Solid-State Characterization and Biological Activity of Betulonic Acid Derivatives
Abstract
:1. Introduction
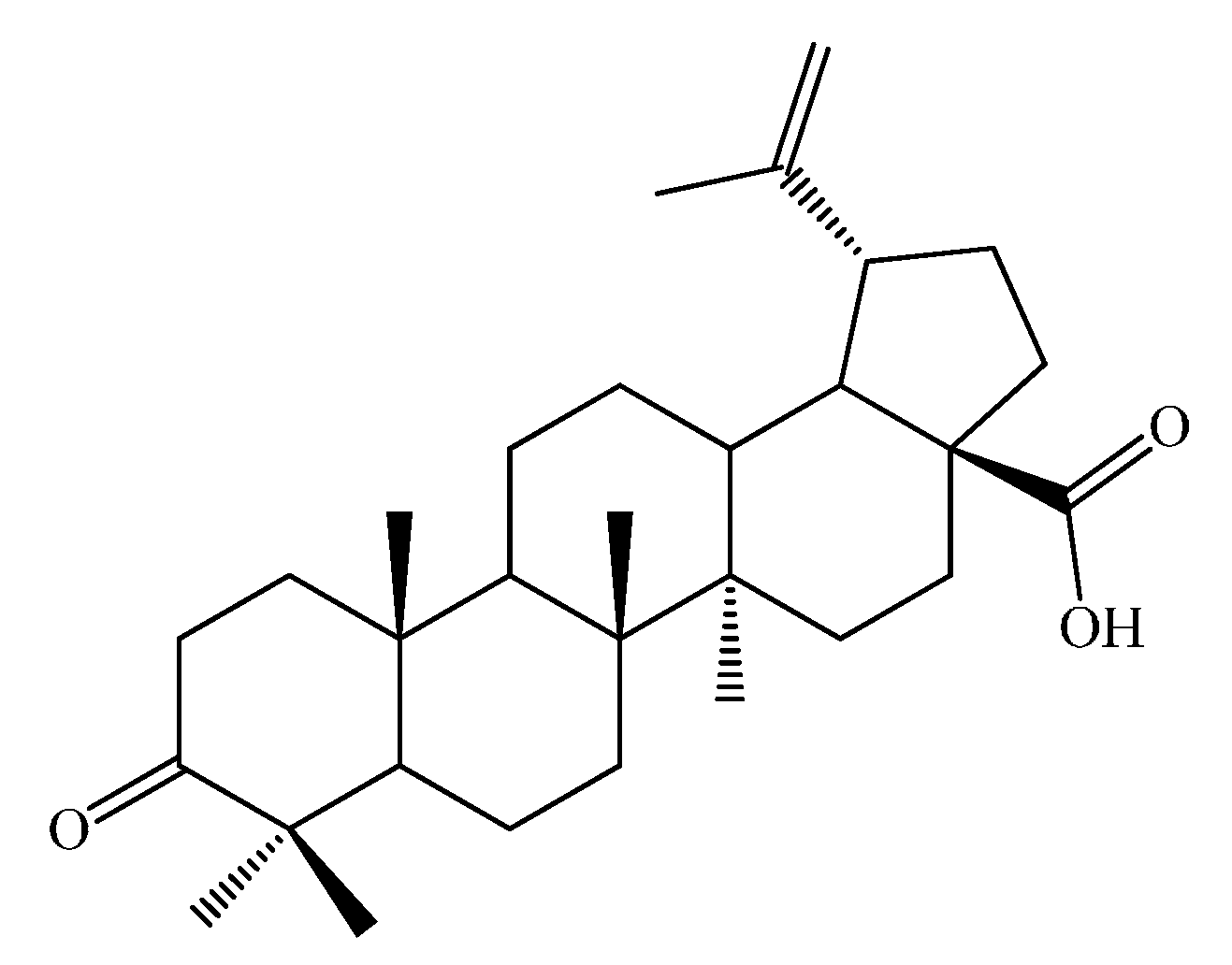
2. Results and Discussion
2.1. Synthesis
2.2. Characterization of Compounds
2.2.1. Physico-Chemical Data
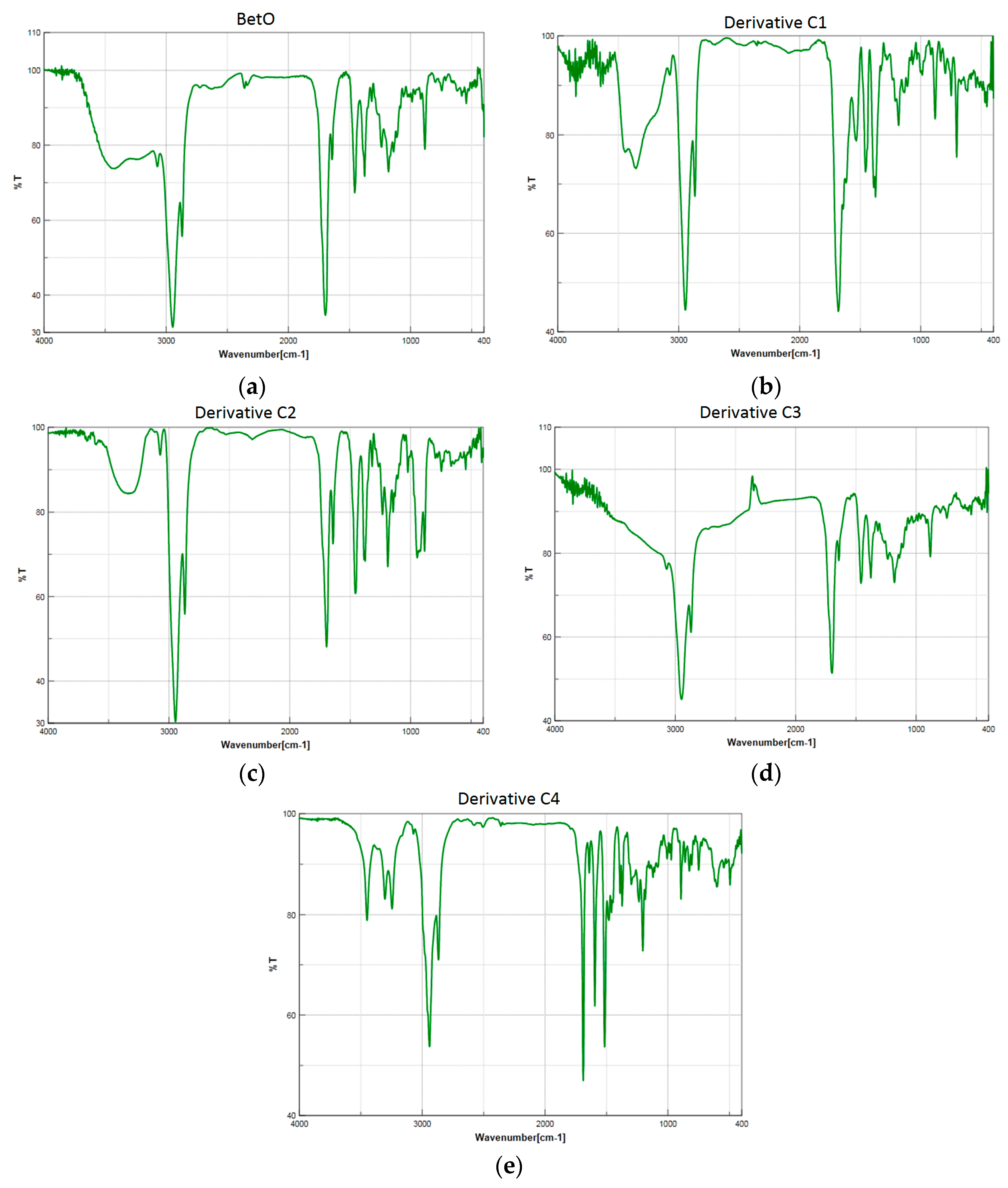
2.2.2. Thermal Analysis of Decomposition in Air

2.3. Biological Activities
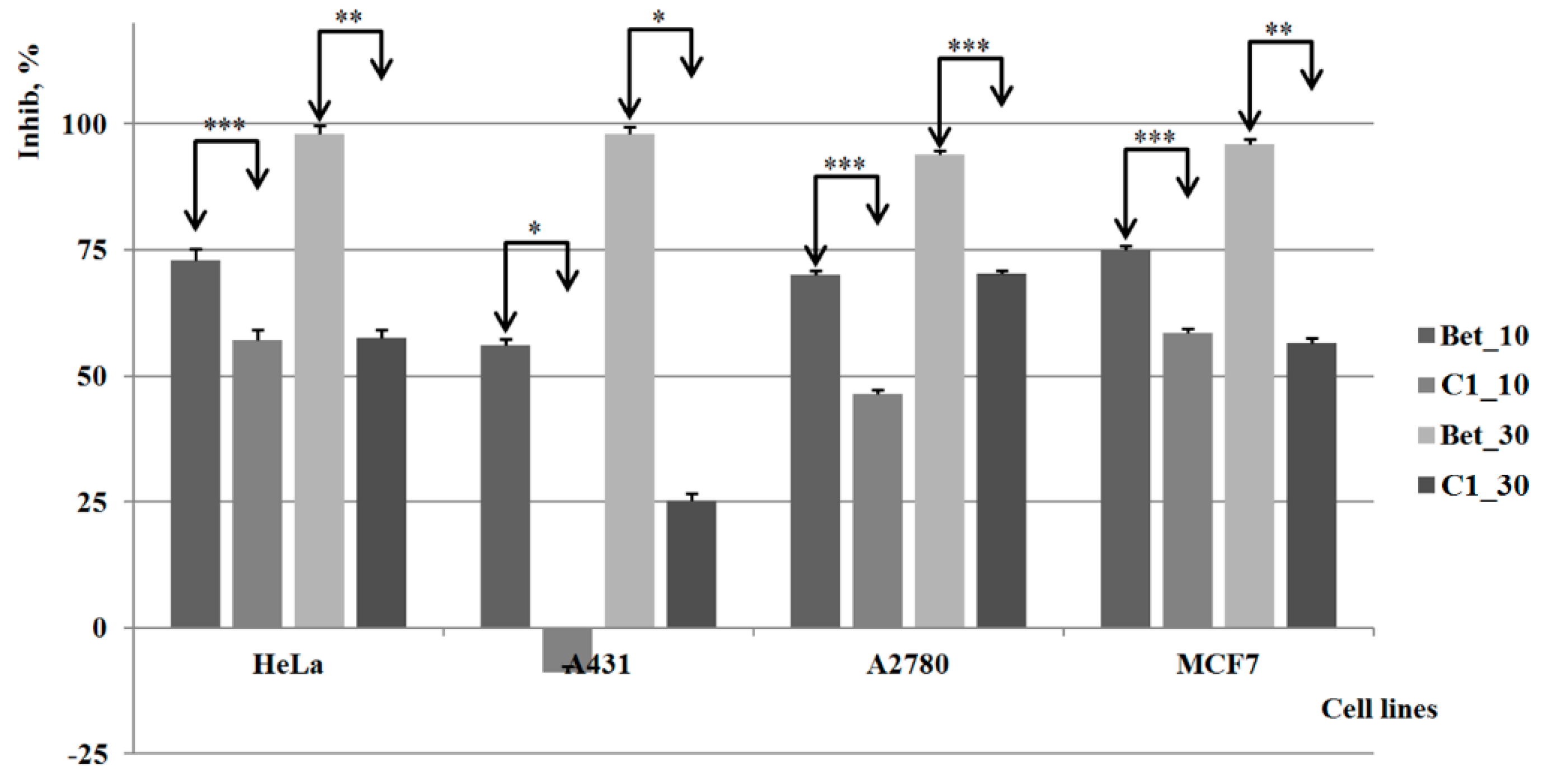

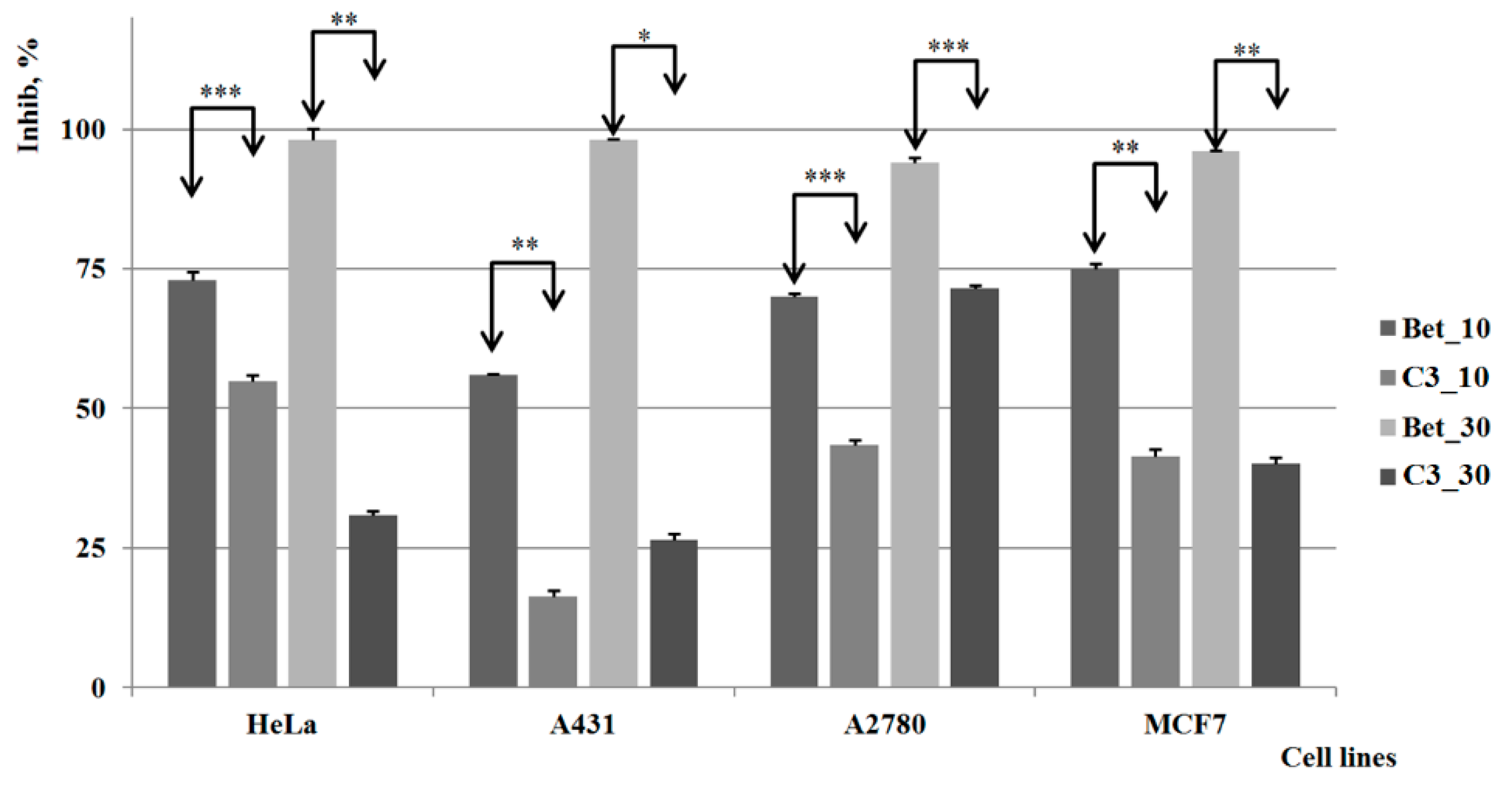
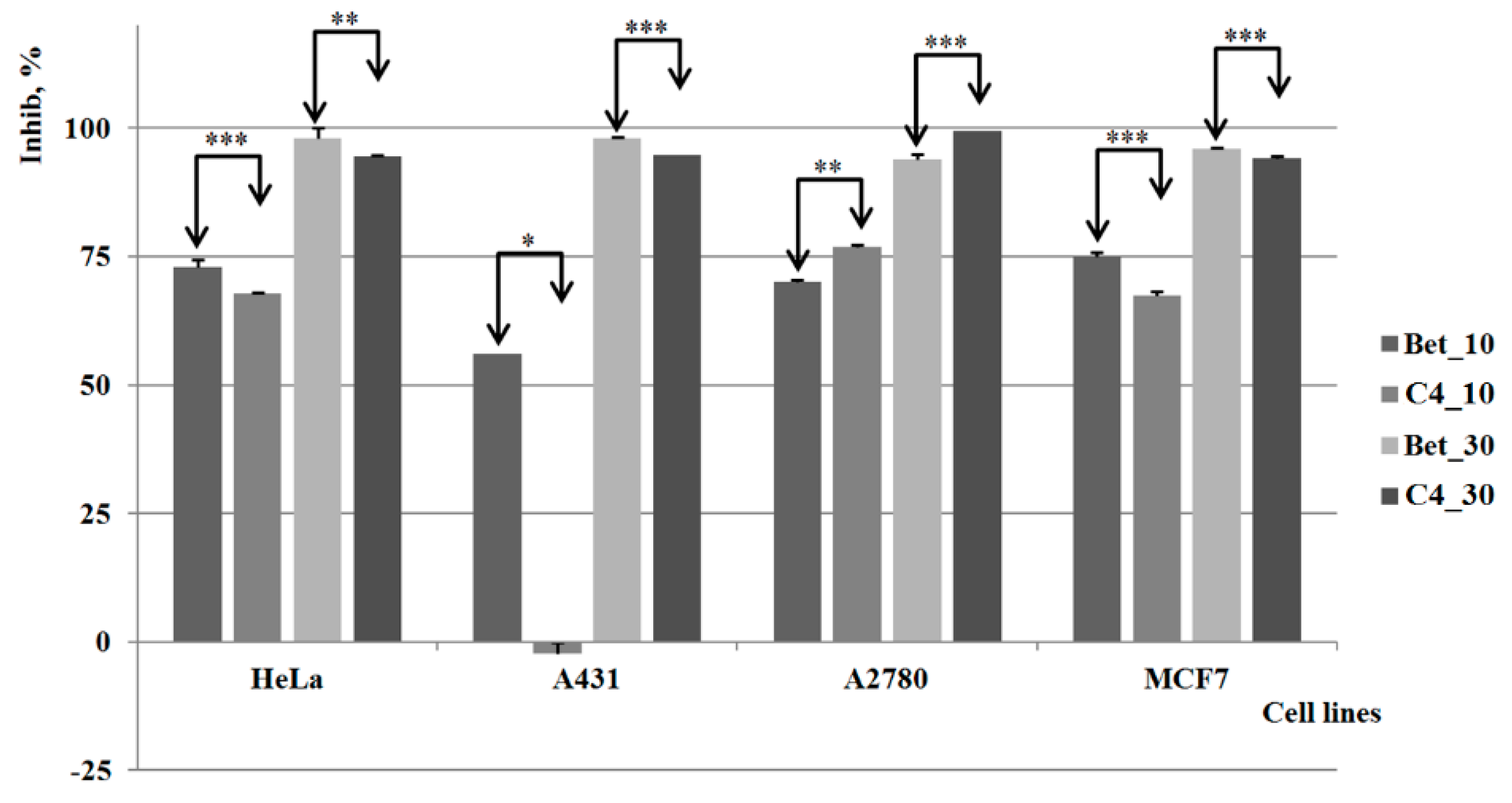
3. Experimental Section
3.1. General Information
3.2. Synthesis and Characterization
| Structure | Analytical Data |
|---|---|
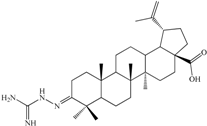 | 3-2-Carbamimidoylhydrazonolup-20(29)-en-28-oic acid (C1). White crystalline solid (yield η = 66.7%), m.p. (Boetius) = 274–277 °C; FTIR (KBr, cm−1): 3442, 3352, 3175, 3070, 2946, 2867, 1680, 1642, 1457, 1537, 1455, 1375, 1184, 989, 882, 749, 703, 617. Elemental Analysis (Calcd./Found) for C31H50N4O2, M = 510.75 g/mol: C = 72.90%, H = 9.87%, N = 10.97%; Found: C = 73.04%, H = 9.91%, N = 11.07% |
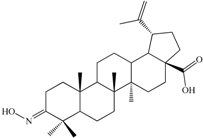 | 3-Hydroxyiminolup-20(29)-en-28-oic acid (C2). White crystalline solid (yield η = 72.4%), m.p. (Boetius) = 210 °C (phase transition); 219–224 °C (crude); m.p.(Boetius) = 225–229 °C (EtOH); FTIR (KBr, cm−1): 3283, 3073, 2946, 2869, 1697, 1642, 1455, 1385, 1376, 1320, 1267, 1233, 1188, 1144, 1023, 945, 885, 795, 746, 665, 567, 544, 501; Elemental Analysis (Calcd./Found) for C30H47NO3, M = 469.70 g/mol: C = 76.71%, H = 10.09%, N = 2.98%; Found: C = 76.15%, H = 10.23%, N = 2.64% |
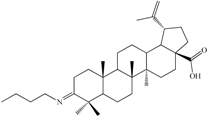 | 3-Butyliminolup-20(29)-en-28-oic acid (C3). Pale yellow solid (yield η = 73.4%), m.p. (Boetius) = 145–150 °C; FTIR (KBr, cm−1): 3072, 2986, 2940, 2868, 1694, 1642, 1454, 1375, 1279, 1233, 1180, 1110, 1024, 882, 744, 543; Elemental Analysis (Calcd./Found) for C34H55NO2, M = 509.81 g/mol: C = 80.10%, H = 10.87%, N = 2.75%; Found: C = 80.98%, H = 11.29%, N = 2.82% |
