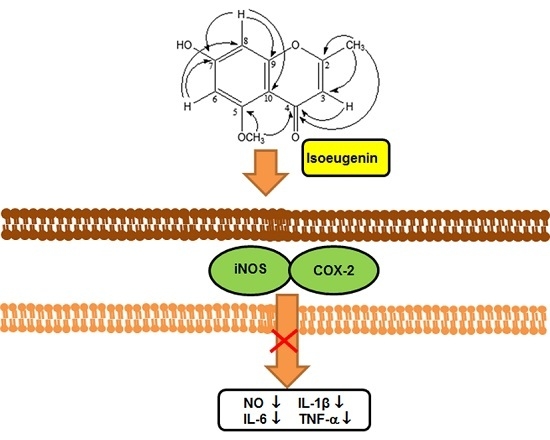
Isoeugenin, a Novel Nitric Oxide Synthase Inhibitor Isolated from the Rhizomes of Imperata cylindrica
Abstract
:1. Introduction
2. Results and Discussion
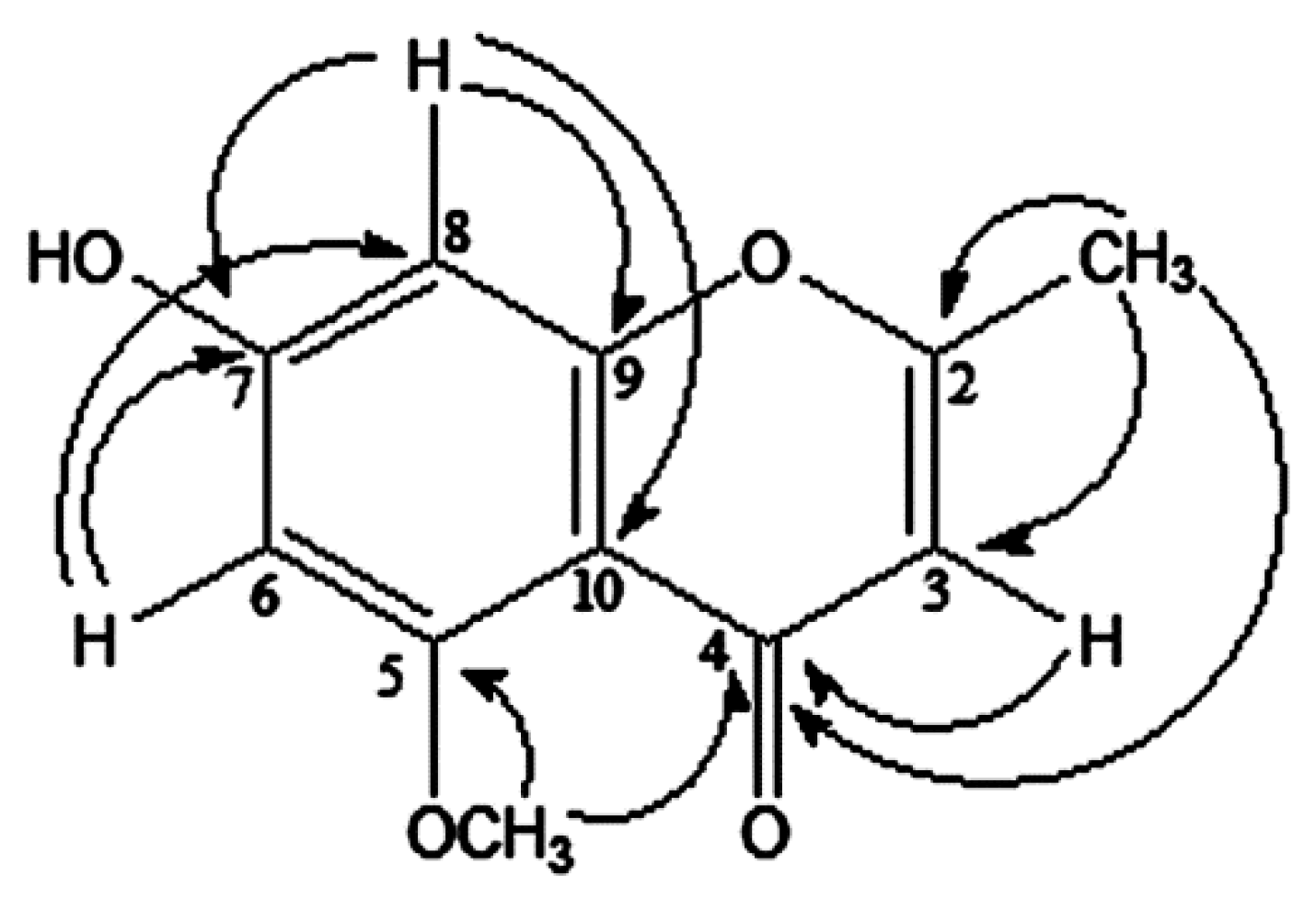
2.1. Isolation and Characterization of Compounds
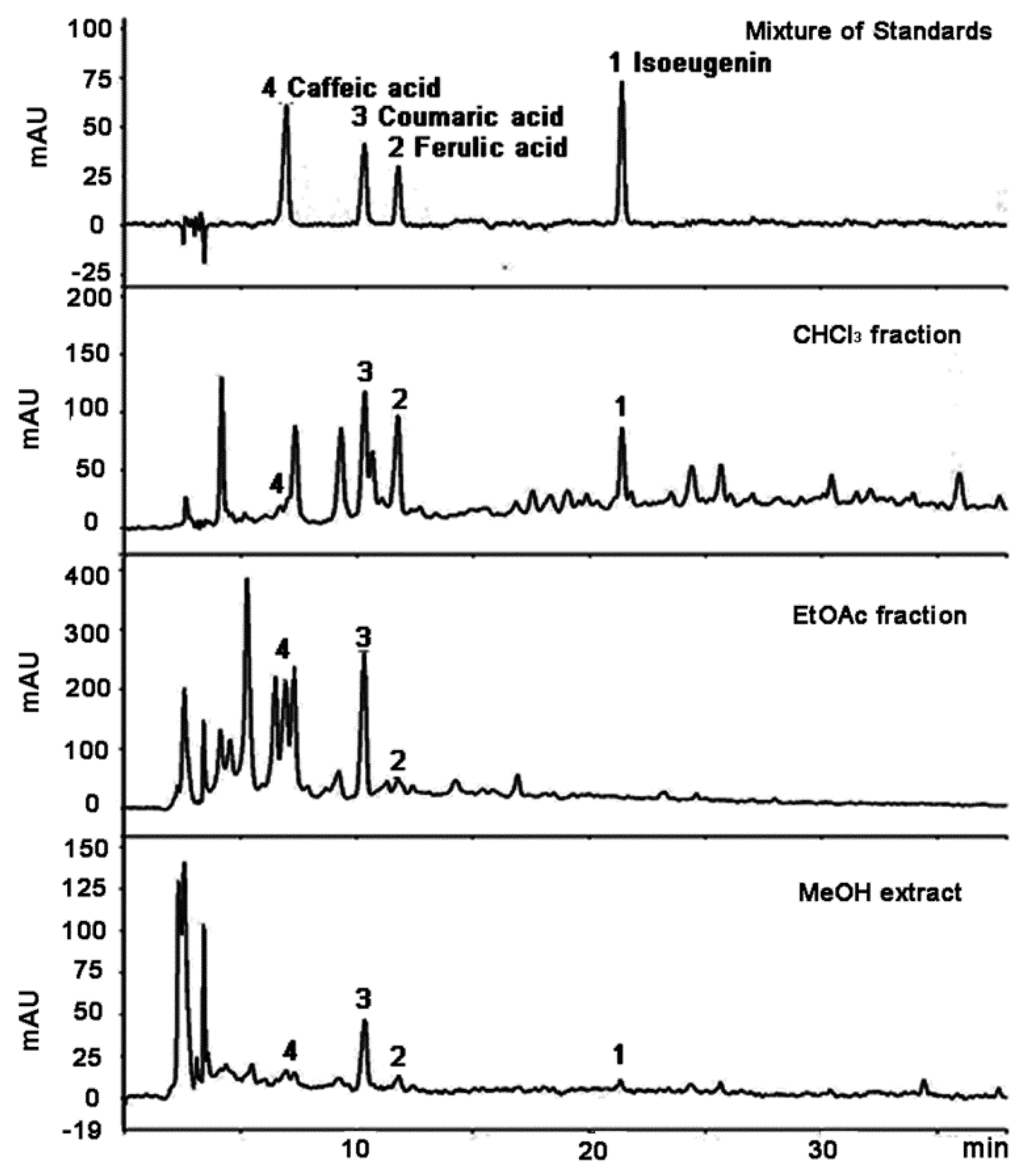
| Standard Compounds | tR (min) | Calibration Equation (Linear Model) a | Linear Range (μg/mL) | R2 b | LOD c (μg/mL) | LOQ d (μg/mL) |
|---|---|---|---|---|---|---|
| Caffeic acid | 6.92 | y = 747.05x + 83.33 | 12.50–200.0 | 0.9998 | 0.16 | 0.52 |
| p-Coumaric acid | 10.30 | y = 245.73x + 95.83 | 12.50–200.0 | 0.9998 | 0.42 | 1.41 |
| Ferulic acid | 11.75 | y = 276.37x + 41.66 | 12.50–200.0 | 0.9993 | 0.57 | 1.91 |
| Isoeugenin | 21.42 | y = 40.468x + 30.26 | 25.00–1000.0 | 0.9998 | 4.19 | 13.97 |
| Analyte | MeOH Extract | Fractions (mg/g Extract) | ||
|---|---|---|---|---|
| (mg/g Dry Weight) | (mg/g Extract) | CHCl3 | EtOAc | |
| Caffeic acid | 0.020 | 0.15 | 0.32 | 4.59 |
| p-Coumaric acid | 0.182 | 1.32 | 3.73 | 16.18 |
| Ferulic acid | 0.042 | 0.30 | 4.89 | 2.97 |
| Isoeugenin | 0.268 | 1.94 | 28.09 | n.d. |
| Total | 0.512 | 3.71 | 37.03 | 23.74 |
