Accurate Mass GC/LC-Quadrupole Time of Flight Mass Spectrometry Analysis of Fatty Acids and Triacylglycerols of Spicy Fruits from the Apiaceae Family
Abstract
:1. Introduction
2. Results and Discussion
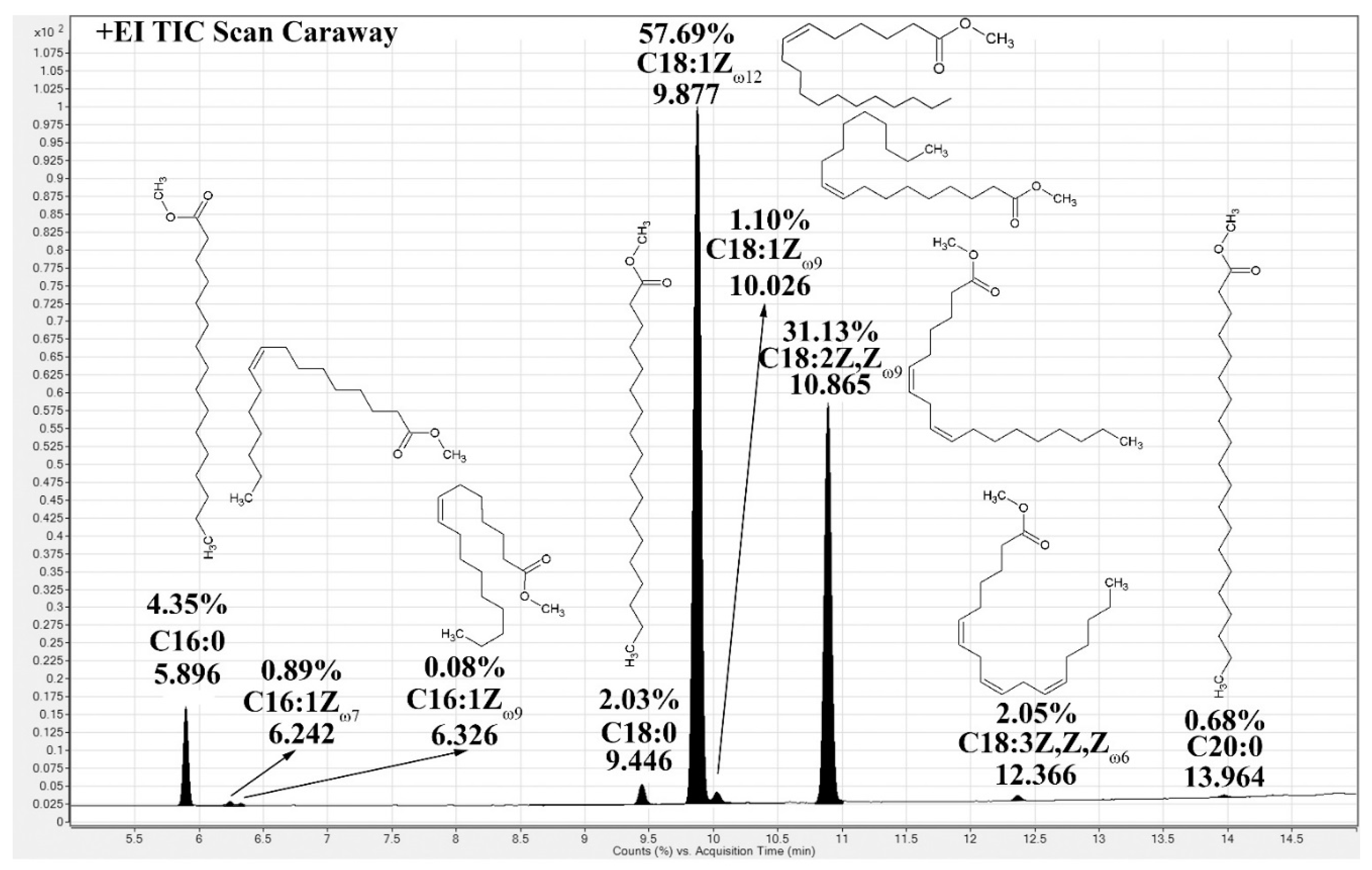
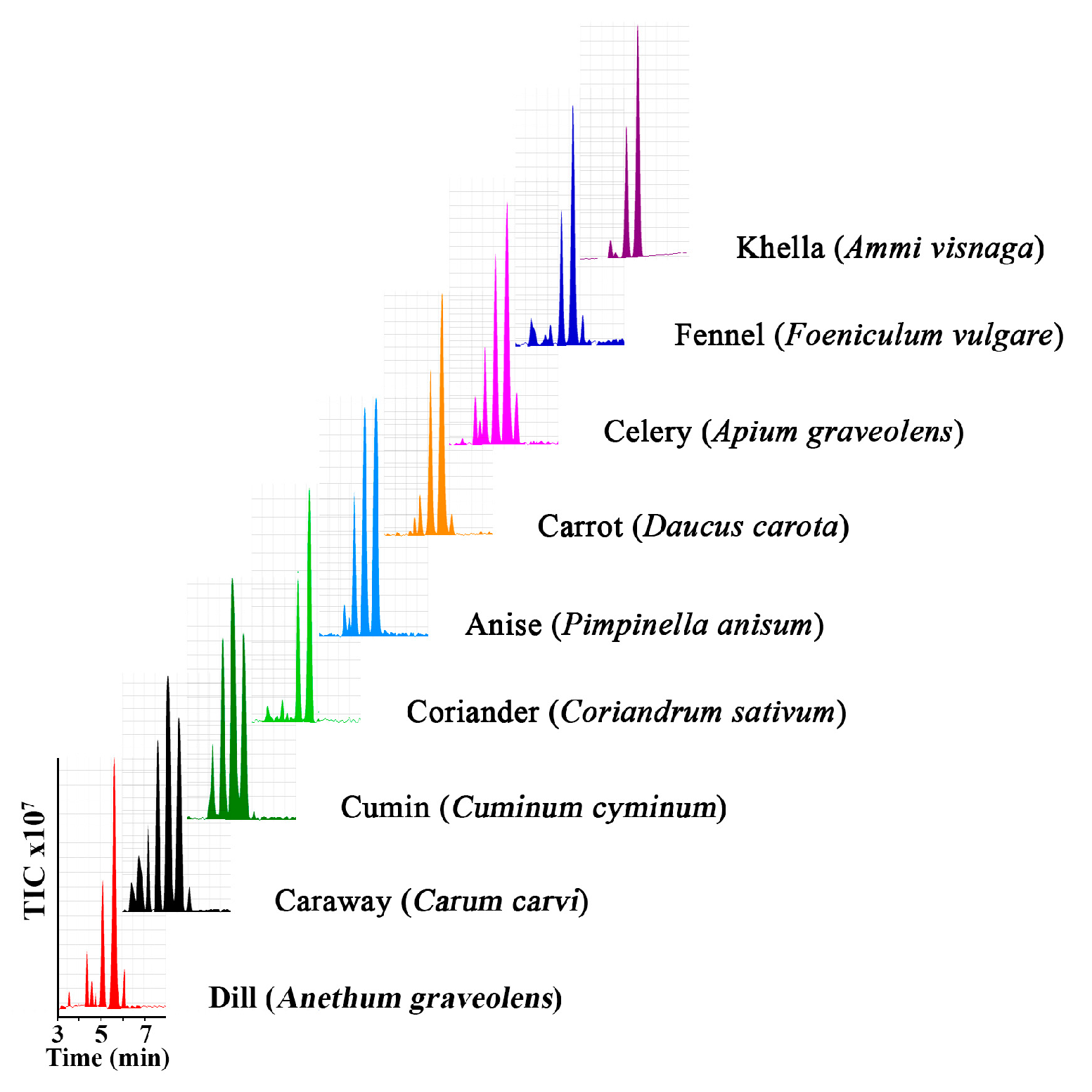
2.1. Fatty Acids Composition
| Fatty Acids | Dill | Caraway | Cumin | Coriander | Anise | Carrot | Celery | Fennel | Khella |
|---|---|---|---|---|---|---|---|---|---|
| C16:0 | 5.79 ± 0.41 | 4.35 ± 0.51 | 4.67 ± 0.11 | 4.25 ± 0.10 | 4.75 ± 0.11 | 5.29 ± 0.15 | 8.51 ± 0.98 | 4.71 ± 0.12 | 4.92 ± 0.09 |
| C16:1 | 0.52 ± 0.05 | 0.89 ± 0.09 | 0.33 ± 0.01 | 0.46 ± 0.05 | 0.29 ± 0.04 | 0.62 ± 0.09 | 0.38 ± 0.07 | 0.45 ± 0.05 | 0.26 ± 0.01 |
| C16:1 | nd * | 0.08 ± 0.01 | 0.31 ± 0.01 | 0.26 ± 0.03 | 0.27 ± 0.05 | 0.64 ± 0.10 | 0.14 ± 0.01 | 0.08 ± 0.01 | 0.23 ± 0.01 |
| C18:0 | 1.61 ± 0.08 | 2.03 ± 0.11 | nd * | nd * | 1.17 ± 0.25 | 0.91 ± 0.11 | 2.03 ± 0.04 | 1.23 ± 0.09 | 1.08 ± 0.11 |
| 6ZC18:1 | 79.91 ± 1.16 | 57.69 ± 0.91 | 61.83 ± 1.97 | 79.78 ± 2.61 | 66.26 ± 1.81 | 81.20 ± 2.41 | 65.79 ± 1.81 | 81.95 ± 2.41 | 74.95 ± 2.15 |
| 9ZC18:1 | nd * | 1.10 ± 0.05 | 1.40 ± 0.04 | nd* | 1.47 | nd * | nd * | nd * | nd * |
| C18:2 | 10.80 ± 0.47 | 31.13 ± 0.81 | 30.44 ± 0.91 | 14.85 ± 0.31 | 24.58 ± 0.12 | 10.02 ± 0.11 | 21.65 ± 0.65 | 10.97 ± 0.31 | 16.68 ± 0.16 |
