A Comprehensive Study of the Use of Cu(I)/4,4’-Dicarboxy-2,2’-biquinoline Complexes to Measure the Total Reducing Capacity: Application in Herbal Extracts
Abstract
:1. Introduction
2. Results and Discussion
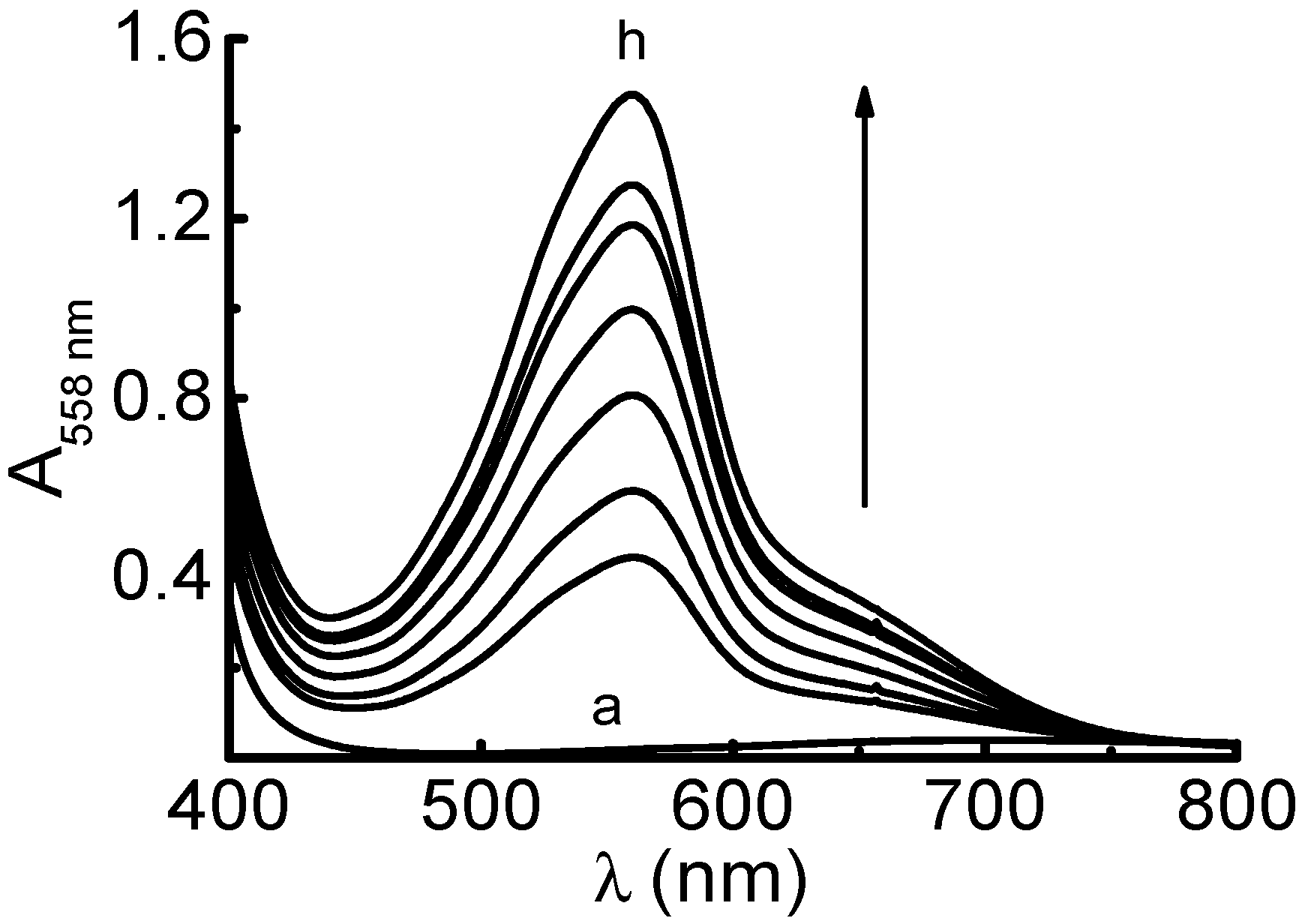
2.1. Determination of the Reduction Capacity of Some Antioxidant Compounds with the Proposed Method
| Phenolic Compound | FHG | FW (g·mol−1) | LR/10−5 (M) | b/103 | AAEC |
|---|---|---|---|---|---|
| Tannic acid | 25 | 1701.23 | 0.1–0.7 | 259 ± 12 | 38.1 ± 1.7 |
| Pyrogallic acid | 3 | 126.11 | 1–4 | 18.7 ± 1.9 | 2.76 ± 0.28 |
| 1,2,4-Benzenetriol | 3 | 126.11 | 0.8–2.2 | 24.5 ± 0.3 | 3.61 ± 0.05 |
| Phloroglucinol | 3 | 126.11 | 1.6–13 | 10.0 ± 0.4 | 1.47 ± 0.06 |
| Gallic acid | 3 | 170.12 | 0.1–0.7 | 39.4 ± 1.5 | 5.81 ± 0.23 |
| 2,3,4-THB | 3 | 254.28 | 0.2–1.6 | 35.6 ± 0.2 | 5.24 ± 0.03 |
| o-Pyrocatechol | 2 | 100.11 | 0.2–1.6 | 36.5 ± 0.6 | 5.38 ± 0.09 |
| Hydroquinone | 2 | 110.11 | 1–8 | 14.3 ± 0.1 | 2.11 ± 0.01 |
| Resorcinol | 2 | 110.11 | 1.8–15 | 6.16 ± 0.50 | 0.91 ± 0.07 |
| Caffeic acid | 2 | 180.16 | 0.2–1.6 | 31.0 ± 0.8 | 4.57 ± 0.12 |
| Sinapic acid | 1 | 224.21 | 1–8 | 16.8 ± 0.2 | 2.47 ± 0.03 |
| Ferulic acid | 1 | 194.19 | 1–8 | 12.3 ± 0.5 | 1.82 ± 0.08 |
| p-Coumaric acid | 1 | 164.16 | 4–32 | 2.43 ± 0.23 | 0.36 ± 0.03 |
| Vanillic acid | 1 | 168.15 | 2–14 | 5.27 ± 0.41 | 0.81 ± 0.06 |
| Vanillin | 1 | 152.13 | 26–40 | 0.66 ± 0.06 | 0.10 ± 0.01 |
| 4-Hydroxyphenylacetic acid | 1 | 152.13 | 12–40 | 0.75 ± 0.07 | 0.11 ± 0.01 |
| Phenol | 1 | 94.11 | 106–848 | (65 ± 3) × 10−3 | (9.6 ± 0.4) × 10−3 |
| Quercetin | 5 | 302.24 | 0.2–1.6 | 66.1± 0.7 | 9.74 ± 0.11 |
| Rutin | 10 | 610.58 | 0.2–1.6 | 41.6 ± 0.7 | 6.13 ± 0.11 |
| (−)-Epigallocatechin gallate | 8 | 458.37 | 0.2–1.6 | 65.5 ± 2.6 | 9.65 ± 0.38 |
| β-Carotene | - | 536.87 | 1–8.1 | 1.49 ± 0.04 | 0.22 ± 0.01 |
| Trolox | 1 | 250.29 | 26–40 | 19.2 ± 1.9 | 2.83 ± 0.28 |
| Ascorbic acid | - | 176.13 | 4–20 | 6.78 ± 0.46 | 1.00 |

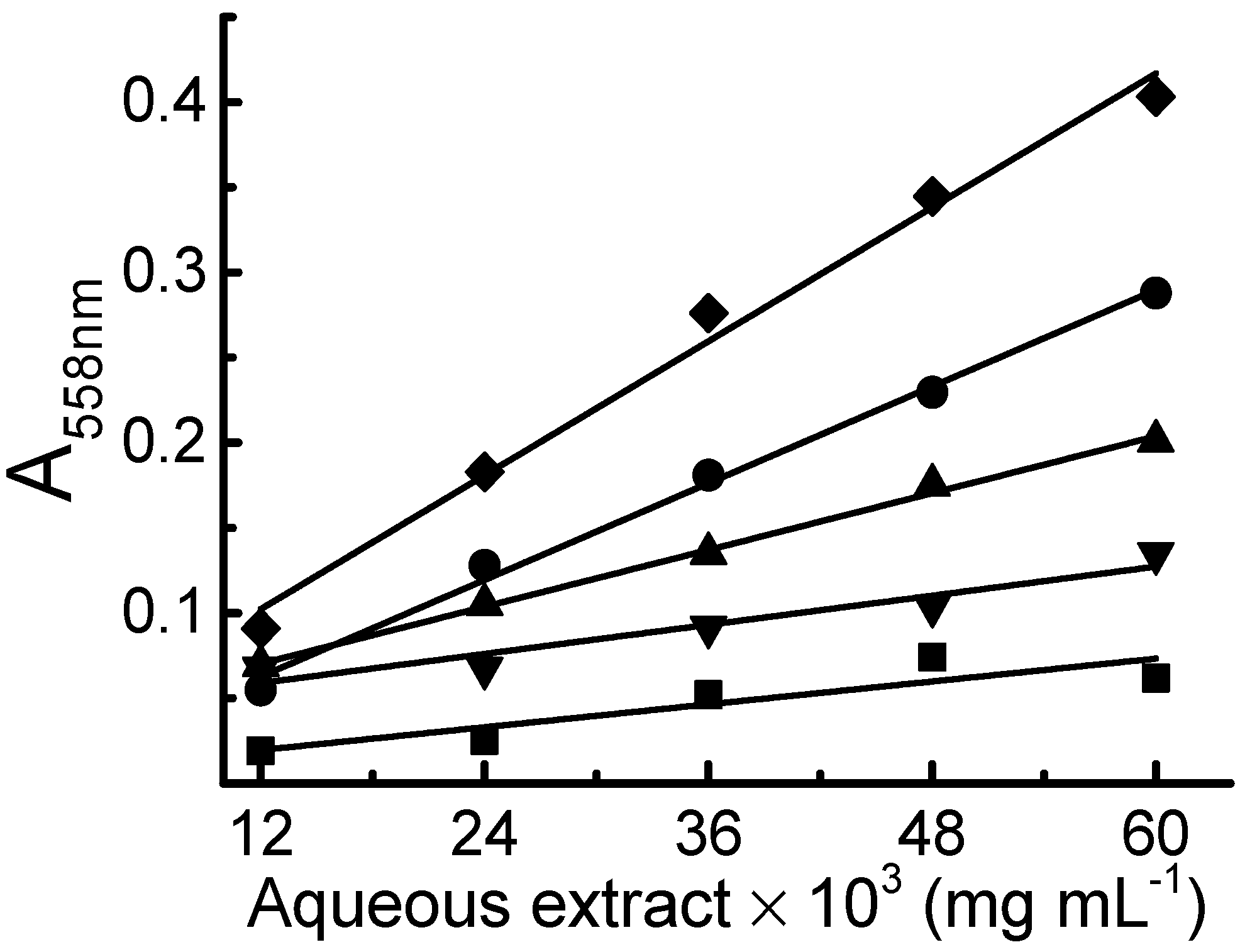