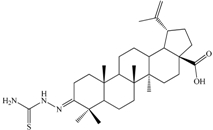 | 3-(2-Carbamothioylhydrazono)lup-20(29)-en-28-oic acid (C4). White solid (yield η = 28.1%), m.p.(Boetius) >330 °C; FTIR (KBr, cm−1): 3449, 3303, 3245, 2940, 2867, 1690, 1640, 1515, 1481, 1458, 1389, 1373, 1236, 1204, 973, 894, 602, 495; Elemental Analysis (Calcd./Found) for C31H49N3O2S, M = 527.80 g/mol: C = 70.54%, H = 9.36%, N = 7.96%; Found: C = 70.15%, H = 9.59%, N = 8.01% |
3.3. Biological Activity
3.4. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Frighetto, R.T.S.; Welendorf, R.M.; Nigro, E.N.; Frighetto, N.; Siani, A.C. Isolation of ursolic acid from apple peels by high speed counter-current chromatography. Food Chem. 2008, 106, 767–771. [Google Scholar]
- Wójciak-Kosior, M. Separation and determination of closely related triterpenic acids by high performance thin-layer chromatography after iodine derivatization. J. Pharm. Biomed. 2007, 45, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, G.; Liu, S.; Liu, D.; Chen, G.; Hu, N.; Suo, Y.; You, J. Simultaneous determination of six triterpenic acids in some Chinese medicinal herbs using ultrasound-assisted dispersive liquid-liquid microextraction and high-performance liquid chromatography with fluorescence detection. J. Pharm. Biomed. 2014, 107, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M. The Mevalonate and Deoxyxylulose. Phosphate Pathways: Terpenoids. and Steroids. In Medicinal Natural Products: A Biosynthetic Approach, 2nd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2001. [Google Scholar]