| C18:3 | 0.70 ± 0.03 | 2.05 ± 0.05 | 0.61 ± 0.02 | 0.17 ± 0.03 | 0.75 ± 0.09 | 0.61 ± 0.05 | 1.01 ± 0.09 | 0.32 ± 0.01 | 1.41 ± 0.11 |
| C20:0 | 0.52 ± 0.06 | 0.68 ± 0.04 | 0.15 ± 0.01 | 0.12 ± 0.02 | nd * | 0.32 ± 0.03 | 0.33 ± 0.07 | 0.22 ± 0.01 | 0.25 ± 0.01 |
| % Yield § | 20.5 | 14.3 | 23.4 | 16.8 | 15.3 | 17.9 | 9.8 | 14.6 | 12.6 |
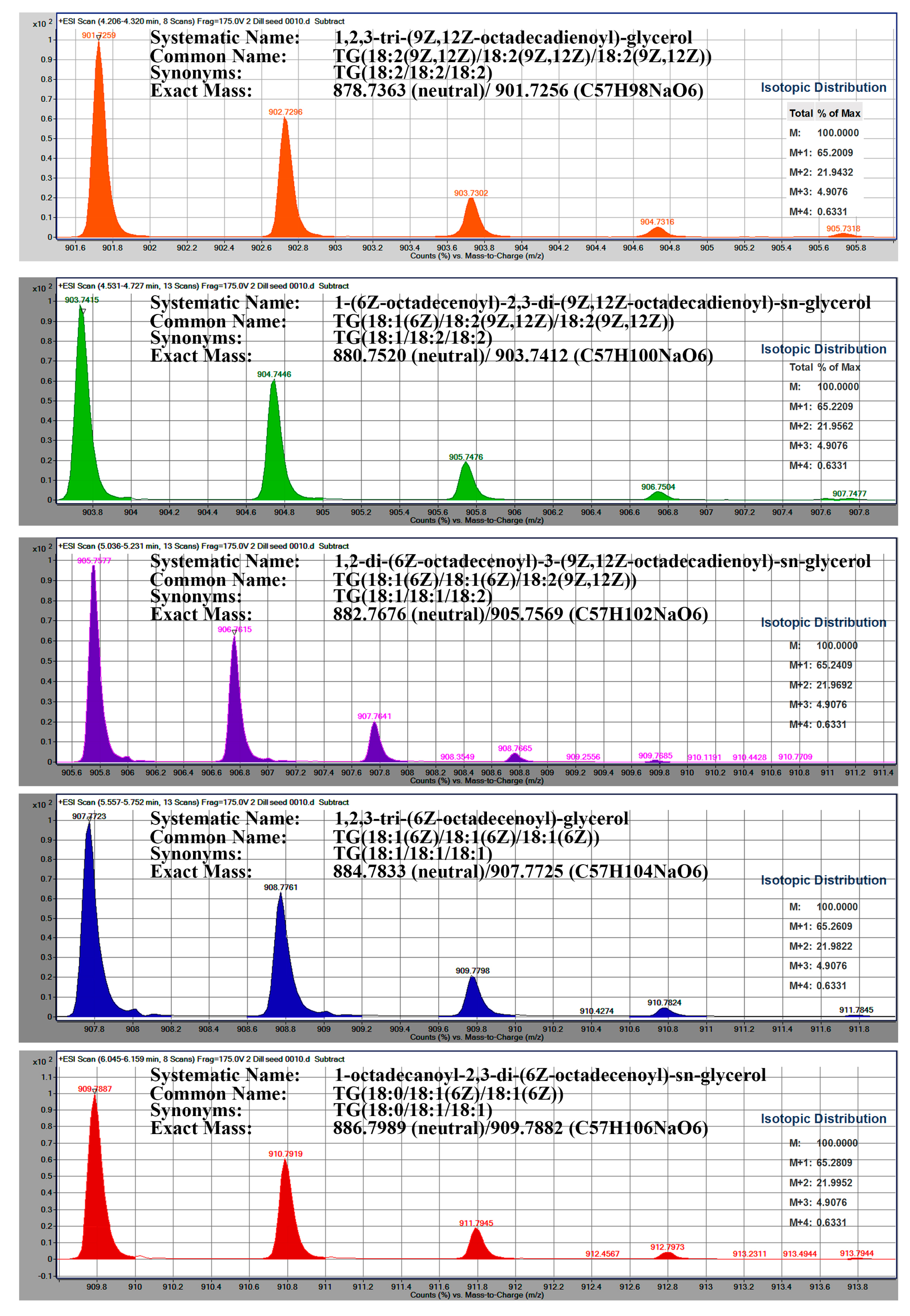
2.2. Identification of Triacylglycerols TAG
| Accurate mass (Possible Isomers) | Suspected Isomers | Regioisomers |
|---|---|---|
| 901.7256 (186 isomers) | 18:1Z9/18:2/18:3 | 6 |
| 18:1Z6/18:2/18:3 | 6 | |
| 18:0/18:3/18:3 | 3 | |
| 18:2/18:2/18:2 | 1 | |
| Total | 16 | |
| 903.7412 (405 isomers) | 18:1Z9/18:2/18:2 | 3 |
| 18:1Z6/18:2/18:2 | 3 | |
| 18:0/18:2/18:3 | 6 | |
| 18:1Z9/18:1Z9/18:3 | 3 | |
| 18:1Z6/18:1Z6/18:3 | 3 | |
| 18:1Z9/18:1Z6/18:3 | 6 | |
| Total | 24 | |
| 905.7569 (405) | 18:0/18:1Z9/18:3 | 6 |
| 18:0/18:1Z6/18:3 | 6 | |
| 16:1/18:3/20:0 | 6 | |
| 18:0/18:2/18:2 | 3 | |
| 18:1Z6/18:1Z6/18:2 | 3 | |
| 18:1Z9/18:1Z9/18:2 | 3 | |
| 18:1Z9/18:1Z6/18:2 | 6 | |
| Total | 33 | |
| 907.7725 (342) | 18:0/18:0/18:3 | 3 |
| 18:1Z6/18:1Z6/18:1Z6 | 1 | |
| 18:1Z9/18:1Z9/18:1Z9 | 1 | |
| 18:1Z9/18:1Z9/18:1Z6 | 3 | |
| 18:1Z9/18:1Z6/18:1Z6 | 3 | |
| 18:0/18:1Z9/18:2 | 6 | |
| 18:0/18:1Z6/18:2 | 6 | |
| 16:0/18:3/20:0 | 6 | |
| 16:1/18:2/20:0 | 6 | |
| Total | Total | 35 |
| 909.7882 (291) | 18:0/18:1 Z9/18:1Z9 | 3 |
| 18:0/18:1 Z6/18:1Z6 | 3 | |