2.2. Inhibitory Effect of Compounds on LPS-Induced NO Production
| Group | Concentration | NO Production (μM) | Inhibition (%) |
|---|---|---|---|
| NOR | - | 11.90 ± 0.65 | - |
| LPS | - | 77.18 ± 0.82 # | - |
| l-NIL (μM) | 20 | 36.12 ± 1.56 *** | 53.21 ± 2.02 |
| Isoeugenin (μg/mL) | 12.5 | 32.96 ± 0.47 *** | 57.29 ± 0.61 |
| 25 | 10.36 ± 0.16 *** | 86.58 ± 0.20 | |
| 50 | 6.12 ± 0.16 *** | 92.07 ± 0.20 | |
| Ferulic Acid (μg/mL) | 25 | 79.05 ± 3.08 | −2.42 ± 3.99 |
| 50 | 79.67 ± 3.16 | −3.22 ± 4.09 | |
| 100 | 72.56 ± 0.71 ** | 5.99 ± 0.91 | |
| Coumaric acid (μg/mL) | 25 | 77.89 ± 1.49 | −0.92 ± 1.92 |
| 50 | 77.00 ± 2.46 | 0.23 ± 3.19 | |
| 100 | 72.83 ± 1.67 ** | 5.64 ± 2.16 | |
| Caffeic acid (μg/mL) | 25 | 76.38 ± 1.49 | 1.04 ± 1.06 |
| 50 | 74.87 ± 0.67 | 2.99 ± 0.87 | |
| 100 | 66.60 ± 0.41 *** | 13.70 ± 0.53 |
2.3. Inhibitory Effect of Isoeugenin on the LPS-Induced iNOS and COX-2 Expressions

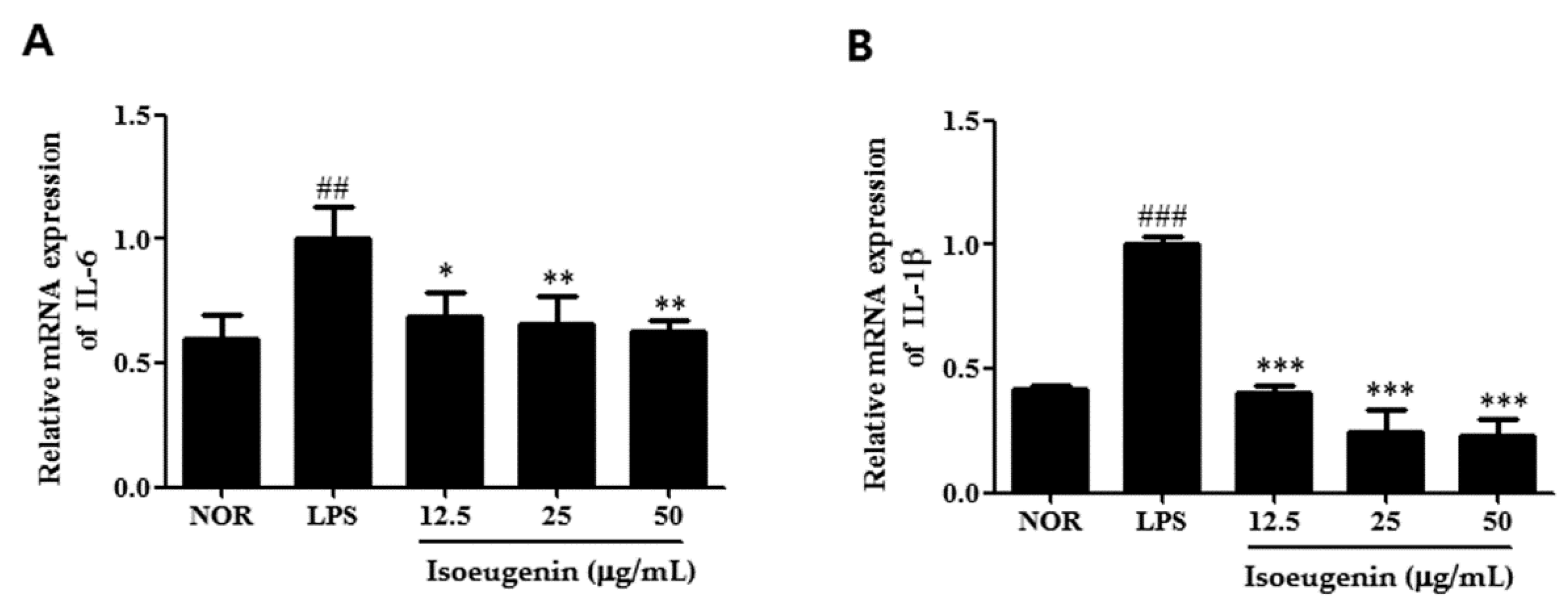
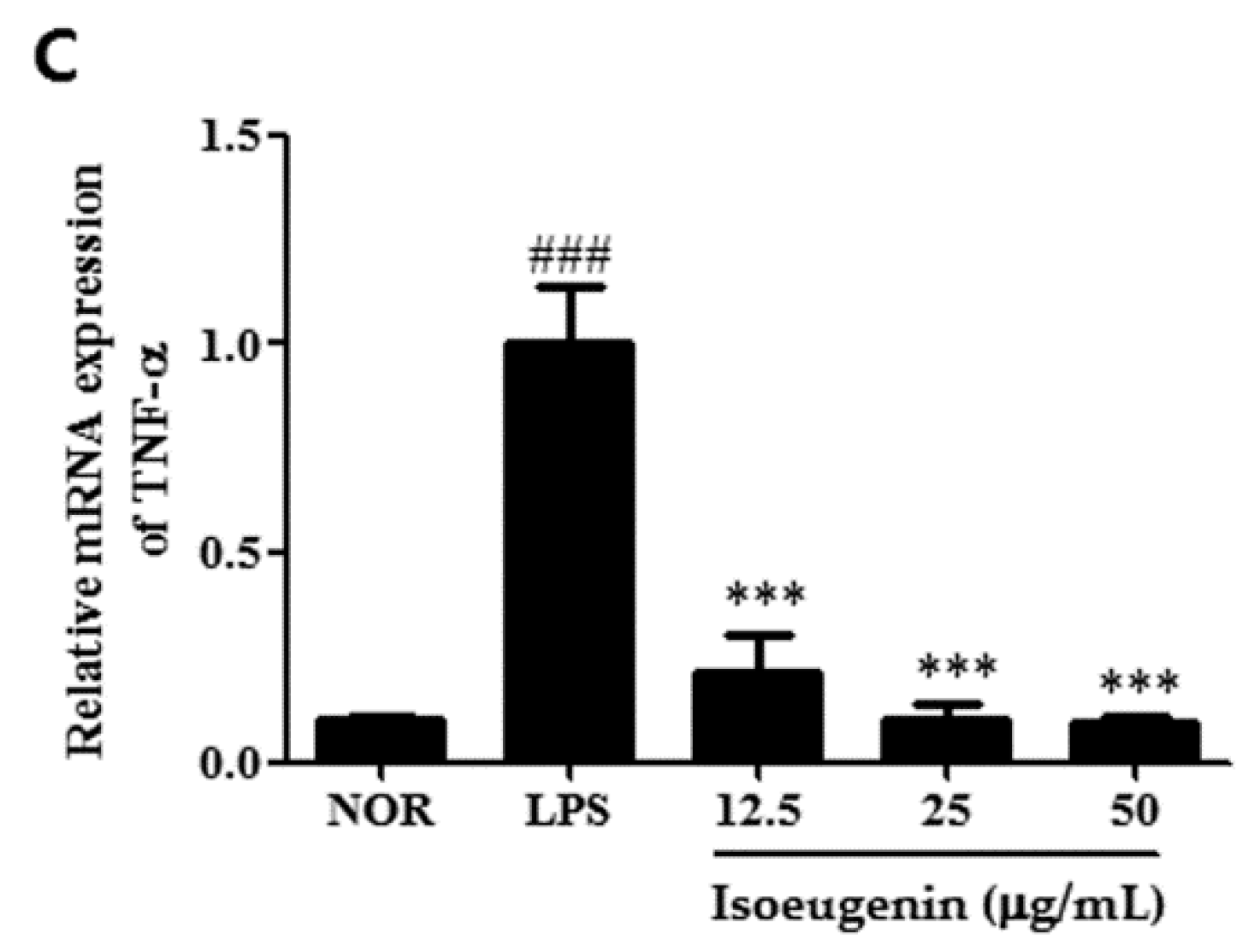
2.4. Inhibitory Effect of Isoeugenin on the LPS-Induced mRNA Levels of Cytokines
3. Experimental Section
3.1. General Information
3.2. Plant Material
3.3. Extraction and Fractionation
3.4. Isolation of the Componds
3.5. HPLC Analysis
