, a sample manager and a column temperature controller. The mobile phase which consisted of 0.1% formic acid aqueous solution (A)-0.1% formic acid Acetonitrile solution (B) using a gradient elution (
Table 13), was pumped at a flow rate of 0.5 mL·min
−1. The temperatures of the column and sample manager were maintained at 40 °C and 4 °C, respectively.
Waters SYNAPT G2-S Mass Spectrometers (Waters Corp., Milford, MA, USA) equipped with an electrospray ionization (ESI) source was employed for detection. The mass spectrometry was operated in negative ionization mode. In order to achieve maximum sensitivity, the mass spectrometric conditions were optimized as follows:cone gas (nitrogen) flow rate, 10 L/h; desolvation gas (nitrogen) flow rate, 800 L/h; capillary voltage, 2500 V; source temperature, 120 °C; desolvation temperature, 500 °C; scan range: m/z 50–1500. Data processing was performed with UNIFI 1.7 software.
Table 13.
Mobile phase gradient elution system.
Table 13.
Mobile phase gradient elution system.
| Time (min) | A (%) | B (%) | flow-Rate (mL/min) |
|---|
| 0 | 99 | 1 | 0.5 |
| 0.3 | 99 | 1 | 0.5 |
| 6 | 75 | 25 | 0.5 |
| 9 | 60 | 40 | 0.5 |
| 12 | 50 | 50 | 0.5 |
| 17 | 1 | 99 | 0.5 |
| 18 | 1 | 99 | 0.5 |
| 20 | 99 | 1 | 0.5 |
3.12. Statistical Analysis
The data is represented as the mean ± SD. Statistical significance was calculated using student’s t-test. p-values of 5% or less were considered statistically significant.
4. Conclusions
Five different fraction-containing serums (petroleum ether fraction, chloroform fraction, ethyl acetate fraction, n-butanol fraction and water fraction of
Sauromatum giganteum (Engl.)
Cusimano & Hett) were prepared and investigated for the inhibition activity on tumor cells, respectively. Only the mice serum containing ethyl acetate fraction of
Sauromatum giganteum (Engl.)
Cusimano & Hett Tuber could inhibit the tumor cells proliferation. The inhibition effection of ethyl acetate fraction on the seven cell lines in descending order is SGC-7901 > SMMC-7721 > MCF-7 > HT-29 > A549 > HeLa > MDA-MB-231. Sarcoma S
180 and hepatoma H
22 tumor-bearing mouse models are adopted in antitumor experiment
in vivo [
36,
37,
38,
39], We found ethyl acetate fraction of
Sauromatum giganteum (Engl.)
Cusimano & Hett Tuber could inhibit the S
180 growth and prolonged the life span of mice bearing H
22 with the dosage increase.
Therefore, Ethyl acetate fraction of Sauromatum giganteum (Engl.) Cusimano & Hett is the anti-tumor activity fraction. Anti-tumor activity on cancer cell line in descending order is SGC-7901 > SMMC-7721 > MCF-7 > HT-29 > A549 > HeLa > MDA-MB-231.