Rational Drug Design and Synthesis of Molecules Targeting the Angiotensin II Type 1 and Type 2 Receptors
Abstract
:1. Introduction
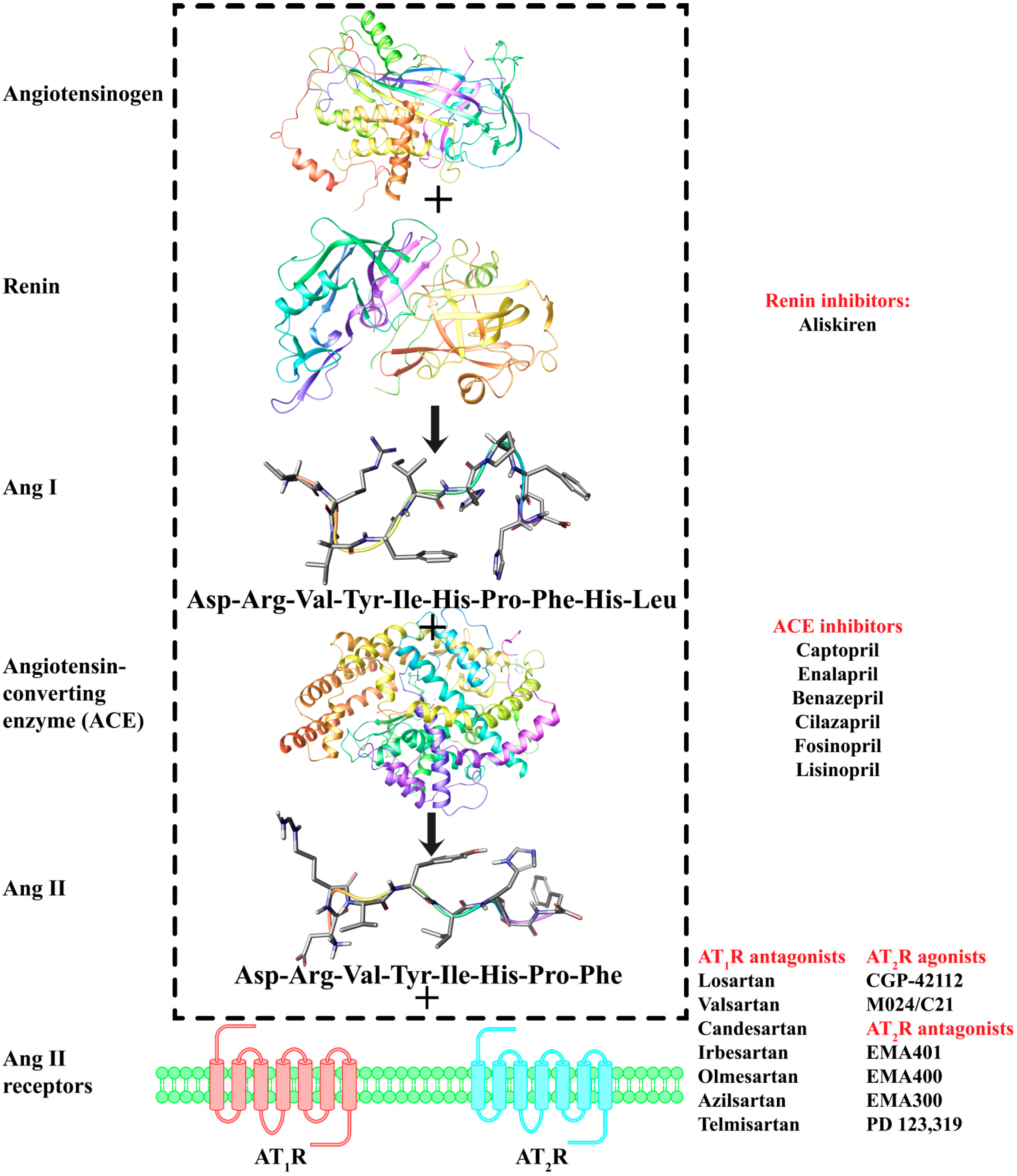
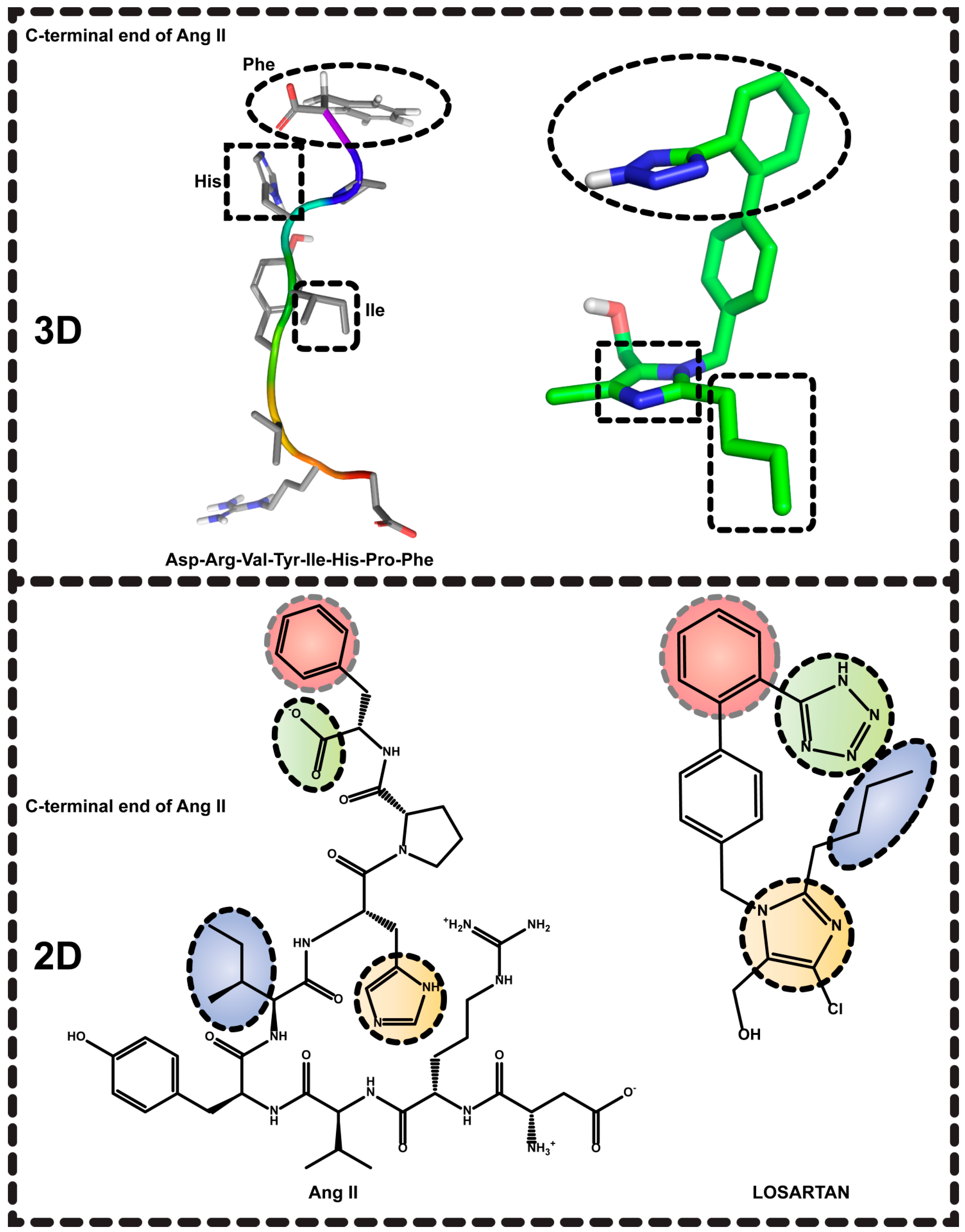
2. Results and Discussion
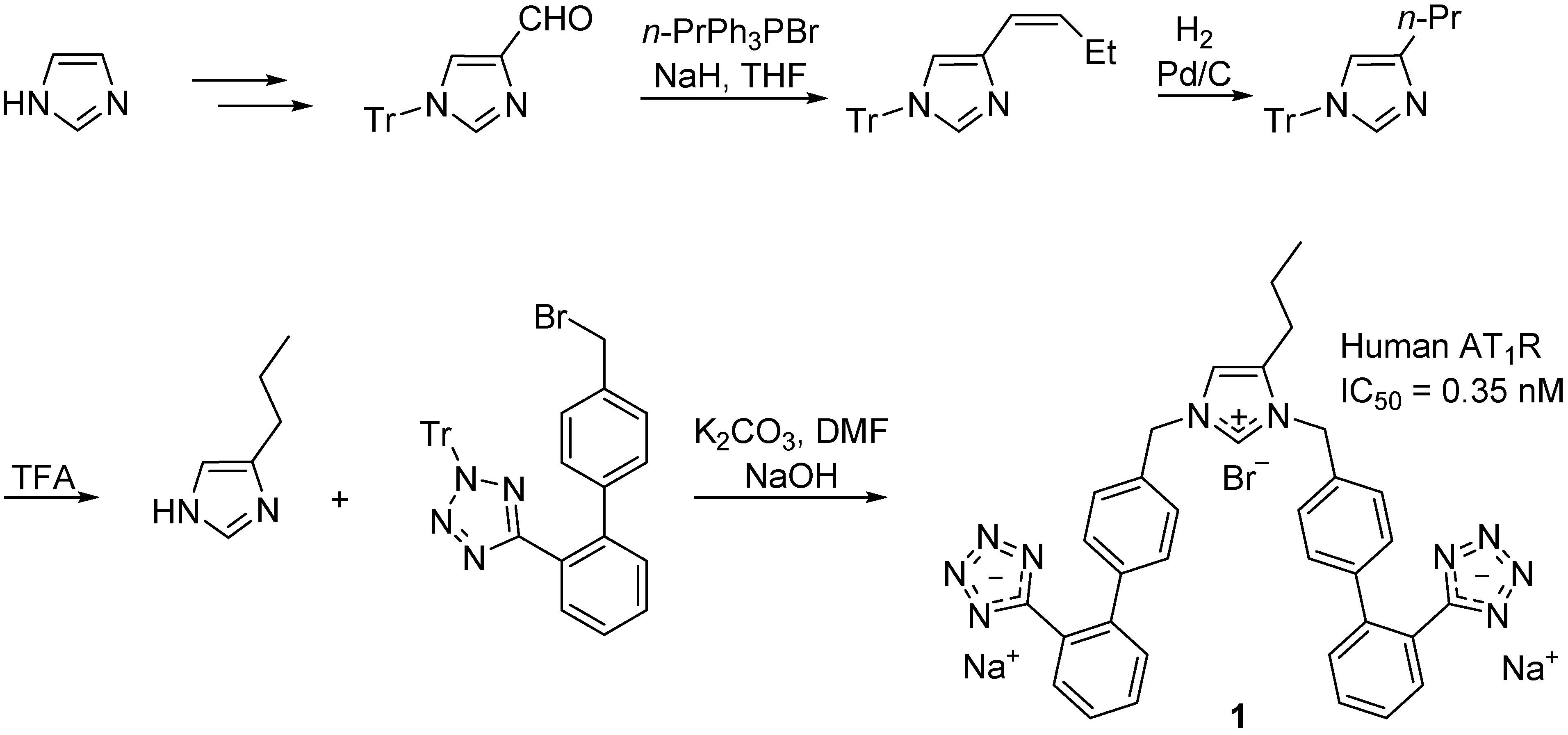
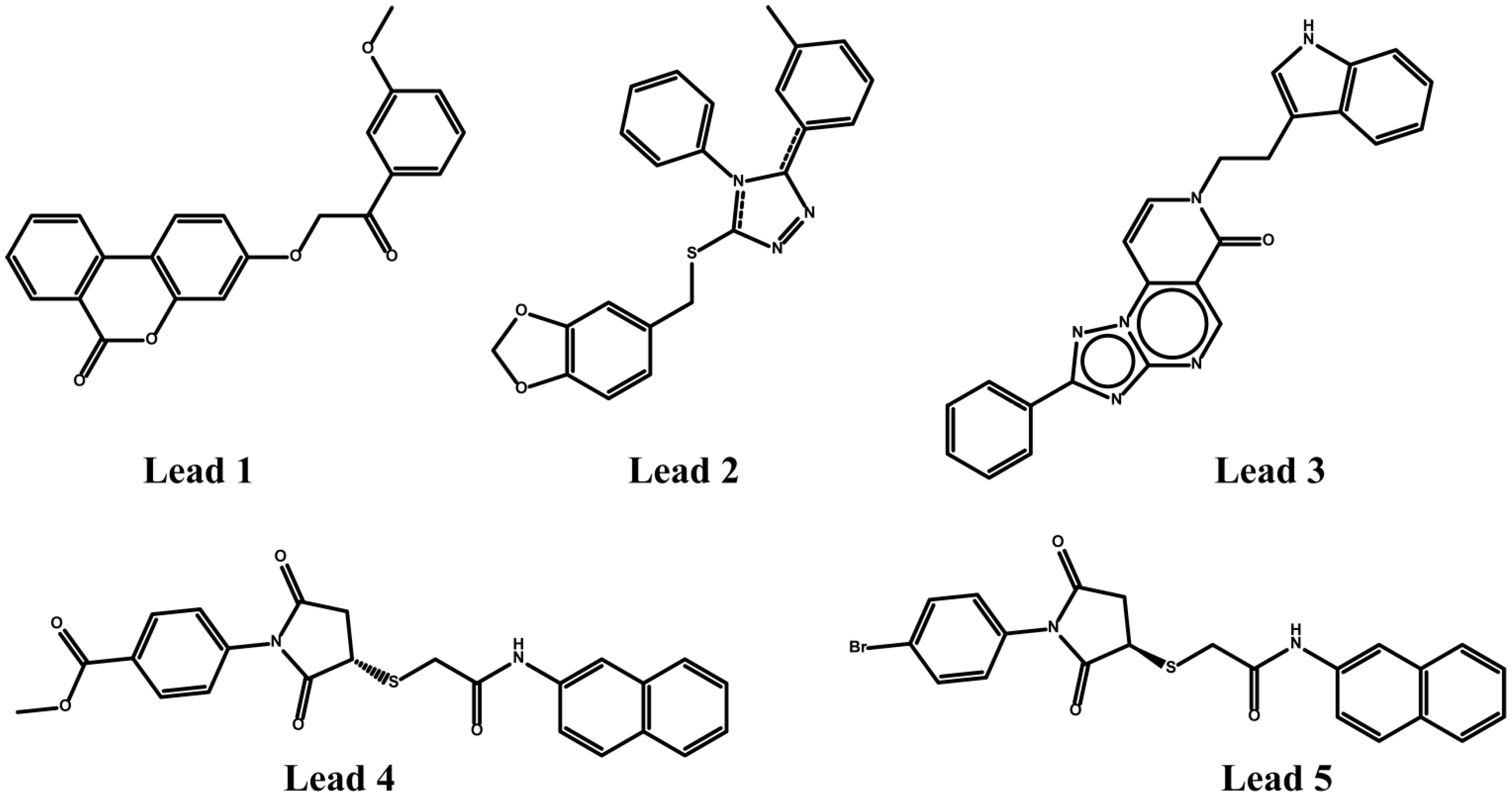
2.1. Novel Synthetic Molecules Acting on the AT1R


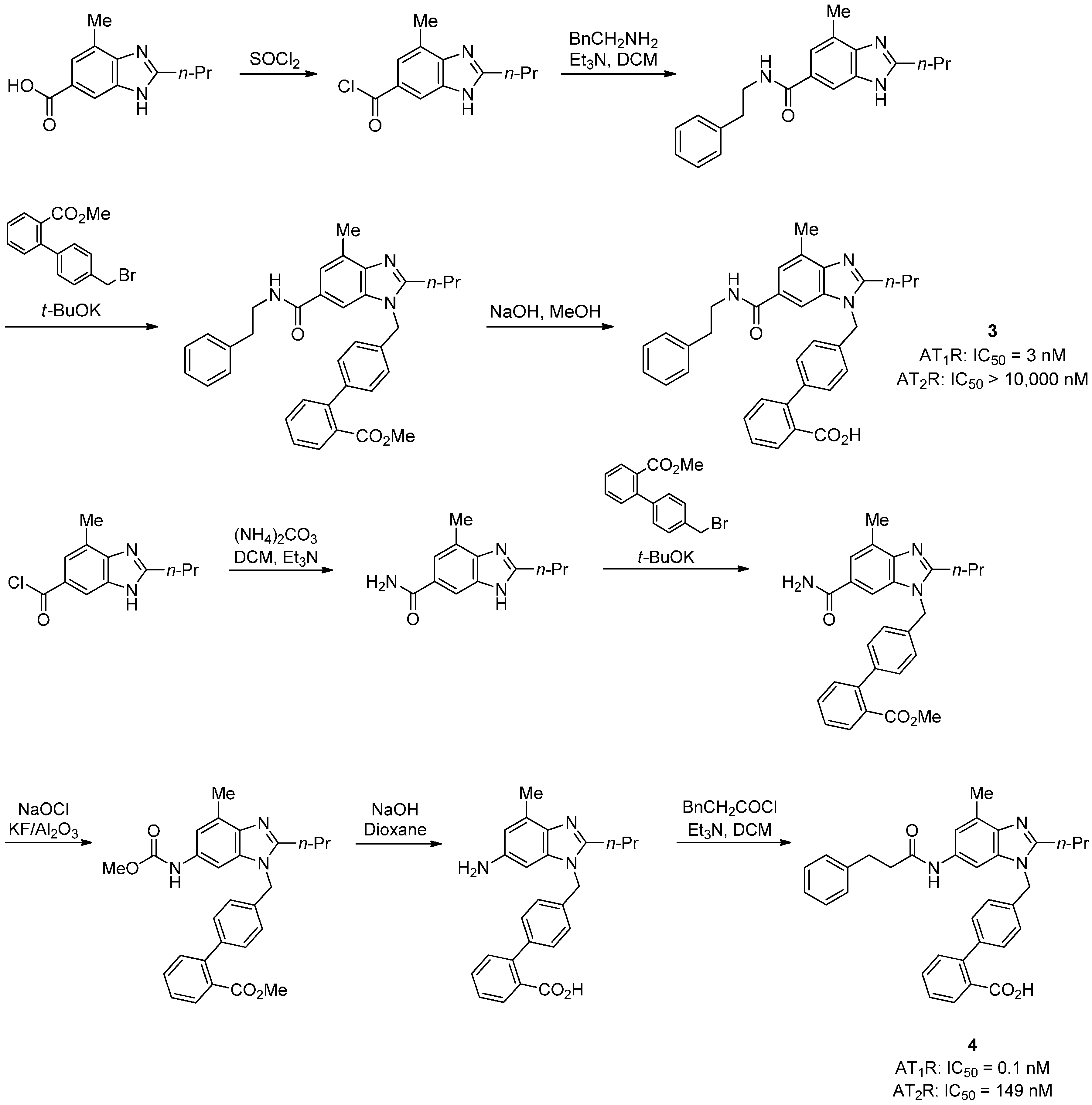
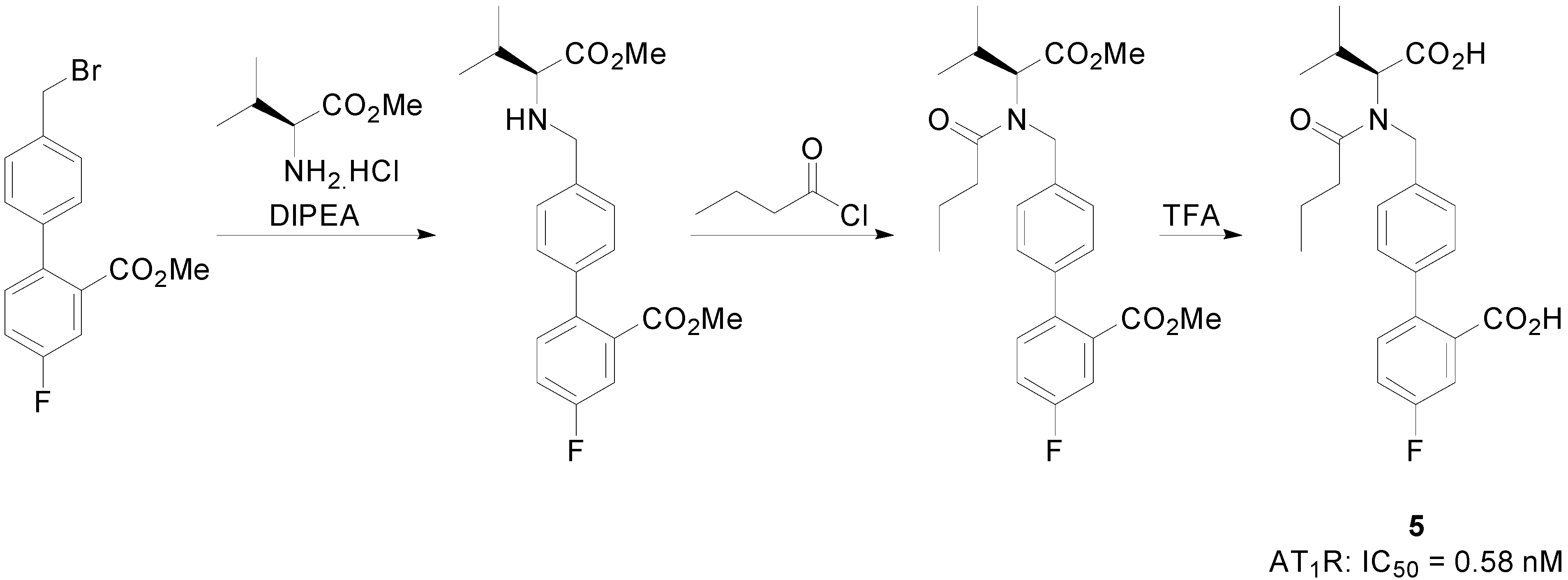


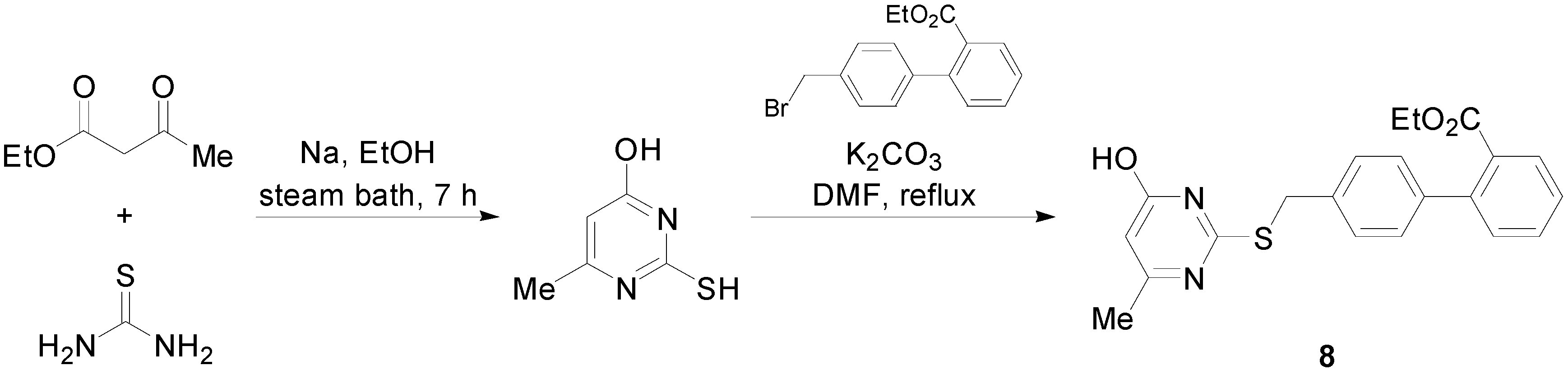
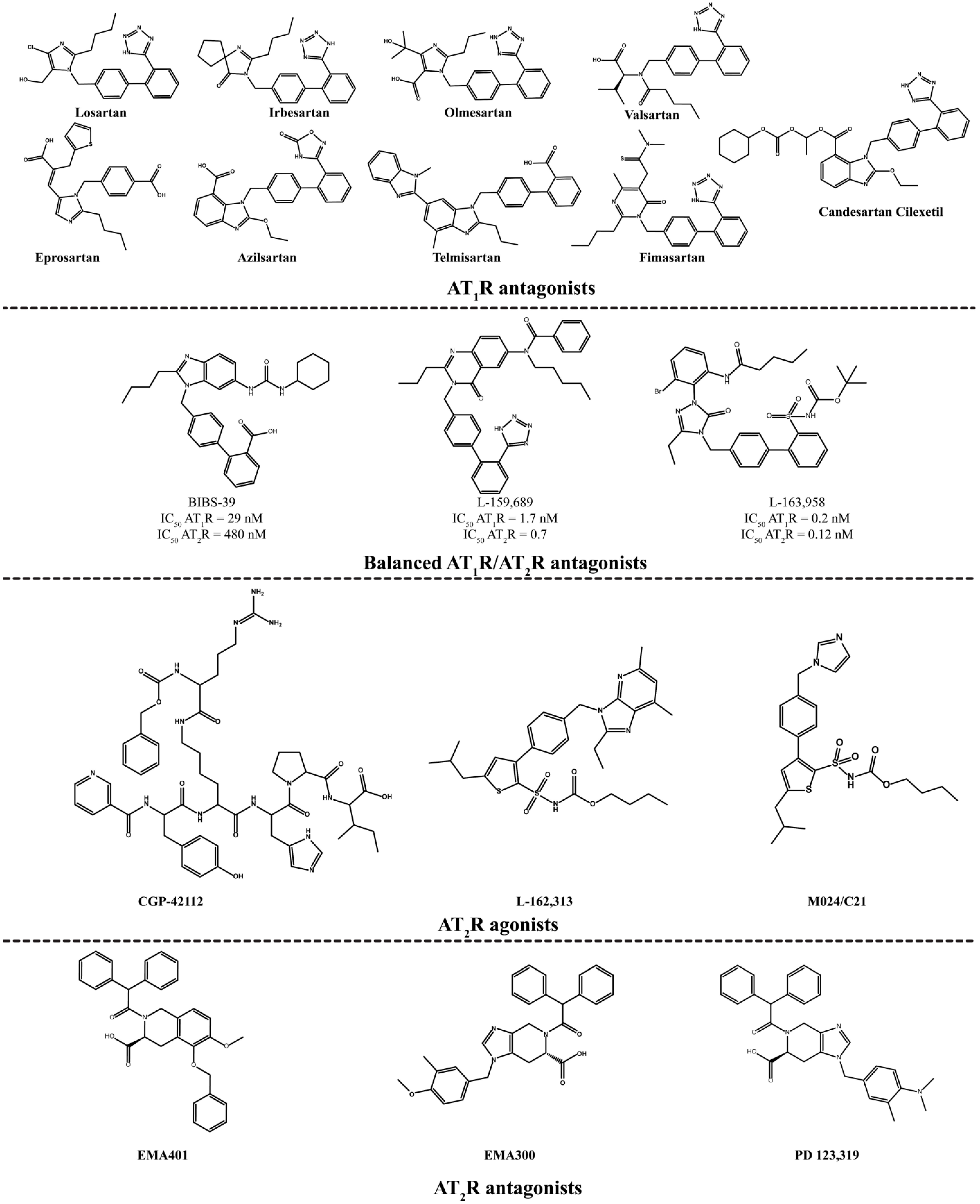
2.2. AT2R Agonists and Antagonists
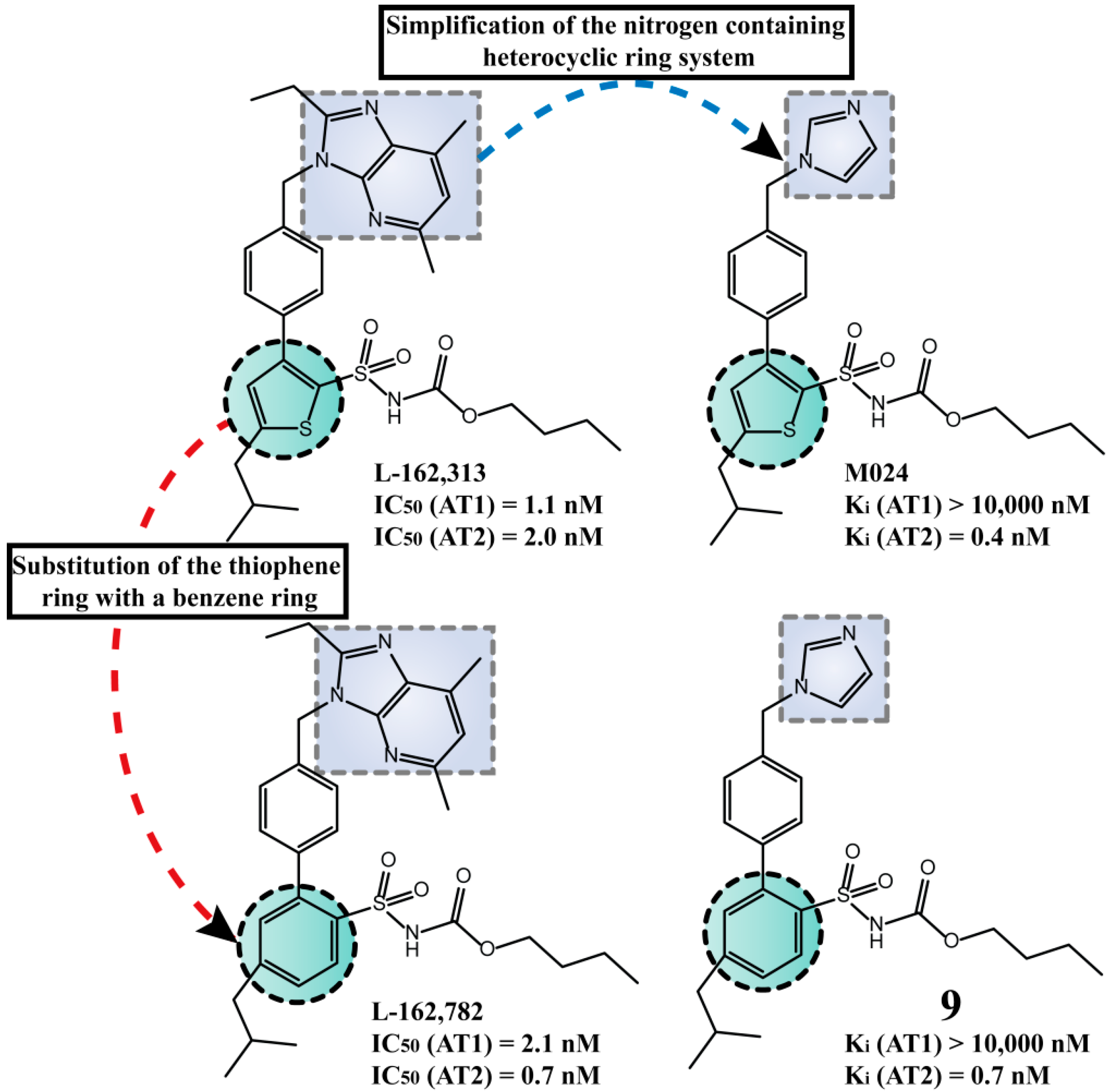
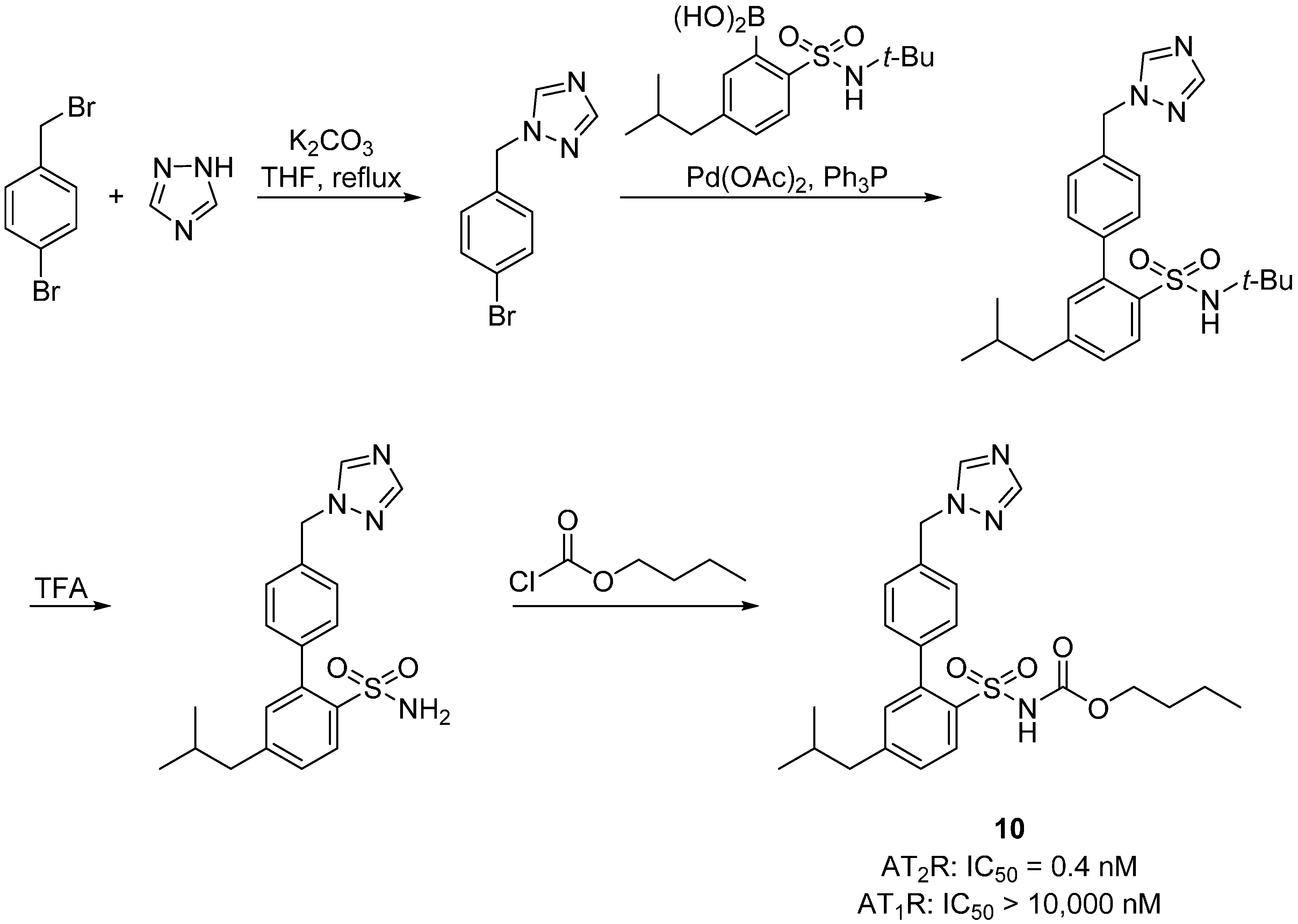
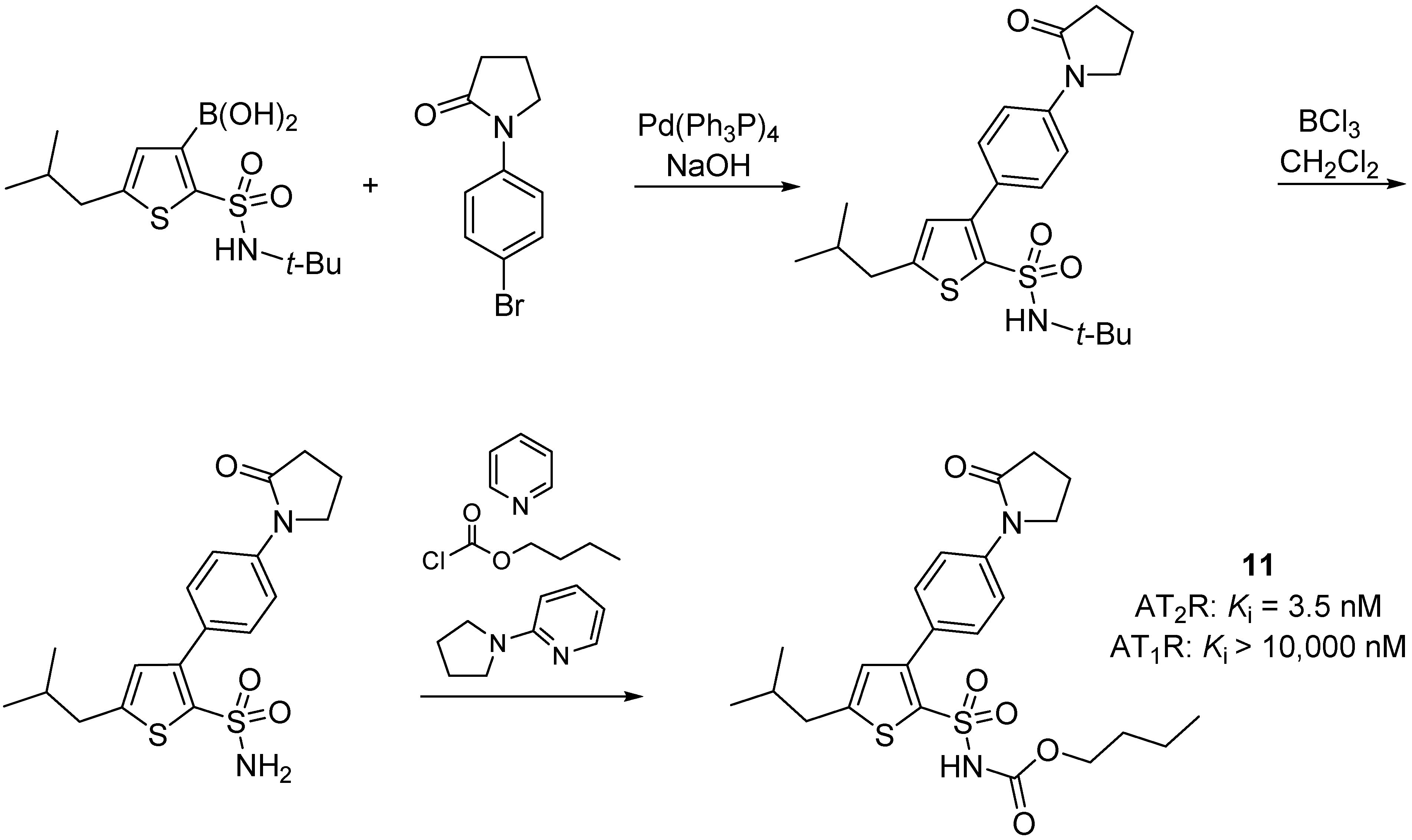
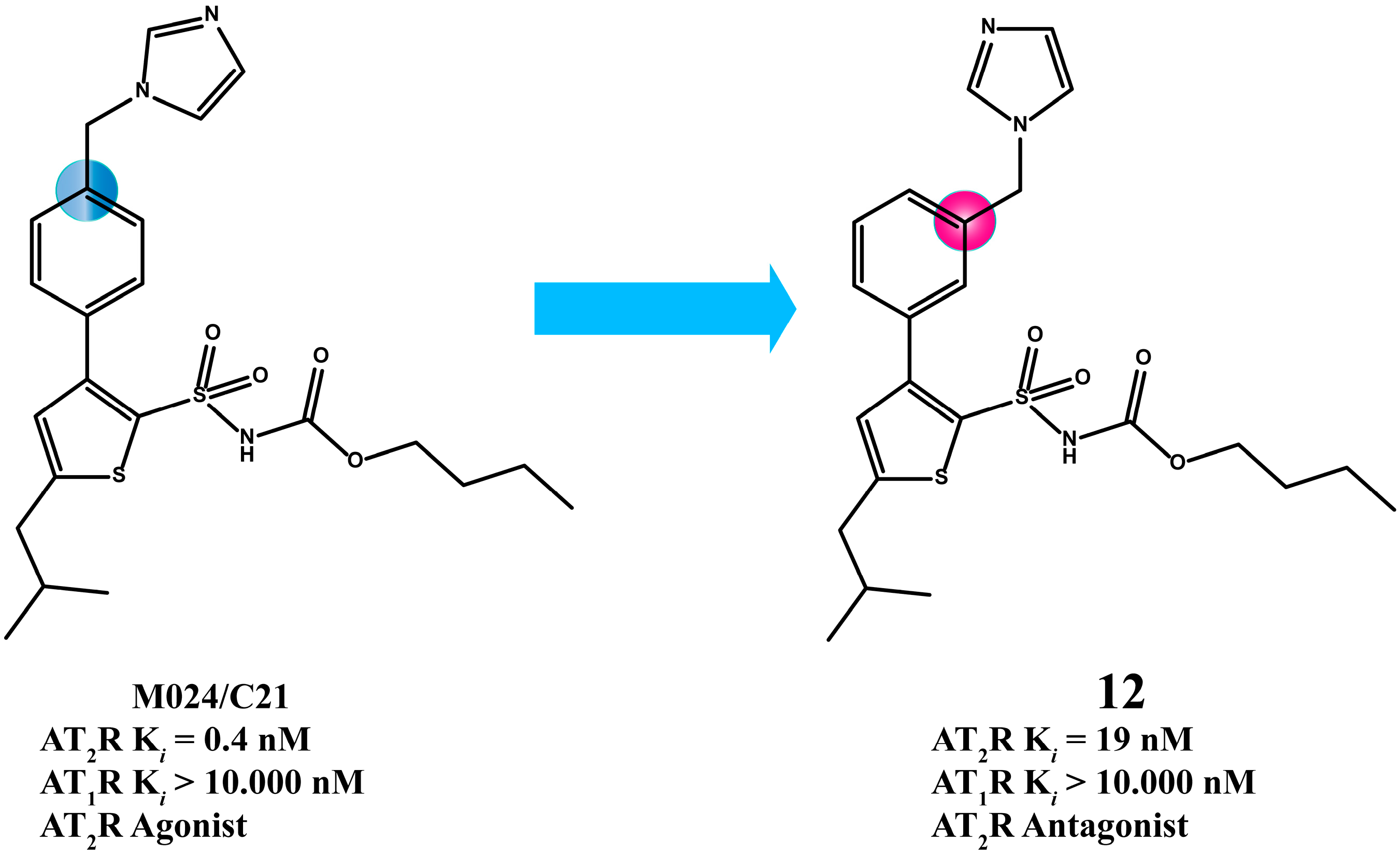

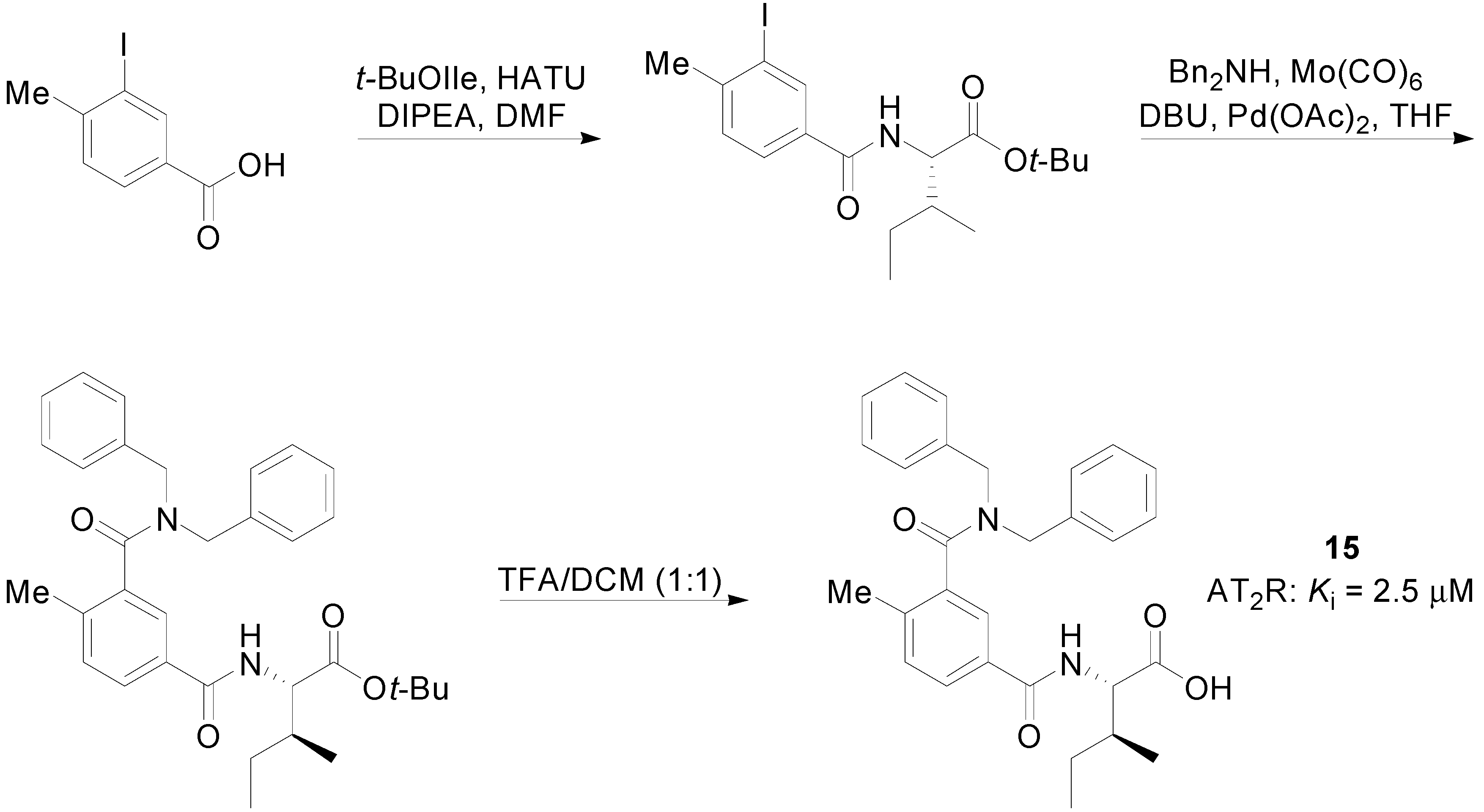
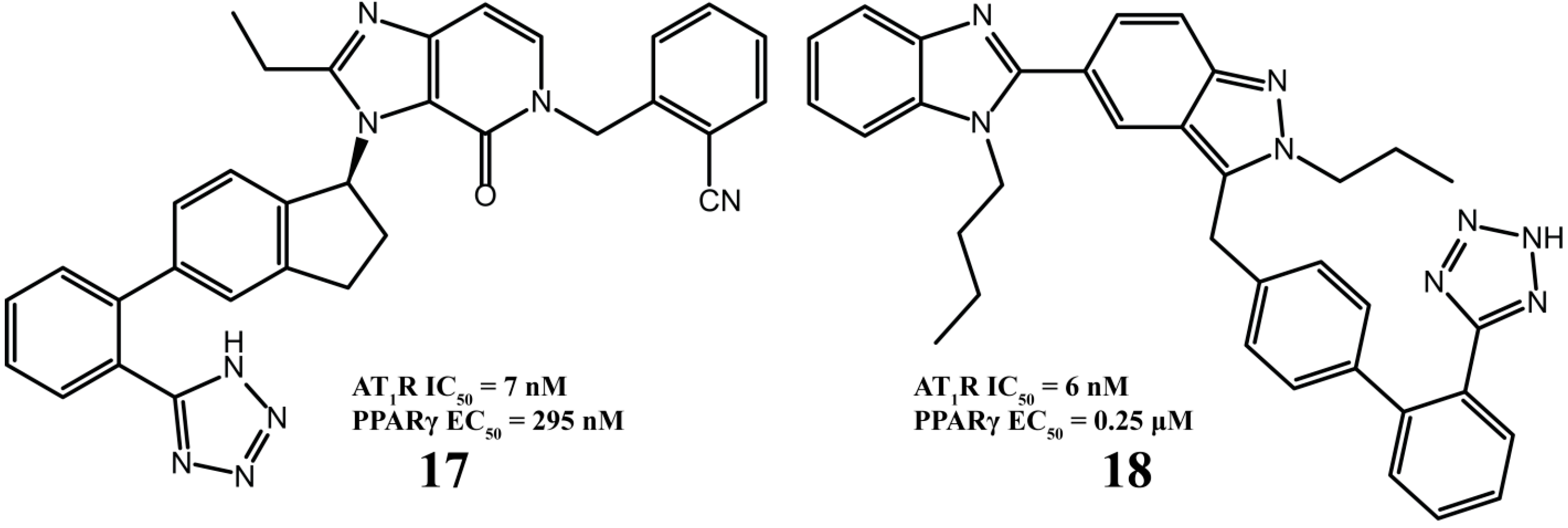
2.3. Multitarget Drugs


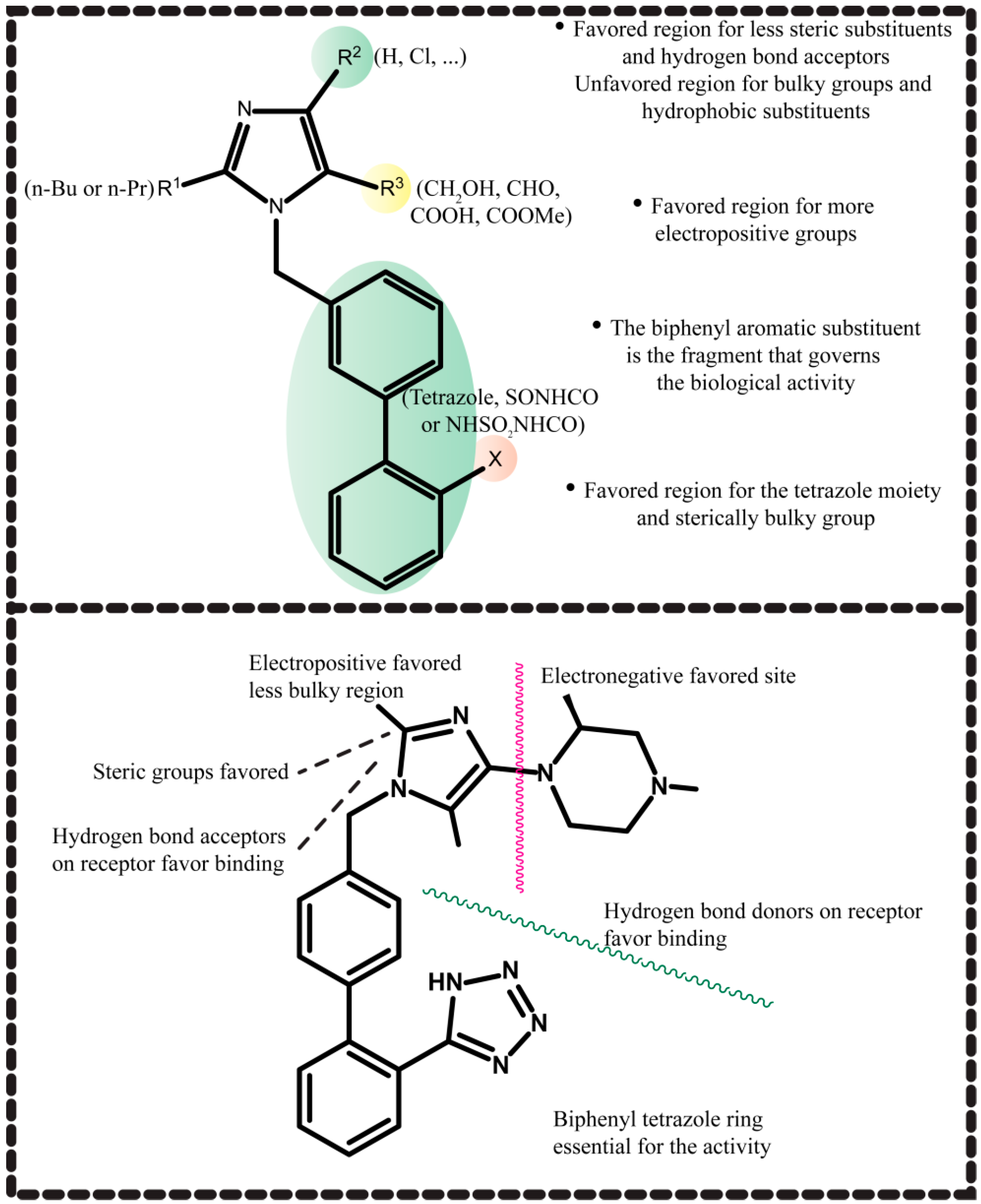
2.4. Molecular Modeling


2.5. Mechanisms of Activation of AT1R and AT2R and Signalling
2.6. The Role of Membrane Bilayers in the Binding to AT Receptors
3. Future Perspectives

Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Gasparo, M.; Catt, K.J.; Inagami, T.; Wright, J.W.; Unger, T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol. Rev. 2000, 52, 415–472. [Google Scholar]
- Burnier, M.; Brunner, H.R. Comparative antihypertensive effects of angiotensin II receptor antagonists. J. Am. Soc. Nephrol. 1999, 10, S278–S282. [Google Scholar] [PubMed]
- Wood, J.M.; Maibaum, J.; Rahuel, J.; Grütter, M.G.; Cohen, N.C.; Rasetti, V.; Rüger, H.; Göschke, R.; Stutz, S.; Fuhrer, W.; et al. Structure-based design of aliskiren, a novel orally effective renin inhibitor. Biochem. Biophys. Res. Commun. 2003, 308, 698–705. [Google Scholar] [CrossRef]
- Unger, T.; Chung, O.; Csikos, T.; Culman, J.; Gallinat, S.; Gohlke, P.; Hohle, S.; Meffert, S.; Stoll, M.; Stroth, U.; et al. Angiotensin receptors. J. Hypertens. Suppl. 1996, 14, S95–S103. [Google Scholar] [CrossRef] [PubMed]
- Swindle, J.D.; Santos, K.L.; Speth, R.C. Pharmacological characterization of a novel non-AT1, non-AT2 angiotensin binding site identified as neurolysin. Endocrine 2013, 44, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Wangler, N.J.; Santos, K.L.; Schadock, I.; Hagen, F.K.; Escher, E.; Bader, M.; Speth, R.C.; Karamyan, V.T. Identification of membrane-bound variant of metalloendopeptidase neurolysin (EC 3.4.24.16) as the non-angiotensin type 1 (non-AT1), non-AT2 angiotensin binding site. J. Biol. Chem. 2012, 287, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Speth, R.C.; Carrera, E.J.; Bretón, C.; Linares, A.; Gonzalez-Reiley, L.; Swindle, J.D.; Santos, K.L.; Schadock, I.; Bader, M.; Karamyan, V.T. Distribution of non-AT1, non-AT2 binding of 125i-sarcosine, isoleucine8 angiotensin ii in neurolysin knockout mouse brains. PLoS One 2014, 9, e105762. [Google Scholar] [CrossRef]
- Unger, T. The angiotensin type 2 receptor: Variations on an enigmatic theme. J. Hypertens. 1999, 17, 1775–1786. [Google Scholar] [CrossRef] [PubMed]
- Steckelings, U.M.; Rompe, F.; Kaschina, E.; Namsolleck, P.; Grzesiak, A.; Funke-Kaiser, H.; Bader, M.; Unger, T. The past, present and future of angiotensin II type 2 receptor stimulation. J. Renin Angiotensin Aldosterone Syst. 2010, 11, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Alterman, M. Development of selective non-peptide angiotensin II type 2 receptor agonsists. J. Renin Angiotensin Aldosterone Syst. 2010, 11, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Unger, T.; Dahlöf, B. Compound 21, the first orally active, selective agonist of the angiotensin type 2 receptor (AT2): Implications for AT2 receptor research and therapeutic potential. J. Renin Angiotensin Aldosterone Syst. 2010, 11, 75–77. [Google Scholar] [CrossRef]