| 18:0/18:1 Z9/18:1Z6 | 6 | |
| 18:0:18:0/18:2 | 3 | |
| 16:0:18:2/20:0 | 6 | |
| 16:1:18:1Z9/20:0 | 6 | |
| 16:1:18:1Z6/20:0 | 6 | |
| Total | 33 |
| Identified TAGs | Dill | Caraway | Cumin | Coriander | Anise | Carrot | Celery | Fennel | Khella |
|---|---|---|---|---|---|---|---|---|---|
| TG18:2/18:2/18:2 C57H98O6Na | 6.17 | 7.78 | 0.68 | 1.25 | 1.85 | 2.01 | nd * | nd * | |
| 901.7256 | |||||||||
| 4.20 min | |||||||||
| TG18:1/18:2/18:2 C57H100O6Na | 3.31 | 16.24 | 20.15 | 10.16 | 2.76 | ||||
| 903.7412 | |||||||||
| 4.63 min | |||||||||
| TG18:0/18:3/18:2 C57H100O6Na | 0.87 | 12.75 | 5.27 | 0.86 | |||||
| 903.7412 | |||||||||
| 4.63 min | |||||||||
| TG18:1/18:1/18:2 C57H102O6Na | 29.71 | 37.88 | 25.99 | ||||||
| 905.7569 | |||||||||
| 5.13 min | |||||||||
| TG18:1/18:2/18:1 C57H102O6Na | 22.36 | 28.38 | 25.41 | 24.38 | 17.68 | 26.33 | |||
| 905.7569 | |||||||||
| 5.13 min | |||||||||
| TG18:1/18:1/18:1 C57H104O6Na | 58.40 | 24.73 | 25.34 | 62.72 | 31.22 | 49.25 | 37.17 | 40.04 | 51.95 |
| 907.7725 | |||||||||
| 5.66 min | |||||||||
| TG18:0/18:1/18:1 C57H106O6Na | 3.27 | 0.63 | 0.32 | 2.64 | 5.34 | 3.71 | nd * | ||
| 909.7882 | |||||||||
| TG18:1/18:0/18:1 C57H106O6Na | 5.06 | 0.79 | nd * | ||||||
| 909.7882 | |||||||||
| 6.50 min |
| TAGs | Dill | Caraway | Cumin | Coriander | Anise | Carrot | Celery | Fennel | Khella |
|---|---|---|---|---|---|---|---|---|---|