2.2. Determination of the Reduction Capacity of Aqueous Extracts of Medicinal Plants with the Proposed Method
2.3. Some Critical Considerations of the Proposed Method
| Plants | Common Brazilian Names | Use in Folk Medicine [25,26] | TPC (g PA/100 g DM) | Cu(BCA)23− (mg DM/g AA) | DPPH EC50 (g DM/g DPPH) |
|---|---|---|---|---|---|
| Geissospermum laeve (Vell.) Miers | Pau Pereira | Tonic properties; antifebrile | 1.53 ± 0.06 | 22.2 ± 0.6 | 6.2 ± 2.3 |
| Schinus terebinthifolius Raddi | Aroeira | Washing wounds; ulcers | 5.57 ± 0.52 | 280 ± 15.7 | 94.8 ± 2.9 |
| Carapa guianensis Aubl. | Andiroba | Bacterial infection; treatment of tumors | 2.55 ± 0.19 | 96.2 ± 3.8 | 25.1 ± 1.0 |
| Bauhinia splendens Kunth | Escada de Jabuti | Diabetes; treating obesity | 1.42 ± 0.14 | 61.3 ± 3.7 | 19.7 ± 1.2 |
| Annona muricata L. | Graviola | Anti-diarrheal; against spasms | 1.90 ± 0.06 | 34.4 ± 0.6 | 8.4 ± 1.5 |
| Salacia impressifolia (Miers) A. C. Sm. | Miraruíra | Muscle relaxant; rheumatism | 3.52 ± 0.39 | 118 ± 4.0 | 34.3 ± 2.7 |
| Dipteryx odorata (Aubl.) Willd. | Cumaru | Antispasmodic; moderator of the cardiac movements and breathing | 1.40 ± 0.12 | 45.2 ± 3.9 | 12.2 ± 1.3 |
| Maytenus ilicifolia Mart. Ex Reissek | Espinheira Santa | Ulcers; indigestion; chronic gastritis; dyspepsia | 0.70 ± 0.04 | 15.8 ± 0.5 | 10.7 ± 5.0 |
| Stachytarpheta cayennensis (Rich.) Vahl | Gervão | Anti-inflammatory; analgesic; gastroprotective | 1.92 ± 0.05 | 41.3 ± 0.2 | 11.8 ± 1.8 |
| Hymenaea courbaril L. | Jatobá | Cystitis; bronchitis; bladder infections; vermifuge | 1.41 ± 0.19 | 32.9 ± 2.1 | 14.0 ± 0.4 |
| Cordiae calyculata Vell. | Porangaba | Diuretic; treating obesity | 1.30 ± 0.08 | 37.9 ± 1.3 | 8.8 ± 1.3 |
| Lippia grandis Schauer | Salva de Marajó | Antimicrobial activity; treatment of liver diseases; stomach disease | 2.40 ± 0.10 | 64.1 ± 2.9 | 7.5 ± 1.2 |
3. Experimental Section
3.1. Apparatus
3.2. Reagents and Solutions
3.3. Preparation of Aqueous Extracts of Medicinal Plants
3.4. Preparation of Extracts for Determining the Reduction Capacity with DPPH