- Stoll, M.; Steckelings, U.M.; Paul, M.; Bottari, S.P.; Metzger, R.; Unger, T. The angiotensin AT2-receptor mediates inhibition of cell proliferation in coronary endothelial cells. J. Clin. Investig. 1995, 95, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Nouet, S.; Nahmias, C. Signal transduction from the angiotensin II AT2 receptor. Trends Endocrinol. Metab. 2000, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Kondo, T.; Numaguchi, Y.; Kobayashi, K.; Aoki, M.; Inoue, N.; Okumura, K.; Murohara, T. Role of bradykinin, nitric oxide, and angiotensin II type 2 receptor in imidapril-induced angiogenesis. Hypertension 2008, 51, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Toyokawa, H.; Yamao, J.; Satoi, S.; Yanagimoto, H.; Yamamoto, T.; Hirooka, S.; Yamaki, S.; Inoue, K.; Matsui, Y.; et al. Antitumor effect of angiotensin II type 1 receptor blocker losartan for orthotopic rat pancreatic adenocarcinoma. Pancreas 2014, 43, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wang, F.; Li, L.; Bum-Erdene, K.; Xu, D.; Wang, B.; Sinn, A.A.; Pollok, K.E.; Sandusky, G.E.; Li, L.; et al. Exploring a structural protein-drug interactome for new therapeutics in lung cancer. Mol. BioSyst. 2014, 10, 581–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Meng, Q.; Zhao, Y.; Liu, M.; Li, D.; Yang, Y.; Sun, L.; Sui, G.; Cai, L.; Dong, X. Angiotensin II type 1 receptor antagonists inhibit cell proliferation and angiogenesis in breast cancer. Cancer Lett. 2013, 328, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, D.R.; Ateeq, B.; Cao, Q.; Tomlins, S.A.; Mehra, R.; Laxman, B.; Kalyana-Sundaram, S.; Lonigro, R.J.; Helgeson, B.E.; Bhojani, M.S.; et al. Agtr1 overexpression defines a subset of breast cancer and confers sensitivity to losartan, an agtr1 antagonist. Proc. Natl. Acad. Sci. USA 2009, 106, 10284–10289. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, H.; Fujita, A.; Bando, S.Y.; Iamashita, P.; Moreira-Filho, C.A. Transcriptional network analysis reveals that AT1 and AT2 angiotensin II receptors are both involved in the regulation of genes essential for glioma progression. PLoS One 2014, 9, e110934. [Google Scholar] [CrossRef] [PubMed]
- Dhande, I.; Ma, W.; Hussain, T. Angiotensin AT2 receptor stimulation is anti-inflammatory in lipopolysaccharide-activated thp-1 macrophages via increased interleukin-10 production. Hypertens. Res. 2015, 38, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Castoldi, G.; di Gioia, C.R.; Bombardi, C.; Maestroni, S.; Carletti, R.; Steckelings, U.M.; Dahlof, B.; Unger, T.; Zerbini, G.; Stella, A. Prevention of diabetic nephropathy by compound 21, selective agonist of angiotensin type 2 receptors, in zucker diabetic fatty rats. Am. J. Physiol. Renal Physiol. 2014, 307, F1123–F1131. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.A.; Vinh, A.; Miller, A.A.; Hallberg, A.; Alterman, M.; Callaway, J.K.; Widdop, R.E. Direct angiotensin AT2 receptor stimulation using a novel AT2 receptor agonist, compound 21, evokes neuroprotection in conscious hypertensive rats. PLoS One 2014, 9, e95762. [Google Scholar] [CrossRef] [PubMed]
- Iwanami, J.; Mogi, M.; Tsukuda, K.; Jing, F.; Ohshima, K.; Wang, X.L.; Nakaoka, H.; Kan-no, H.; Chisaka, T.; Bai, H.Y.; et al. Possible synergistic effect of direct angiotensin II type 2 receptor stimulation by compound 21 with memantine on prevention of cognitive decline in type 2 diabetic mice. Eur. J. Pharmacol. 2014, 724, 9–15. [Google Scholar] [CrossRef]
- Namsolleck, P.; Boato, F.; Schwengel, K.; Paulis, L.; Matho, K.S.; Geurts, N.; Thone-Reineke, C.; Lucht, K.; Seidel, K.; Hallberg, A.; et al. AT2-receptor stimulation enhances axonal plasticity after spinal cord injury by upregulating bdnf expression. Neurobiol. Dis. 2013, 51, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, A.; Baoum, A.; Ohta, N.; Jacquez, S.; Seo, G.M.; Berkland, C.; Tamura, M. Intratracheal administration of a nanoparticle-based therapy with the angiotensin II type 2 receptor gene attenuates lung cancer growth. Cancer Res. 2012, 72, 2057–2067. [Google Scholar] [CrossRef] [PubMed]
- Magnani, F.; Pappas, C.G.; Crook, T.; Magafa, V.; Cordopatis, P.; Ishiguro, S.; Ohta, N.; Selent, J.; Bosnyak, S.; Jones, E.S.; et al. Electronic sculpting of ligand-gpcr subtype selectivity: The case of angiotensin II. ACS Chem. Biol. 2014, 9, 1420–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agelis, G.; Resvani, A.; Durdagi, S.; Spyridaki, K.; Tůmová, T.; Slaninová, J.; Giannopoulos, P.; Vlahakos, D.; Liapakis, G.; Mavromoustakos, T.; et al. The discovery of new potent non-peptide angiotensin II AT1 receptor blockers: A concise synthesis, molecular docking studies and biological evaluation of N-substituted 5-butylimidazole derivatives. Eur. J. Med. Chem. 2012, 55, 358–374. [Google Scholar] [CrossRef]