| TG16:0/16:0/16:0 C51H98O6Na | x | x | x | x | x | x | x | ||
| 829.7256 | |||||||||
| 4.20 min | |||||||||
| TG16:0/18:2/18:2 C55H98O6Na | x | x | x | x | |||||
| 877.7248 | |||||||||
| 4.73 min | |||||||||
| TG16:1/18:1/18:2 C55H98O6Na | x | x | |||||||
| 877.7248 | |||||||||
| 4.73 min | |||||||||
| TG16:1/18:0/18:3 C55H98O6Na | x | x | |||||||
| 877.7248 | |||||||||
| 4.73 min | |||||||||
| TG18:3/18:3/18:3 C57H92O6Na | x | x | x | x | |||||
| 895.6786 | |||||||||
| 3.73 min | |||||||||
| TG16:1/18:0/20:0 C57H108O6Na | x | ||||||||
| 911.8038 | |||||||||
| 6.50 min | |||||||||
| TG18:0/18:0/18:1 C57H108O6Na | x | ||||||||
| 911.8038 | |||||||||
| 6.50 min | |||||||||
| TG18:0/18:0/18:0 C57H110O6Na | x | x | x | ||||||
| 913.8195 | |||||||||
| 7.12 min | |||||||||
| TG18:1/18:1/20:0 C59H110O6Na | x | x | x | x | x | x | x | x | x |
| 937.8186 | |||||||||
| 6.60 min | |||||||||
| TG18:2/20:0/20:0 C61H114O6Na | x | x | x | x | x | x | x | x | |
| 965.8508 | |||||||||
| 6.73 min |
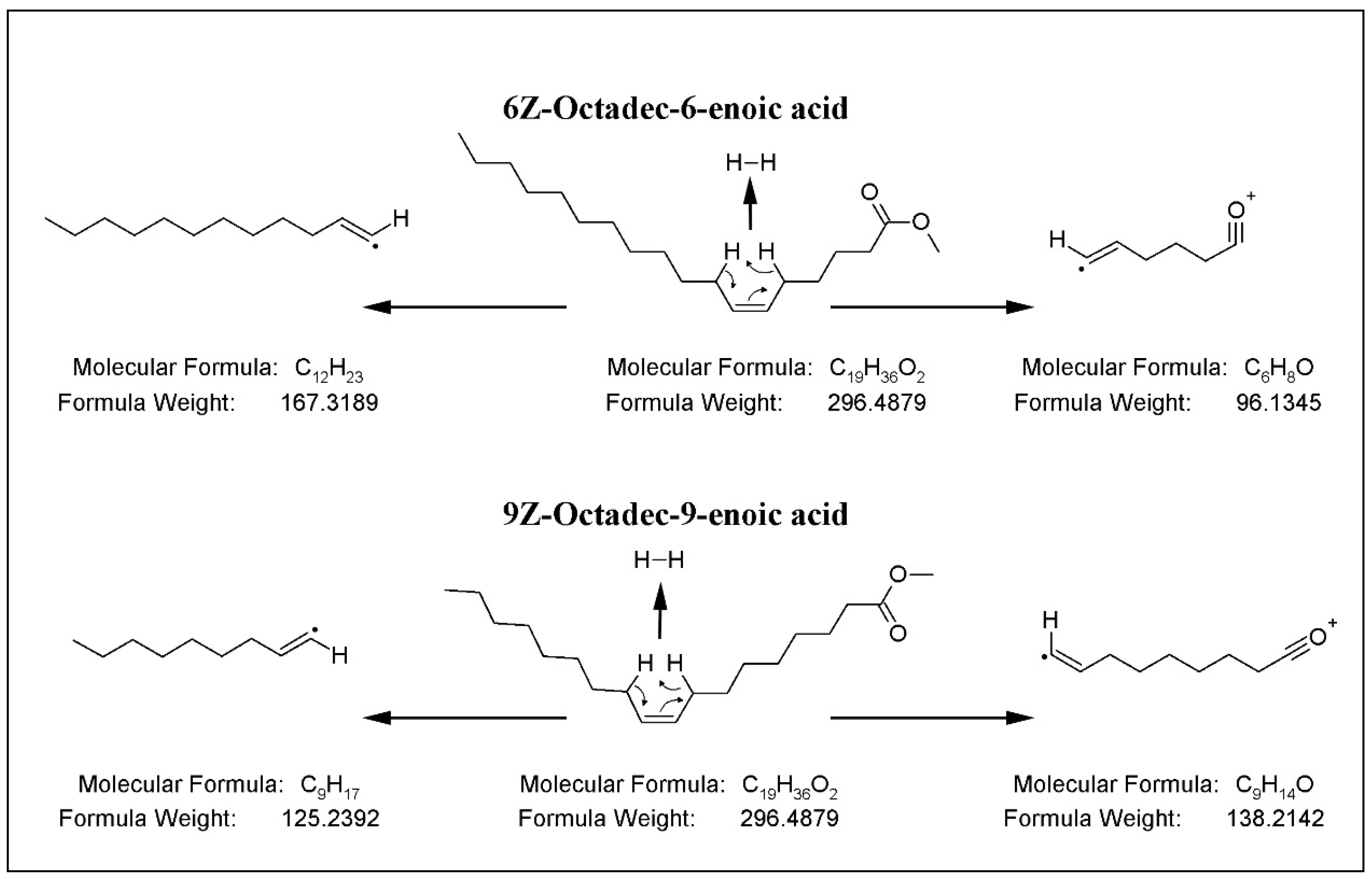
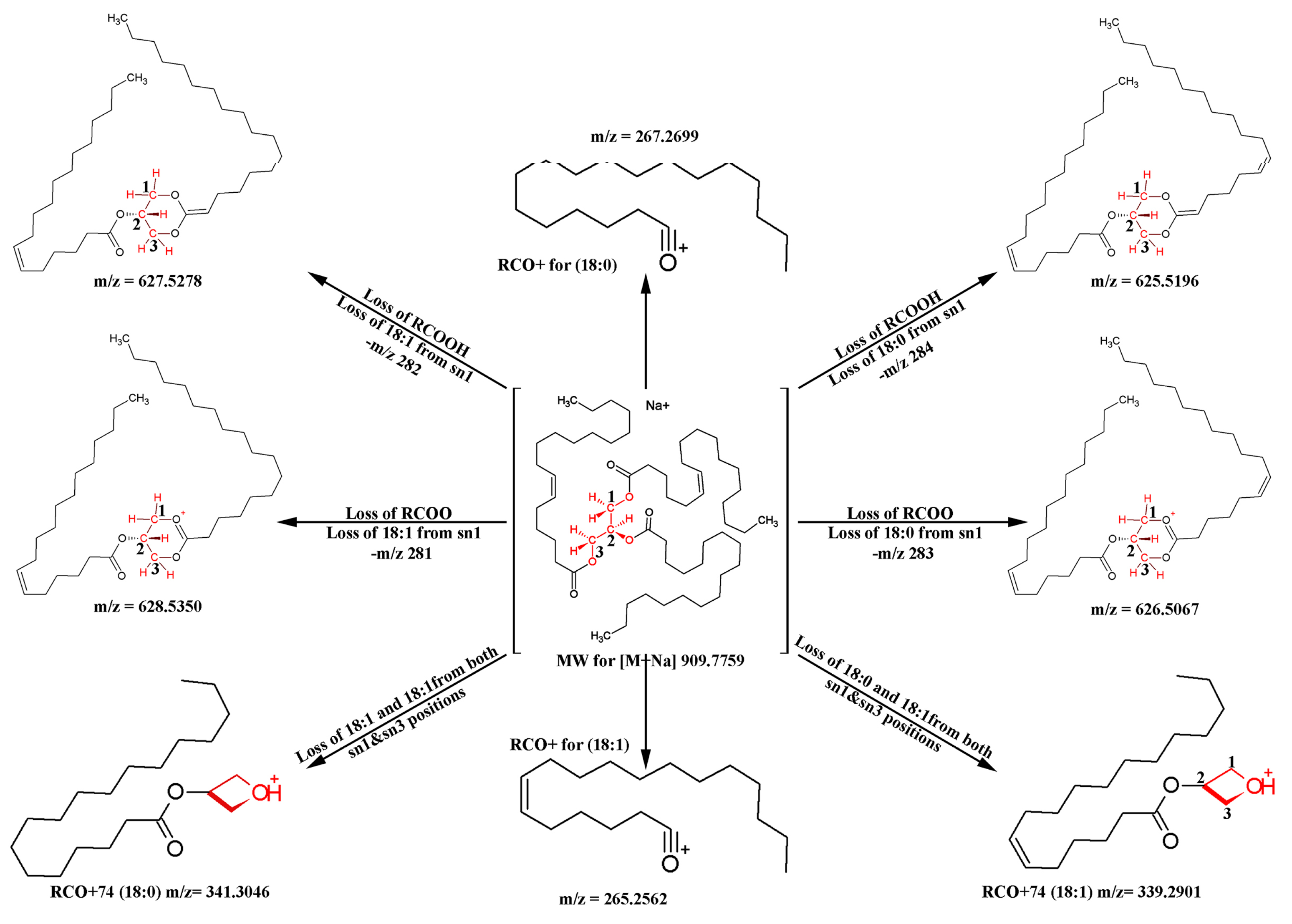
2.3. Determination of Regioisomers by MS/MS Experiments
3. Experimental Section
3.1. Materials
3.2. Oil extraction
3.3. Fatty Acids Methyl Esters (FAMES) Preparation Analysis
3.4. Accurate Mass GC-QTOF
3.5. LC-QTOF
3.6. LC-QTOF MS/MS Experiment