3.6. Preparation of Standard and Test Solutions
3.7. Cell Culture and Sample Treatment
3.8. Measurement of Cell Viability by 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) Assay
3.9. Measurement of Nitrite in Culture Media
3.10. Western Blot Analysis
3.11. Quantitative Real-Time PCR Analysis
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Kim, B.K.; Lim, J.S.; Kil, K.J. Effects of Imperatae Rhizoma Extract on T helper 2 cell differentiation. Korean J. Herbol. 2014, 29, 27–33. [Google Scholar] [CrossRef]
- Yoon, J.S.; Lee, M.K.; Sung, S.H.; Kim, Y.C. Neuroprotective 2-(2-phenylethyl)chromones of Imperata cylindrica. J. Nat. Prod. 2006, 69, 290–291. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Han, K.M.; Song, M.C.; Lee, D.G.; Rho, Y.D.; Baek, N.I. A new lignan glycoside from the rhizomes of Imperata cylindrica. J. Asian Nat. Prod. Res. 2008, 10, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, K.; Ikeda, M.; Shibuya, M.; Ohizumi, Y. Cylindol A, a novel biphenyl ether with 5-lipoxygenase inhibitory activity, and a related compound from Imperata Cylindrica. J. Nat. Prod. 1994, 57, 1290–1293. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, K.; Shibuya, M.; Ohizumi, Y. Cylindrene, a novel sesquiterpenoid from Imperata cylindrica with inhibitory activity on contractions of vascular smooth muscle. J. Nat. Prod. 1994, 57, 1183–1184. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, B.F.; Yang, L.; Chou, G.X.; Wang, Z.T. Four new compounds from Imperata cylindrica. J. Nat. Med. 2014, 68, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Cerdeira, A.L.; Cantrell, C.L.; Dayan, F.E.; Byrd, J.D. Tabanone, a new phytotoxic constituent of Cogongrass (Imperata cylindrica). Weed Sci. 2012, 60, 212–218. [Google Scholar] [CrossRef]
- Kanwar, J.R.; Kanwar, R.K.; Burrow, H.; Baratchi, S. Recent advances on the roles of NO in cancer and chronic inflammatory disorders. Cur. Med. Chem. 2009, 16, 2373–2394. [Google Scholar] [CrossRef]
- An, H.J.; Kim, I.T.; Park, H.J.; Kim, H.M.; Choi, J.H.; Lee, K.T. Tormentic acid, a triterpenoid saponin, isolated from Rosa rugosa, inhibited LPS-induced iNOS, COX-2, and TNF-alpha expression through inactivation of the nuclear factor-kappab pathway in RAW264.7 macrophages. Int. Immunopharmacol. 2011, 11, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Vuolteenaho, K.; Moilanen, T.; Knowles, R.G.; Moilanen, E. The role of nitric oxide in osteoarthritis. Scand. J. Rheumatol. 