3.5. Total Polyphenol Content Quantification with the FC Reagent
3.6. Determination of the Total Reducing Capacity of Plant Extracts with DPPH Reagent
3.7. Proposed Method for Reducing Capacity Quantification
3.7.1. Calibration Curve with Ascorbic Acid Standard Solution
3.7.2. Calibration Curves with Standard Antioxidant Compounds
3.7.3. Calibration Curve with Aqueous Plant Extracts of Medicinal Plants
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food. Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.J.; Ou, B.X.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food. Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, A.M.; Lysenko, M.N. Determination of copper in soil extracts by using 2,2′-bicinchoninic acid. Agrokhimiya 1987, 11, 115–120. [Google Scholar]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- McFeeters, R.F. A manual method for reducing sugar determinations with 2,2′-bicinchoninate reagent. Anal. Biochem. 1980, 103, 302–306. [Google Scholar] [CrossRef]
- Holz, F. Automated photometric determination of ascorbic acid and dehydroascorbic acid (vitamin C) in food of plant origin, Part I: Determination of ascorbic acid. Landwirtsch. Forsch. 1982, 38, 558–578. [Google Scholar]
- Rocha, D.L.; Rocha, F.R.P.A. Flow-based procedure with solenoid micro-pumps for the spectrophotometric determination of uric acid in urine. Microchem. J. 2010, 94, 53–59. [Google Scholar] [CrossRef]
- Gershuns, A.L.; Verezubova, A.A.; Tolstykh, Zh.A. Photocolorimetric determination of copper with 2,2’-bicinchoninic acid. Izv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol. 1961, 4, 25–27. [Google Scholar]
- Moya, H.D.; Dantoni, P.; Rocha, F.R.P.; Coichev, N. A multicommuted flow-system for spectrophotometric determination of tannin exploiting the Cu(I)/BCA complex formation. Microchem. J. 2008, 88, 21–25. [Google Scholar] [CrossRef]