- Kohara, Y.; Kubo, K.; Imamiya, E.; Wada, T.; Inada, Y.; Naka, T. Synthesis and angiotensin II receptor antagonistic activities of benzimidazole derivatives bearing acidic heterocycles as novel tetrazole bioisosteres. J. Med. Chem. 1996, 39, 5228–5235. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, A.; Pericot Mohr Gl, G.; Gallelli, A.; Rizzo, M.; Anzini, M.; Vomero, S.; Mennuni, L.; Ferrari, F.; Makovec, F.; Menziani, M.C.; et al. Design, synthesis, structural studies, biological evaluation, and computational simulations of novel potent AT(1) angiotensin II receptor antagonists based on the 4-phenylquinoline structure. J. Med. Chem. 2004, 47, 2574–2586. [Google Scholar] [CrossRef] [PubMed]
- Hein, L.; Meinel, L.; Pratt, R.E.; Dzau, V.J.; Kobilka, B.K. Intracellular trafficking of angiotensin II and its AT1 and AT2 receptors: Evidence for selective sorting of receptor and ligand. Mol. Endocrinol. 1997, 11, 1266–1277. [Google Scholar] [CrossRef] [PubMed]
- Kaschina, E.; Unger, T. Angiotensin AT1/AT2 receptors: Regulation, signalling and function. Blood Press. 2003, 12, 70–88. [Google Scholar] [CrossRef] [PubMed]
- Steckelings, U.M.; Kaschina, E.; Unger, T. The AT2 receptor—A matter of love and hate. Peptides 2005, 26, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Dudley, D.T.; Panek, R.L.; Major, T.C.; Lu, G.H.; Bruns, R.F.; Klinkefus, B.A.; Hodges, J.C.; Weishaar, R.E. Subclasses of angiotensin II binding sites and their functional significance. Mol. Pharmacol. 1990, 38, 370–377. [Google Scholar] [PubMed]
- Whitebread, S.E.; Taylor, V.; Bottari, S.P.; Kamber, B.; de Gasparo, M. Radioiodinated cgp 42112a: A novel high affinity and highly selective ligand for the characterization of angiotensin AT2 receptors. Biochem. Biophys. Res. Commun. 1991, 181, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wallinder, C.; Plouffe, B.; Beaudry, H.; Mahalingam, A.K.; Wu, X.; Johansson, B.; Holm, M.; Botoros, M.; Karlén, A.; et al. Design, synthesis, and biological evaluation, of the first selective nonpeptide AT2 receptor agonist. J. Med. Chem. 2004, 47, 5995–6008. [Google Scholar] [CrossRef]
- Rice, A.S.C.; Dworkin, R.H.; McCarthy, T.D.; Anand, P.; Bountra, C.; McCloud, P.I.; Hill, J.; Cutter, G.; Kitson, G.; Desem, N.; et al. Ema401, an orally administered highly selective angiotensin II type 2 receptor antagonist, as a novel treatment for postherpetic neuralgia: A randomised, double-blind, placebo-controlled phase 2 clinical trial. The Lancet 2014, 383, 1637–1647. [Google Scholar] [CrossRef]
- McCarthy, T. Development of ema401 as an orally-administered, highly-selective angiotensin II type 2 receptor antagonist for the treatment of neuropathic pain. J. Peripher. Nerv. Syst. 2014, 19, S13–S14. [Google Scholar] [CrossRef] [PubMed]
- Ichiki, T.; Labosky, P.A.; Shiota, C.; Okuyama, S.; Imagawa, Y.; Fogo, A.; Niimura, F.; Ichikawa, I.; Hogan, B.L.M.; Inagami, T. Effects on blood pressure and exploratory behaviour of mice lacking angiotensin II type-2 receptor. Nature 1995, 377, 748–750. [Google Scholar] [CrossRef] [PubMed]
- Rein, L.; Barsh, G.S.; Pratt, R.E.; Dzau, V.J.; Kobilka, B.K. Behavioural and cardiovascular effects of disrupting the angiotensin II type-2 receptor gene in mice. Nature 1995, 377, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Speth, R.C.; Kim, K.H. Discrimination of two angiotensin II receptor subtypes with a selective agonist analogue of angiotensin II, p-aminophenylalanine6 angiotensin II. Biochem. Biophys. Res. Commun. 1990, 169, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Ohinata, K.; Yamada, Y. The pharmacological effects of novokinin; a designed peptide agonist of the angiotensin AT2 receptor. Curr. Pharm. Des. 2013, 19, 3009–3012. [Google Scholar] [CrossRef] [PubMed]