- Yogeeswari, P.; Sriram, D. Betulinic acid and its derivatives: A review on their biological properties. Curr. Med. Chem. 2005, 12, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Soica, C.; Danciu, C.; Savoiu-Balint, G.; Borcan, F.; Ambrus, R.; Zupko, I.; Bojin, F.; Coricovac, D.; Ciurlea, S.; Avram, S.; et al. Betulinic Acid in Complex with a Gamma-Cyclodextrin Derivative Decreases Proliferation and in Vivo Tumor Development of Non-Metastatic and Metastatic B164A5 Cells. Int. J. Mol. Sci. 2014, 15, 8235–8255. [Google Scholar] [CrossRef] [PubMed]
- Alakurtti, S.; Taru, M.; Salme, K.; Jari, Y.K. Pharmacological properties of the ubiquitous natural product betulin. Eur. J. Pharm. Sci. 2006, 29, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chichewicz, R.H.; Kouzi, S.A. Chemistry, biological activity and chemotherapeutic potential of betulinic acid for the prevention and treatment of cancer and HIV infection. Med. Res. Rev. 2004, 24, 90–114. [Google Scholar] [CrossRef] [PubMed]
- Soica, C.; Oprean, C.; Borcan, F.; Danciu, C.; Trandafirescu, C.; Coricovac, D.; Crăiniceanu, Z.; Dehelean, C.A.; Munteanu, M. The Synergistic Biologic Activity of Oleanolic and Ursolic Acids in Complex with Hydroxypropyl-γ-Cyclodextrin. Molecules 2014, 19, 4924–4940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.M.; Xu, H.G.; Wang, L.; Li, Y.J.; Sun, P.H.; Wu, X.M.; Wang, G.J.; Chen, W.M.; Ye, W.C. Betulinic Acid and Its Derivatives as Potential Antitumor Agents. Med. Res. Rev. 2015, 35, 1127–1155. [Google Scholar] [CrossRef] [PubMed]
- Chudzik, M.; Korzonek-Szlacheta, I.; Król, W. Triterpenes as potentially cytotoxic compounds. Molecules 2015, 20, 1610–1625. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.R.; Chen, X.P.; Lu, J.J.; Wang, Y.; Wang, Y.T. Potent natural products and herbal medicines for treating liver fibrosis. Chin. Med. 2015, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hsu, R.J.; Hsu, Y.C.; Chen, S.P.; Fu, C.L.; Yu, J.C.; Chang, F.W.; Chen, Y.H.; Liu, J.M.; Ho, J.Y.; Yu, C.P. The triterpenoids of hibiscus syriacus induce apoptosis and inhibit cell migration in breast cancer cells. BMC Complement. Altern. Med. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Fulias, A.; Ledeti, I.; Vlase, G.; Vlase, T.; Soica, C.; Dehelean, C.; Oprean, C.; Bojin, F.; Suta, L.-M.; Bercean, V.; et al. Thermal degradation, kinetic analysis, and apoptosis induction in human melanoma for oleanolic and ursolic acids. J. Therm. Anal. Calorim. 2015, in press. [Google Scholar] [CrossRef]
- Kim, D.S.H.L.; Pezzuto, J.M.; Pisha, E. Synthesis of betulinic acid derivatives with activity against human melanoma. Bioorg. Med. Chem. Lett. 1998, 8, 1707–1712. [Google Scholar] [CrossRef]
- Król, S.K.; Kiełbus, M.; Rivero-Müller, A.; Stepulak, A. Comprehensive review on betulin as a potent anticancer agent. BioMed Res. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Webster, D.; Johnson, J.A.; Gray, C.A. Anti-mycobacterial triterpenes from the Canadian medicinal plant Alnus incana. J. Ethnopharmacol. 2015, 165, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Ragasa, C.Y.; Lim, K.F.; Shen, C.-C.; Raga, D.D. Hypoglycemic Potential of Triterpenes from Alstonia scholaris. Pharm. Chem. J. 2015, 47. [Google Scholar]
- Ständer, S.; Blome, C.; Phan, N.Q.; Laszczyk, M.N.; Siepmann, D.; Augustin, M. Antipruritic Effects and Individual Needs of Patients on Topical Therapy of Chronic Pruritus: Results of an Observational Study with the Triterpene Betulin. Aktuel. Dermatol. 2015, 41, 89–97. [Google Scholar]
- Shamsabadipour, S.; Ghanadian, M.; Saeedi, H.; Reza Rahimnejad, M.; Mohammadi-Kamalabadi, M.; Ayatollahi, S.M.; Salimzadeh, L. Triterpenes and steroids from euphorbia denticulata lam. with anti-herpes symplex virus activity. Iran J. Pharm. Res. 2013, 12, 759–767. [Google Scholar] [PubMed]
- Ghanadian, M.; Akhavan, A.; Abdalla, O.M.; Ayatollahi, A.M.; Mohammadi-Kamalabadi, M.; Ghazanfari, H. Triterpenes from Euphorbia spinidens with immunomodulatory activity. Res. Pharm. Sci. 2013, 8, 205–210. [Google Scholar]