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dempewolf, H.; Eastwood, R.J.; Guarino, L.; Khoury, C.K.; Müller, J.V.; Toll, J. Adapting agriculture to climate change: A global initiative to collect, conserve and use crop wild relatives. Agroecol. Sustain. Food Syst. 2014, 38, 369–377. [Google Scholar] [CrossRef]
- Kleiman, R.; Spencer, G. Search for new industrial oils. JAOSC 1982, 59, 29. [Google Scholar]
- Heywood, V.H. Biology and Chemistry of the Umbelliferae; Academic Press: London, UK, 1971. [Google Scholar]
- Murphy, D.J. Designer Oil Crops: Breeding, Processing and Biotechnology; VCH: Weinheim, Germany, 1994. [Google Scholar]
- Weber, N.; Richter, K.; Schulte, D.E.; Mukherjee, K.D. Petroselinic acid from dietary triacylglycerols reduces the concentration of arachidonic acid in tissue lipids of rats. J. Nutr. 1995, 125, 1563–1568. [Google Scholar] [PubMed]
- Takeuchi, Y.; Yamaoka, Y.; Fukushima, S.; Miyawaki, K.; Taguchi, K.; Yasukawa, H.; Kishimoto, S.; Suzuki, M. Skin penetration enhancing action of cis-unsaturated fatty acids with omega-9, omega-12 chain lengths. Biol. Pharm. Bull. 1998, 21, 484–491. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shahat, A.A.; Ibrahim, A.Y.; Hendawy, S.F.; Elsayed, A.; Omer, E.A.; Hammouda, F.M.; Abdel-Rahman, F.H.; Saleh, M.A. Chemical composition, antimicrobial and antioxidant activities of essential oils from organically cultivated fennel cultivars. Molecules 2011, 16, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Reiter, B.; Lechner, M.; Lorbeer, E. The fatty acid profiles including petroselinic and cis-vaccenic acid of different Umbelliferae seed oils. Lipid 1998, 100, 498–502. [Google Scholar] [CrossRef]
- Kleiman, R.; Spencer, G.F. Search for new industrial oils. XVI. Umbelliflorae-seed oils rich in petroselinic acid. J. Am. Oil Chem. Soc. 1982, 59, 29–38. [Google Scholar] [CrossRef]
- Knapp, S.J. New temperate oilseed crops. In Advances in New Crops; Janick, J., Simon, J.E., Eds.; Timber Press: Portland, OR, USA, 1990; pp. 203–210. [Google Scholar]
- Smith, M.A.; Zhang, H.; Randy, L.F.; Purves, W. Characterization of novel triacylglycerol estolides from the seed oil of Mallotus philippensis and Trewia nudiflora. Lipids 2013, 48, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Triacylglycerols composition, oxidation and oxidation compounds in camellia oil using liquid chromatography–mass spectrometry. Chem. Phys. Lipids 2012, 165, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Ngo-Duy, C.; Destaillats, F.; Keskitalo, M.; Arul, J.; Angers, P. Triacylglycerols of Apiaceae seed oils: Composition and regiodistribution of fatty acids. Eur. J. Lipid Sci. Technol. 2009, 111, 164–169. [Google Scholar] [CrossRef]
- Tobias, K.; Fiehn, O. Advances in structure elucidation of small molecules using mass spectrometry. Bioanal. Rev. 2010, 2, 23–60. [Google Scholar]