- Guimond, M.O.; Hallberg, M.; Gallo-Payet, N.; Wallinder, C. Saralasin and sarile are AT2 receptor agonists. ACS Med. Chem. Lett. 2014, 5, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Mavromoustakos, T.; Agelis, G.; Durdagi, S. AT1 antagonists: A patent review (2008–2012). Expert Opin. Ther. Pat. 2013, 23, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- Naik, P.; Murumkar, P.; Giridhar, R.; Yadav, M.R. Angiotensin II receptor type 1 (AT1) selective nonpeptidic antagonists—A perspective. Bioorg. Med. Chem. 2010, 18, 8418–8456. [Google Scholar] [CrossRef] [PubMed]
- Trstenjak, U.; Kikelj, D. Multitarget cardiovascular drugs. Curr. Med. Chem. 2011, 18, 2531–2542. [Google Scholar] [CrossRef] [PubMed]
- Tuccinardi, T.; Martinelli, A. Computational approaches on angiotensin receptors and their ligands: Recent developments and results. Curr. Med. Chem. 2007, 14, 3105–3121. [Google Scholar] [CrossRef] [PubMed]
- Agelis, G.; Resvani, A.; Koukoulitsa, C.; Tůmová, T.; Slaninová, J.; Kalavrizioti, D.; Spyridaki, K.; Afantitis, A.; Melagraki, G.; Siafaka, A.; et al. Rational design, efficient syntheses and biological evaluation of N,N'-symmetrically bis-substituted butylimidazole analogs as a new class of potent angiotensin II receptor blockers. Eur. J. Med. Chem. 2013, 62, 352–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agelis, G.; Resvani, A.; Matsoukas, M.T.; Tselios, T.; Kelaidonis, K.; Kalavrizioti, D.; Vlahakos, D.; Matsoukas, J. Towards non-peptide ang II AT1 receptor antagonists based on urocanic acid: Rational design, synthesis and biological evaluation. Amino Acids 2011, 40, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Agelis, G.; Resvani, A.; Ntountaniotis, D.; Chatzigeorgiou, P.; Koukoulitsa, C.; Androutsou, M.E.; Plotas, P.; Matsoukas, J.; Mavromoustakos, T.; Čendak, T.; et al. Interactions of the potent synthetic AT1 antagonist analog bv6 with membrane bilayers and mesoporous silicate matrices. BBA-Bioenergetics 2013, 1828, 1846–1855. [Google Scholar] [PubMed]
- Deprez-Poulain, R.; Cousaert, N.; Toto, P.; Willand, N.; Deprez, B. Application of ullmann and ullmann-finkelstein reactions for the synthesis of N-aryl-N-(1H-pyrazol-3-yl) acetamide or N-(1-aryl-1H-pyrazol-3-yl) acetamide derivatives and pharmacological evaluation. Eur. J. Med. Chem. 2011, 46, 3867–3876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.L.; Yu, W.F.; Zhou, Z.M.; Tao, W.C.; Wang, Y.C.; Xue, W.Z.; Xu, D.; Hao, L.P.; Han, X.F.; et al. Nonpeptidic angiotensin II AT1 receptor antagonists derived from 6-substituted aminocarbonyl and acylamino benzimidazoles. Eur. J. Med. Chem. 2013, 69, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Da, Y.J.; Yuan, W.D.; Xin, T.; Nie, Y.Y.; Ye, Y.; Yan, Y.J.; Liang, L.S.; Chen, Z.L. Synthesis and biological evaluation of new fluorine substituted derivatives as angiotensin II receptor antagonists with anti-hypertension and anti-tumor effects. Bioorg. Med. Chem. 2012, 20, 7101–7111. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Da, Y.; Wu, D.; Zheng, H.; Zhu, L.; Wang, L.; Yan, Y.; Chen, Z. Design, synthesis and biological evaluation of new 5-nitro benzimidazole derivatives as AT1 antagonists with anti-hypertension activities. Bioorg. Med. Chem. 2014, 22, 2294–2302. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.A.H.; Aboul-Enein, M.N.; El-Azzouny, A.A.E.; Abouzid, K.A.M.; Ismail, N.S.M. Design, synthesis, and antihypertensive evaluation of 2'-tetrazolyl and 2'-carboxy-biphenylylmethyl-pyrrolidine scaffolds substituted at their N1, C3, and C4 positions as potential angiotensin II AT1 receptor antagonists. Med. Chem. Res. 2015, 24, 442–458. [Google Scholar] [CrossRef]
- Ismail, M.A.H.; Abou El Ella, D.A.; Abouzid, K.A.M.; Al-Ansary, G.H.A. Computer-based drug design, synthesis and biological evaluation of new pyrimidinone derivatives linked to arylpiperazine and 2'-carbethoxy-biphenylylmethyl moeities as α1-adrenoceptor antagonists and angiotensin II AT1 receptor antagonists. Pharmazie 2010, 65, 794–800. [Google Scholar] [PubMed]
- Namsolleck, P.; Recarti, C.; Foulquier, S.; Steckelings, U.M.; Unger, T. AT2 receptor and tissue injury: Therapeutic implications. Curr. Hypertens. Rep. 2014, 16, 416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamargo, J.; López-Sendón, J. Novel therapeutic targets for the treatment of heart failure. Nat. Rev. Drug Discov. 2011, 10, 536–555. [Google Scholar] [CrossRef] [PubMed]