2007, 36, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Tsui, W.G.; Brown, G.D. Chromones and chromaones from Baeckea frutescens. Phytochemistry 1996, 43, 871–876. [Google Scholar] [CrossRef]
- Hauser, D.; Zardin, T. Isolation of 6-hydroxymethyl-eugenin from Chaetomium minutum. Experientia 1972, 28, 1114. [Google Scholar] [CrossRef]
- Park, H.J.; Young, H.S.; Kim, J.O.; Rhee, S.H.; Choi, J.S. A Study on the Chemical Constituents of Orostachys japonicus A. Berger. Korean J. Pharmacogn. 1992, 22, 78–84. [Google Scholar]
- Yi, B.; Hu, L.; Mei, W.; Zhou, K.; Wang, H.; Luo, Y.; Wei, X.; Dai, H. Antioxidant phenolic compounds of cassava (Manihot esculenta) from Hainan. Molecules 2011, 16, 10157–10167. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, B. Chemical constituents of Solanum xanthocarpum. J. Chem. Pharm. Res. 2011, 3, 176–181. [Google Scholar]
- Aggarwal, B.B.; Natarajan, K. Tumor necrosis factors: Developments during the last decade. Eur. Cytokine Netw. 1996, 7, 93–124. [Google Scholar] [PubMed]
- Kaplanski, G.; Marin, V.; Montero-Julian, F.; Mantovani, A.; Farnarier, C. IL-6: A regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 2003, 24, 25–29. [Google Scholar] [CrossRef]