- De Moraes, W.F.; Galdino, P.M.; Nascimento, M.V.M.; Vanderlinde, F.A.; Bara, M.T.F.; Costa, E.A.; de Paula, J.R. Triterpenes involved in the anti-inflammatory effect of ethanolic extract of Pterodon emarginatus Vogel stem bark. J. Nat. Med. 2012, 66, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Tolmacheva, I.A.; Grishko, V.V.; Boreko, E.I.; Savinova, O.V.; Pavlova, N.I. Synthesis and antiviralactivity of 2,3-seco-derivatives of betulonic acid. Chem. Nat. Compd. 2009, 45, 673–676. [Google Scholar] [CrossRef]
- Flekhter, O.B.; Ashavina, O.Y.; Boreko, E.I.; Karachurina, L.T.; Pavlova, N.I.; Kabal’nova, N.N.; Savinova, O.V.; Galin, F.Z.; Nikolaeva, S.N.; Zarudii, F.S.; et al. Synthesis of 3-O-Acetylbetulinic and betulonic aldehydes according to Svern and the pharmacological activity of related oximes. Pharm. Chem. J. 2002, 36, 303–306. [Google Scholar] [CrossRef]
- Kazakova, O.B.; Medvedeva, N.I.; Lopatina, T.V.; Apryshko, G.N.; Pugacheva, R.B.; Yavorskaya, N.P.; Golubeva, I.S.; Tolstikov, G.A. Synthesis and the antineoplastic activity of imidazolides of betulonic acid. Russ. J. Bioorg. Chem. 2015, 41, 305–314. [Google Scholar] [CrossRef]
- Yang, S.-J.; Liu, M.-C.; Zhao, Q.; Hu, D.-Y.; Xue, W.; Yang, S. Synthesis and biological evaluation of betulonic acid derivatives as antitumor agents. Eur. J. Med. Chem. 2015, 96, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Semenov, D.E.; Zhukova, N.A.; Ivanova, E.P.; Sorokina, I.V.; Baiev, D.S.; Nepomnyashchikh, G.I.; Tolstikova, T.G.; Biryukova, M.S. Hepatoprotective Properties of Betulonic Acid Amide and Heptral in Toxic Liver Injury Induced by Carbon Tetrachloride in Combination with Ethanol. Bull. Exp. Biol. Med. 2015, 158, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-L.; Fang, S.-C.; Huang, H.-W.; Yen, G.-C. Anti-inflammatory effects of triterpenes and steroid compounds isolated from the stem bark of Hiptage benghalensis. J. Funct. Food. 2015, 12, 420–427. [Google Scholar] [CrossRef]