- Wallinder, C.; Botros, M.; Rosenström, U.; Guimond, M.O.; Beaudry, H.; Nyberg, F.; Gallo-Payet, N.; Hallberg, A.; Alterman, M. Selective angiotensin II AT2 receptor agonists: Benzamide structure-activity relationships. Bioorg. Med. Chem. 2008, 16, 6841–6849. [Google Scholar] [CrossRef] [PubMed]
- Steckelings, U.M.; Larhed, M.; Hallberg, A.; Widdop, R.E.; Jones, E.S.; Wallinder, C.; Namsolleck, P.; Dahlöf, B.; Unger, T. Non-peptide AT2-receptor agonists. Curr. Opin. Pharm. 2011, 11, 187–192. [Google Scholar] [CrossRef]
- Wu, X.; Wan, Y.; Mahalingam, A.K.; Plouffe, B.; Botros, M.; Karlén, A.; Hallberg, M.; Gallo-Payet, N.; Alterman, M. Selective angiotensin II AT2 receptor agonists: Arylbenzylimidazole structure-activity relationships. J. Med. Chem. 2006, 49, 7160–7168. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Q.; Yang, X.; Xu, S.; Zhang, H.; Bai, R.; Yao, H.; Jiang, J.; Shen, M.; Wu, X.; et al. Design, synthesis, and biological evaluation of 1,2,4-triazole bearing 5-substituted biphenyl-2-sulfonamide derivatives as potential antihypertensive candidates. Bioorg. Med. Chem. 2013, 21, 7742–7751. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, A.K.; Wan, Y.; Murugaiah, A.M.S.; Wallinder, C.; Wu, X.; Plouffe, B.; Botros, M.; Nyberg, F.; Hallberg, A.; Gallo-Payet, N.; et al. Selective angiotensin II AT2 receptor agonists with reduced cyp 450 inhibition. Bioorg. Med. Chem. 2010, 18, 4570–4590. [Google Scholar] [CrossRef] [PubMed]
- Murugaiah, A.M.S.; Wu, X.; Wallinder, C.; Mahalingam, A.K.; Wan, Y.; Sköld, C.; Botros, M.; Guimond, M.O.; Joshi, A.; Nyberg, F.; et al. From the first selective non-peptide at 2 receptor agonist to structurally related antagonists. J. Med. Chem. 2012, 55, 2265–2278. [Google Scholar] [CrossRef] [PubMed]
- Verdonk, K.; Durik, M.; Abd-Alla, N.; Batenburg, W.W.; van den Bogaerdt, A.J.; van Veghel, R.; Roks, A.J.; Danser, A.H.; van Esch, J.H. Compound 21 induces vasorelaxation via an endothelium- and angiotensin II type 2 receptor-independent mechanism. Hypertension 2012, 60, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Veron, J.-B.; Joshi, A.; Wallinder, C.; Larhed, M.; Odell, L.R. Synthesis and evaluation of isoleucine derived angiotensin II AT2 receptor ligands. Bioorg. Med. Chem. Lett. 2014, 24, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Georgsson, J.; Sköld, C.; Botros, M.; Lindeberg, G.; Nyberg, F.; Karlén, A.; Hallberg, A.; Larhed, M. Synthesis of a new class of druglike angiotensin II C-terminal mimics with affinity for the AT2 receptor. J. Med. Chem. 2007, 50, 1711–1715. [Google Scholar] [CrossRef] [PubMed]
- Behrends, M.; Wallinder, C.; Wieckowska, A.; Guimond, M.O.; Hallberg, A.; Gallo-Payet, N.; Larhed, M. N-aryl isoleucine derivatives as angiotensin II AT2 receptor ligands. ChemistryOpen 2014, 3, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Janga, S.C.; Tzakos, A. Structure and organization of drug-target networks: Insights from genomic approaches for drug discovery. Mol. BioSyst. 2009, 5, 1536–1548. [Google Scholar] [CrossRef] [PubMed]
- Mojarrad, J.S.; Zamani, Z.; Nazemiyeh, H.; Ghasemi, S.; Asgari, D. Synthesis of novel 1,4-dihydropyridine derivatives bearing biphenyl-2'-tetrazole substitution as potential dual angiotensin II receptors and calcium channel blockers. Adv. Pharm. Bull. 2011, 1, 1–9. [Google Scholar] [PubMed]
- Hadizadeh, F.; Imenshahidi, M.; Esmaili, P.; Taghiabadi, M. Synthesis and effects of novel dihydropyridines as dual calcium channel blocker and angiotensin antagonist on isolated rat aorta. Iranian J. Basic Med. Sci. 2010, 13, 195–201. [Google Scholar]
- Casimiro-Garcia, A.; Heemstra, R.J.; Bigge, C.F.; Chen, J.; Ciske, F.A.; Davis, J.A.; Ellis, T.; Esmaeil, N.; Flynn, D.; Han, S.; et al. Design, synthesis, and evaluation of imidazo[4,5-c]pyridin-4-one derivatives with dual activity at angiotensin II type 1 receptor and peroxisome proliferator-activated receptor-γ. Bioorg. Med. Chem. Lett. 2013, 23, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Lamotte, Y.; Faucher, N.; Sançon, J.; Pineau, O.; Sautet, S.; Fouchet, M.H.; Beneton, V.; Tousaint, J.J.; Saintillan, Y.; Ancellin, N.; et al. Discovery of novel indazole derivatives as dual angiotensin II antagonists and partial pparγ agonists. Bioorg. Med. Chem. Lett. 2014, 24, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, J.; Pan, W.; Wu, X.; Wang, S. Synthesis and biological study of 3-butyl-1-(2,6-dichlorophenyl)-1H-[1,2,4]triazol-5(4H)-one derivatives as anti-hypertension drugs. Lett. Drug Des. Discov. 2010, 7, 18–22. [Google Scholar] [CrossRef]