- Velinska, L.M.; Holcapek, M.H. Regioisomeric characterization of triacylglycerols using silver-ion HPLC/MS and randomization synthesis of standards. Anal. Chem. 2009, 81, 3903–3910. [Google Scholar]
- Leskinen, H.; Suomela, J.P.; Kallio, H. Quantification of triacylglycerol regioisomers in oils and fat using different mass spectrometric and liquid chromatographic methods. Rapid Commun. Mass Spectrom. 2007, 21, 2361–2373. [Google Scholar] [CrossRef] [PubMed]
- Paavo, J.; Asmo, K. Regiospecific analysis of TAGs using chromatography/MS. Eur. J. Lipid Sci. Technol. 2012, 114, 399–411. [Google Scholar]
- Weimin, W.; Shiying, T.; Stark, R.E. Isolation and identification of triglycerides and ester oligomers from partial degradation of potato suberin. J. Agric. Food Chem. 2010, 27, 58, 1040–1045. [Google Scholar]
- Kalo, P.; Kemppinen, A. Triglycerides/structures and properties. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Trugo, L.P., Finglas, M., Eds.; Academic Press: London, UK, 2003; pp. 5857–5868. [Google Scholar]
- Kuksis, A.; Itabashi, Y. Regio and stereospecific analysis of glycerolipids. Methods 2005, 36, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Kallio, H.; Rua, P. Distribution of major fatty acids of human milk between sn-2 and sn-1,3 positions of triacylglycerols. J. Am. Oil Chem. Soc. 1994, 71, 985–992. [Google Scholar] [CrossRef]