- Govdi, A.I.; Vasilevsky, S.F.; Sokolova, N.V.; Sorokina, I.V.; Tolstikova, T.G.; Nenajdenko, V.G. Betulonic acid-peptide conjugates: Synthesis and evaluation of anti-inflammatory activity. Mendeleev Commun. 2013, 23, 260–261. [Google Scholar] [CrossRef]
- Dinh Ngoc, T.; Moons, N.; Kim, Y.; de Borggraeve, W.; Mashentseva, A.; Andrei, G.; Snoeck, R.; Balzarini, J.; Dehaen, W. Synthesis of triterpenoid triazine derivatives from allobetulone and betulonic acid with biological activities. Bioorg. Med. Chem. 2014, 22, 3292–3300. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, O.B.; Giniyatullina, G.V.; Tolstikov, G.A.; Medvedeva, N.I.; Utkina, T.M.; Kartashova, O.L. Synthesis, Modification, and Antimicrobial Activity of the N-Methylpiperazinyl Amides of Triterpenic Acids. Russ. J. Bioorg. Chem. 2010, 36, 383–389. [Google Scholar] [CrossRef]
- Anikina, L.V.; Tolmacheva, I.A.; Vikharev, Y.B.; Grishko, V.V. The immunotropic activity of lupane and oleanane 2,3-seco-triterpenoids. Russ. J. Bioorg. Chem. 2010, 36, 240–244. [Google Scholar] [CrossRef]
- Ledeti, I.; Bosca, C.S.; Cosma, C.; Badea, V.; Todea, A.; Bercean, V.-N. Study on obtaining 3-oxolup-20(29)-en-28-oic acid (betulonic acid) from (3β)-lup-20(29)-en-3,28-diol (betulin). Rev. Chim. Bucharest 2014, 65, 1289–1293. [Google Scholar]
- Flekhter, O.B.; Boreko, E.I.; Nigmatullina, L.R.; Pavlova, N.I.; Medvedeva, N.I.; Nikolaeva, S.N.; Tretyakova, E.V.; Savinova, O.V.; Baltina, L.A.; Karachurina, L.T.; et al. Synthesis and pharmacological activity of acylated betulonic acid oxides and 28-oxo-allobetulone. Pharm. Chem. J. 2004, 38, 148–152. [Google Scholar] [CrossRef]