- Murugesan, N. Chapter 19 designing drugs with dual activity: Novel dual angiotensin II and endothelin receptor antagonists. In Designing Multi-Target Drugs; The Royal Society of Chemistry: Cambridge, UK, 2012; pp. 316–334. [Google Scholar]
- Ohno, K.; Amano, Y.; Kakuta, H.; Niimi, T.; Takakura, S.; Orita, M.; Miyata, K.; Sakashita, H.; Takeuchi, M.; Komuro, I.; et al. Unique “delta lock” structure of telmisartan is involved in its strongest binding affinity to angiotensin II type 1 receptor. Biochem. Biophys. Res. Commun. 2011, 404, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Kritsi, E.; Potamitis, C.; Durdagi, S.; Zoumpoulakis, P.; Golic Grdadolnik, S.; Mavromoustakos, T. Molecular insights into the AT1 antagonism based on biophysical and in silico studies of telmisartan. Med. Chem. Res. 2013, 22, 4842–4857. [Google Scholar] [CrossRef]
- Sharma, M.C.; Sharma, S.; Sharma, P.; Kumar, A.; Bhadoriya, K.S. Structural insights for substituted acyl sulfonamides and acyl sulfamides derivatives of imidazole as angiotensin II receptor antagonists using molecular modeling approach. J. Taiwan Inst. Chem. E 2014, 45, 12–23. [Google Scholar] [CrossRef]
- Sharma, M.C.; Kohli, D.V. A comprehensive structure-activity analysis 2,3,5-trisubstituted 4,5-dihydro-4-oxo-3H-imidazo[4,5-c]pyridine derivatives as angiotensin II receptor antagonists: Using 2D- and 3D-QSAR approach. Med. Chem. Res. 2013, 22, 588–605. [Google Scholar] [CrossRef]
- Sharma, M.C.; Kohli, D.V. Comprehensive structure-activity relationship analysis of substituted 5-(biphenyl-4-ylmethyl)pyrazoles derivatives as AT1 selective angiotensin II receptor antagonists: 2D and KNNMFA QSAR approach. Med. Chem. Res. 2013, 22, 2124–2138. [Google Scholar] [CrossRef]
- Sharma, M.C.; Kohli, D.V. Predicting substituted 2-butylbenzimidazoles derivatives as angiotensin II receptor antagonists: 3D-QSAR and pharmacophore modeling. J. Saudi Chem. Soc. 2011. [Google Scholar] [CrossRef]
- Vyas, V.K.; Gupta, N.; Ghate, M.; Patel, S. Design, synthesis, pharmacological evaluation and in silico admet prediction of novel substituted benzimidazole derivatives as angiotensin II-AT1 receptor antagonists based on predictive 3D QSAR models. SAR QSAR Environ. Res. 2014, 25, 117–146. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.D.; Muthusamy, K. Molecular modeling, quantum polarized ligand docking and structure-based 3D-QSAR analysis of the imidazole series as dual KT1 and ET a receptor antagonists. Acta Pharmacol. Sin. 2013, 34, 1592–1606. [Google Scholar] [CrossRef] [PubMed]
- Da C. Silva, D.; Maltarollo, V.G.; de Lima, E.F.; Weber, K.C.; Honorio, K.M. Understanding electrostatic and steric requirements related to hypertensive action of AT1 antagonists using molecular modeling techniques. J. Mol. Model. 2014, 20, 2231–2244. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Kwon, G.H.; Han, N.S.; Yoo, B.W.; Kim, J.H.; Paik, S.H.; Chi, Y.H.; Lee, K.-T.; Lee, J.Y. Development of 3D-QSAR CoMSIA models for 5-(biphenyl-2-yl)-1H-tetrazole derivatives as angiotensin II receptor type 1 (AT1) antagonists. Bioorg. Med. Chem. Lett. 2013, 23, 4540–4546. [Google Scholar] [CrossRef] [PubMed]
- Sköld, C.; Nikiforovich, G.; Karlén, A. Modeling binding modes of angiotensin II and pseudopeptide analogues to the AT2 receptor. J. Mol. Graphics Model. 2008, 26, 991–1003. [Google Scholar] [CrossRef]
- Miura, S.I.; Imaizumi, S.; Saku, K. Recent progress in molecular mechanisms of angiotensin II type 1 and 2 receptors. Curr. Pharm. Des. 2013, 19, 2981–2987. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, H.; Yano, M.; Yabumoto, C.; Kudo-Sakamoto, Y.; Komuro, I. Angiotensin II type 1 and type 2 receptor-induced cell signaling. Curr. Pharm. Des. 2013, 19, 2988–2995. [Google Scholar] [CrossRef] [PubMed]