- Marino, D.C.; Sabino, L.Z.L.; Armando, J., Jr.; Ruggiero, A.A.; Moya, H.D. Analysis of the polyphenols content in medicinal plants based on the reduction of Cu(II)/bicinchoninic complexes. J. Agric. Food. Chem. 2009, 57, 11061–11066. [Google Scholar] [CrossRef] [PubMed]
- Sabino, L.Z.L.; Marino, D.C.; Moya, H.D. Determination of diltiazem based on the reduction of Cu(II)-BCA complexes in micellar medium. Can. J. Chem. 2010, 88, 533–539. [Google Scholar] [CrossRef]
- Braga, M.C.; Requeijo, T.B.; Franco, A.M.C.; Coichev, N.; Moya, H.D. Quantification of some nonsteroidal anti-inflammatory drugs as reducing agents of Cu(II)/4,4’-dicarboxy-2,2’-biquinoline complexes in cationic micellar medium. Anal. Methods 2011, 3, 1637–1642. [Google Scholar] [CrossRef]
- Moreno, C.S.; Larrauri, J.A.; Calixto, F.S. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Santo, M.G.; Nunez, C.V.; Moya, H.D. A new method for quantification of total polyphenol content in medicinal plants based on the reduction of Fe(III)/1,10-phenanthroline complexes. Adv. Biol. Chem. 2013, 3, 525–535. [Google Scholar] [CrossRef]
- ANVISA, Agência Nacional de Vigilância Sanitária. Brazilian Pharmacopoeia, 5th ed.; Atheneu: São Paulo, Brazil, 2010; pp. 355–357. [Google Scholar]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing, capability in the presence of neocuproine: CUPRAC Method. J. Agric. Food. Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Morrison, R.T.; Boyd, R.N. Organic Chemistry, 3th ed.; Allyn and Bacon Inc.: Boston, MA, USA, 1976; pp. 73–115. [Google Scholar]
- Prior, R.L.; Cao, G. In vivo total antioxidant capacity: Comparison of different analytical methods. Free Radic. Biol. Med. 1999, 27, 1173–1181. [Google Scholar] [CrossRef]
- Berker, K.I.; Guclgu, K.; Demirata, B.; Apak, R. A novel antioxidant assay of ferric reducing capacity measurement using ferrozine as the colour forming complexation reagent. Anal. Methods. 2010, 2, 1770–1778. [Google Scholar] [CrossRef]
- Hudlicky, M. Oxidations in Organic Chemistry. J. Am. Chem. Soc. 1990, 186, 163–166. [Google Scholar]
- Smith, R.M.; Martell, A.E. Critically Selected Stability Constants of Metal Complexes. NIST Standard Reference Database 46. 2004; 8. [Google Scholar]