- Melnikova, N.; Burlova, I.; Kiseleva, T.; Klabukova, I.; Gulenova, M.; Kislitsin, A.; Vasin, V.; Tanaseichuk, B. A practical synthesis of betulonic acid using selective oxidation of betulin on aluminium solid support. Molecules 2012, 17, 11849–11863. [Google Scholar] [CrossRef] [PubMed]
- Genet, C.; Schmidt, C.; Strehle, A.; Schoonjans, K.; Auwerx, J.; Saladin, R.; Wagner, A. Redefining the TGR5 Triterpenoid Binding Pocket at the C-3 Position. ChemMedChem 2010, 5, 1983–1988. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Z.X.; Luo, J.; Yang, F.; Liu, T.; Liu, M.; Qiu, W.W.; Tang, J. Synthesis and Biological Evaluation of Heterocyclic Ring-Fused Betulinic Acid Derivatives as Novel Inhibitors of Osteoclast Differentiation and Bone Resorption. J. Med. Chem. 2012, 55, 3122–3134. [Google Scholar] [CrossRef] [PubMed]
- Frederickson, L.D. An Infrared Study of the C=N Stretching Vibration in Azine Derivatives of Aldehydes and Ketones. Anal. Chem. 1964, 36, 1349–1355. [Google Scholar] [CrossRef]
- Zoya, A.; Saveleva, G.V.; Romanenko, L.A.; Sheludyakova, S.V. Synthesis and structure of a complex with the coordinated triaminoguanidinium(2+) ion, [Cu(TAGH2)Cl3]Cl·H2O. Polyhedron 2000, 17, 1737–1740. [Google Scholar]
- Dehelean, C.A.; Feflea, S.; Molnar, J.; Zupko, I.; Soica, C. Betulin as an Antitumor Agent Tested in vitro on A431, HeLa and MCF7, and as an Angiogenic Inhibitor in vivo in the CAM Assay. Nat. Prod. Commun. 2012, 7, 981–985. [Google Scholar] [PubMed]
- Yang, S.; Zhao, Q.; Xiang, H.; Liu, M.; Zhang, Q.; Xue, W.; Song, B.; Yang, S. Antiproliferative activity and apoptosis-inducing mechanism of constituents from Toona sinensis on human cancer cells. Cancer Cell Int. 2013, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Şoica, C.; Peev, C.; Dehelean, C.; Aluas, M.; Zupko, I.; Kasa, P.; Alexa, E. Physico-chemical comparison study of betulinic acid, betulin and birch bark extract and in vitro investigation of their cytotoxic effects towards