- Matsoukas, M.T.; Potamitis, C.; Plotas, P.; Androutsou, M.E.; Agelis, G.; Matsoukas, J.; Zoumpoulakis, P. Insights into AT1 receptor activation through ANGII binding studies. J. Chem. Inf. Model. 2013, 53, 2798–2811. [Google Scholar] [CrossRef] [PubMed]
- Matsoukas, M.T.; Cordomí, A.; Ríos, S.; Pardo, L.; Tselios, T. Ligand binding determinants for angiotensin II type 1 receptor from computer simulations. J. Chem. Inf. Model. 2013, 53, 2874–2883. [Google Scholar] [CrossRef] [PubMed]
- Sokkar, P.; Mohandass, S.; Ramachandran, M. Multiple templates-based homology modeling enhances structure quality of AT1 receptor: Validation by molecular dynamics and antagonist docking. J. Mol. Model. 2011, 17, 1565–1577. [Google Scholar] [CrossRef] [PubMed]
- Prokop, J.W.; Santos, R.A.S.; Milsted, A. Differential mechanisms of activation of the ang peptide receptors AT1, AT2, and MAS: Using in silico techniques to differentiate the three receptors. PLoS One 2013, 8, e65307. [Google Scholar] [CrossRef] [PubMed]
- Fillion, D.; Cabana, J.; Guillemette, G.; Leduc, R.; Lavigne, P.; Escher, E. Structure of the human angiotensin II type 1 (AT1) receptor bound to angiotensin II from multiple chemoselective photoprobe contacts reveals a unique peptide binding mode. J. Biol. Chem. 2013, 288, 8187–8197. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, P.; Jagadeesh, G. Structural determinants for binding, activation, and functional selectivity of the angiotensin AT1 receptor. J. Mol. Endocrinol. 2014, 53, R71–R92. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-F.; Jiang, Y.-R.; Pan, Y.-F.; Chen, D.; Li, C.-J. Multiple templates-based homology modeling and docking analysis of angiotensin II type 1 receptor. J. Cent. South. Univ. 2012, 19, 3033–3039. [Google Scholar] [CrossRef]
- Mavromoustakos, T.; Durdagi, S.; Koukoulitsa, C.; Simcic, M.; Papadopoulos, M.G.; Hodoscek, M.; Grdadolnik, S.G. Strategies in the rational drug design. Curr. Med. Chem. 2011, 18, 2517–2530. [Google Scholar] [CrossRef] [PubMed]
- Zoumpoulakis, P.; Mavromoustakos, T. Seeking the active site of the AT1 receptor for computational docking studies. Drug Design Reviews Online 2005, 2, 537–545. [Google Scholar] [CrossRef]
- Mavromoustakos, T.; Zervou, M.; Zoumpoulakis, P.; Kyrikou, I.; Benetis, N.P.; Polevaya, L.; Roumelioti, P.; Giatas, N.; Zoga, A.; Moutevelis Minakakis, P.; et al. Conformation and bioactivity. Design and discovery of novel antihypertensive drugs. Curr. Top. Med. Chem. 2004, 4, 385–401. [Google Scholar] [CrossRef] [PubMed]
- Mavromoustakos, T.M.; Zervou, M.V.; Zoumpoulakis, P.G. Design and discovery of novel antihypertensive drugs through conformation and bioactivity studies. In Frontiers in Medicinal Chemistry; Atta-Ur-Rahman, Reitz, A.B., Eds.; Bentham Science Publishers: Sharjah, UAE, 2006; Volume 3, pp. 87–111. [Google Scholar]
- Mavromoustakos, T.; Zoumpoulakis, I.; Kyrikou, I.; Zoga, A.; Siapi, E.; Zervou, M.; Daliani, I.; Dimitriou, D.; Pitsas, A.; Kamoutsis, C.; et al. Efforts to understand the molecular basis of hypertension through drug: Membrane interactions. Curr. Top. Med. Chem. 2004, 4, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Fotakis, C.; Megariotis, G.; Christodouleas, D.; Kritsi, E.; Zoumpoulakis, P.; Ntountaniotis, D.; Zervou, M.; Potamitis, C.; Hodzic, A.; Pabst, G.; et al. Comparative study of the AT1 receptor prodrug antagonist candesartan cilexetil with other sartans on the interactions with membrane bilayers. BBA-Bioenergetics 2012, 1818, 3107–3120. [Google Scholar] [PubMed]
- Hodzic, A.; Zoumpoulakis, P.; Pabst, G.; Mavromoustakos, T.; Rappolt, M. Losartan’s affinity to fluid bilayers modulates lipid-cholesterol interactions. PCCP 2012, 14, 4780–4788. [Google Scholar] [CrossRef] [PubMed]
- Ntountaniotis, D.; Mali, G.; Grdadolnik, S.G.; Maria, H.; Skaltsounis, A.L.; Potamitis, C.; Siapi, E.; Chatzigeorgiou, P.; Rappolt, M.; Mavromoustakos, T. Thermal, dynamic and structural properties of drug AT1 antagonist olmesartan in lipid bilayers. BBA-Bioenergetics 2011, 1808, 2995–3006. [Google Scholar] [PubMed]