- Kurvinen, J.P.; Sjovall, O.; Kallio, H. Molecular weight distribution and regioisomeric structure of triacylglycerols in some common human milk substitutes. J. Am. Oil Chem. Soc. 2002, 79, 13–22. [Google Scholar] [CrossRef]
- Sample Availability: No samples are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.; Aparicio, M.; Saleh, M.A. Accurate Mass GC/LC-Quadrupole Time of Flight Mass Spectrometry Analysis of Fatty Acids and Triacylglycerols of Spicy Fruits from the Apiaceae Family. Molecules 2015, 20, 21421-21432. https://doi.org/10.3390/molecules201219779
Nguyen T, Aparicio M, Saleh MA. Accurate Mass GC/LC-Quadrupole Time of Flight Mass Spectrometry Analysis of Fatty Acids and Triacylglycerols of Spicy Fruits from the Apiaceae Family. Molecules. 2015; 20(12):21421-21432. https://doi.org/10.3390/molecules201219779
Chicago/Turabian StyleNguyen, Thao, Mario Aparicio, and Mahmoud A. Saleh. 2015. "Accurate Mass GC/LC-Quadrupole Time of Flight Mass Spectrometry Analysis of Fatty Acids and Triacylglycerols of Spicy Fruits from the Apiaceae Family" Molecules 20, no. 12: 21421-21432. https://doi.org/10.3390/molecules201219779
APA StyleNguyen, T., Aparicio, M., & Saleh, M. A. (2015). Accurate Mass GC/LC-Quadrupole Time of Flight Mass Spectrometry Analysis of Fatty Acids and Triacylglycerols of Spicy Fruits from the Apiaceae Family. Molecules, 20(12), 21421-21432. https://doi.org/10.3390/molecules201219779