skin epidermoid carcinoma (A431), breast carcinoma (MCF7) and cervix adenocarcinoma (HeLa) cell-lines. Nat. Prod. Res. 2012, 26, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. Betulinic acid for cancer treatment and prevention. Int. J. Mol. Sci. 2008, 9, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Drag, M.; Surowiak, P.; Drag-Zalesinka, M.; Dietel, M.; Lage, H.; Oleksyszyn, J. Comparison of the cytotoxic effects of birch bark extract, betulin and betulinic acid towards human gastric carcinoma and pancreatic carcinoma drug-sensitive and drug resistant cell lines. Molecules 2009, 14, 1639–1651. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ledeţi, I.; Avram, Ş.; Bercean, V.; Vlase, G.; Vlase, T.; Ledeţi, A.; Zupko, I.; Mioc, M.; Şuta, L.-M.; Şoica, C.; et al. Solid-State Characterization and Biological Activity of Betulonic Acid Derivatives. Molecules 2015, 20, 22691-22702. https://doi.org/10.3390/molecules201219876
Ledeţi I, Avram Ş, Bercean V, Vlase G, Vlase T, Ledeţi A, Zupko I, Mioc M, Şuta L-M, Şoica C, et al. Solid-State Characterization and Biological Activity of Betulonic Acid Derivatives. Molecules. 2015; 20(12):22691-22702. https://doi.org/10.3390/molecules201219876
Chicago/Turabian StyleLedeţi, Ionuţ, Ştefana Avram, Vasile Bercean, Gabriela Vlase, Titus Vlase, Adriana Ledeţi, Istvan Zupko, Marius Mioc, Lenuţa-Maria Şuta, Codruţa Şoica, and et al. 2015. "Solid-State Characterization and Biological Activity of Betulonic Acid Derivatives" Molecules 20, no. 12: 22691-22702. https://doi.org/10.3390/molecules201219876
APA StyleLedeţi, I., Avram, Ş., Bercean, V., Vlase, G., Vlase, T., Ledeţi, A., Zupko, I., Mioc, M., Şuta, L.-M., Şoica, C., & Dehelean, C. (2015). Solid-State Characterization and Biological Activity of Betulonic Acid Derivatives. Molecules, 20(12), 22691-22702. https://doi.org/10.3390/molecules201219876