- Fotakis, C.; Christodouleas, D.; Zoumpoulakis, P.; Kritsi, E.; Benetis, N.P.; Mavromoustakos, T.; Reis, H.; Gili, A.; Papadopoulos, M.G.; Zervou, M. Comparative biophysical studies of sartan class drug molecules losartan and candesartan (CV-11974) with membrane bilayers. J. Phys. Chem. B 2011, 115, 6180–6192. [Google Scholar] [CrossRef] [PubMed]
- Potamitis, C.; Chatzigeorgiou, P.; Siapi, E.; Viras, K.; Mavromoustakos, T.; Hodzic, A.; Pabst, G.; Cacho-Nerin, F.; Laggner, P.; Rappolt, M. Interactions of the AT1 antagonist valsartan with dipalmitoyl-phosphatidylcholine bilayers. BBA-Bioenergetics 2011, 1808, 1753–1763. [Google Scholar] [PubMed]
- Mavromoustakos, T.; Chatzigeorgiou, P.; Koukoulitsa, C.; Durdagi, S. Partial interdigitation of lipid bilayers. Int. J. Quantum Chem. 2011, 111, 1172–1183. [Google Scholar] [CrossRef]
- Fotakis, C.; Gega, S.; Siapi, E.; Potamitis, C.; Viras, K.; Moutevelis-Minakakis, P.; Kokotos, C.G.; Durdagi, S.; Grdadolnik, S.G.; Sartori, B.; et al. Interactions at the bilayer interface and receptor site induced by the novel synthetic pyrrolidinone analog MMK3. BBA-Bioenergetics 2010, 1798, 422–432. [Google Scholar] [PubMed]
- Mavromoustakos, T.; Golic Grdadolnik, S.; Zervou, M.; Zoumpoulakis, P.; Potamitis, C.; Politi, A.; Mantzourani, E.; Platts, J.A.; Koukoulitsa, C.; Minakakis, P.; et al. Putative bioactive conformers of small molecules: A concerted approach using NMR spectroscopy and computational chemistry. In Medicinal Chemistry Research Progress; Ricci, S., Colombo, G.P., Eds.; Nova Science Publishers: New York, NY, USA, 2008; pp. 175–207. [Google Scholar]
- Ntountaniotis, D.; Kellici, T.; Tzakos, A.; Kolokotroni, P.; Tselios, T.; Becker-Baldus, J.; Glaubitz, C.; Lin, S.; Makriyannis, A.; Mavromoustakos, T. The application of solid-state NMR spectroscopy to study candesartan cilexetil (TCV-116) membrane interactions. Comparative study with the AT1R antagonist drug olmesartan. BBA-Bioenergetics 2014, 1838, 2439–2450. [Google Scholar] [PubMed]
- Zervou, M.; Cournia, Z.; Potamitis, C.; Patargias, G.; Durdagi, S.; Grdadolnik, S.G.; Mavromoustakos, T. Insights into the molecular basis of action of the AT1 antagonist losartan using a combined NMR spectroscopy and computational approach. BBA-Bioenergetics 2014, 1838, 1031–1046. [Google Scholar] [PubMed]
- Stegbauer, J.; Coffman, T.M. New insights into angiotensin receptor actions: From blood pressure to aging. Curr. Opin. Nephrol. Hypertens. 2011, 20, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Wei, W. Angiotensin II in inflammation, immunity and rheumatoid arthritis. Clin. Exp. Immunol. 2014. [Google Scholar] [CrossRef]
- Valero-Esquitino, V.; Lucht, K.; Namsolleck, P.; Monnet-Tschudi, F.; Stubbe, T.; Lucht, F.; Liu, M.; Ebner, F.; Brandt, C.; Danyel, L.A.; et al. Direct angiotensin type 2 receptor (AT2R) stimulation attenuates T-cell and microglia activation and prevents demyelination in experimental autoimmune encephalomyelitis in mice. Clin. Sci. (Lond.) 2015, 128, 95–109. [Google Scholar] [CrossRef]
- Marion, E.; Song, O.R.; Christophe, T.; Babonneau, J.; Fenistein, D.; Eyer, J.; Letournel, F.; Henrion, D.; Clere, N.; Paille, V.; et al. Mycobacterial toxin induces analgesia in buruli ulcer by targeting the angiotensin pathways. Cell 2014, 157, 1565–1576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kellici, T.F.; Tzakos, A.G.; Mavromoustakos, T. Rational Drug Design and Synthesis of Molecules Targeting the Angiotensin II Type 1 and Type 2 Receptors. Molecules 2015, 20, 3868-3897. https://doi.org/10.3390/molecules20033868
Kellici TF, Tzakos AG, Mavromoustakos T. Rational Drug Design and Synthesis of Molecules Targeting the Angiotensin II Type 1 and Type 2 Receptors. Molecules. 2015; 20(3):3868-3897. https://doi.org/10.3390/molecules20033868
Chicago/Turabian StyleKellici, Tahsin F., Andreas G. Tzakos, and Thomas Mavromoustakos. 2015. "Rational Drug Design and Synthesis of Molecules Targeting the Angiotensin II Type 1 and Type 2 Receptors" Molecules 20, no. 3: 3868-3897. https://doi.org/10.3390/molecules20033868
APA StyleKellici, T. F., Tzakos, A. G., & Mavromoustakos, T. (2015). Rational Drug Design and Synthesis of Molecules Targeting the Angiotensin II Type 1 and Type 2 Receptors. Molecules, 20(3), 3868-3897. https://doi.org/10.3390/molecules20033868