- Apak, R.; Güçlü, K.; Demirata, B.; Özyürek, M.; Çelik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Özyurt, D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.W.; Moya, H.D. The evaluation of reduction of Fe(III) in 3-hydroxy-4-nitroso-2,7-naphthalenedisulfonic medium as an alternative Ferric Reducing Activity Power assay. Phytochem. Anal. 2015, 26, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Lorenzi, H.; Matos, F.J.A. Medicinal Plants in Brazil: Native and Exotic Cultivated; Instituto Plantarum: Nova Odessa, Brazil, 2002; pp. 56–57; 60–61; 220; 481. [Google Scholar]
- Silva, D.X.; Souza, M.W.; Corrêa, C.S.; Moya, H.D. A critical study of use of the Fe(II)/3-hydroxy-4-nitrose-2,7-naphthalenedisufonic acid complexes in the quantification of polyphenols in medical plants. Food Chem. 2003, 138, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manoel, H.R.; Moya, H.D. A Comprehensive Study of the Use of Cu(I)/4,4’-Dicarboxy-2,2’-biquinoline Complexes to Measure the Total Reducing Capacity: Application in Herbal Extracts. Molecules 2015, 20, 22411-22421. https://doi.org/10.3390/molecules201219855
Manoel HR, Moya HD. A Comprehensive Study of the Use of Cu(I)/4,4’-Dicarboxy-2,2’-biquinoline Complexes to Measure the Total Reducing Capacity: Application in Herbal Extracts. Molecules. 2015; 20(12):22411-22421. https://doi.org/10.3390/molecules201219855
Chicago/Turabian StyleManoel, Hariane R., and Horacio D. Moya. 2015. "A Comprehensive Study of the Use of Cu(I)/4,4’-Dicarboxy-2,2’-biquinoline Complexes to Measure the Total Reducing Capacity: Application in Herbal Extracts" Molecules 20, no. 12: 22411-22421. https://doi.org/10.3390/molecules201219855
APA StyleManoel, H. R., & Moya, H. D. (2015). A Comprehensive Study of the Use of Cu(I)/4,4’-Dicarboxy-2,2’-biquinoline Complexes to Measure the Total Reducing Capacity: Application in Herbal Extracts. Molecules, 20(12), 22411-22421. https://doi.org/10.3390/molecules201219855