- Connell, L.; McInnes, I.B. New cytokine targets in inflammatory rheumatic diseases. Best Pract. Res. Clin. Rheumatol. 2006, 20, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann. N. Y. Acad. Sci. 1998, 856, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cheon, S.Y.; Chung, K.S.; Jeon, E.; Nugroho, A.; Park, H.J.; An, H.J. Anti-inflammatory Activity of Saxifragin via Inhibition of NF-κB Involves Caspase-1 Activation. J. Nat. Prod. 2015, 78, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the isoeugenin, ferulic acid, coumaric acid, caffeic acid are available from Hee-Juhn Park.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, H.-J.; Nugroho, A.; Song, B.-M.; Park, H.-J. Isoeugenin, a Novel Nitric Oxide Synthase Inhibitor Isolated from the Rhizomes of Imperata cylindrica. Molecules 2015, 20, 21336-21345. https://doi.org/10.3390/molecules201219767
An H-J, Nugroho A, Song B-M, Park H-J. Isoeugenin, a Novel Nitric Oxide Synthase Inhibitor Isolated from the Rhizomes of Imperata cylindrica. Molecules. 2015; 20(12):21336-21345. https://doi.org/10.3390/molecules201219767
Chicago/Turabian StyleAn, Hyo-Jin, Agung Nugroho, Byong-Min Song, and Hee-Juhn Park. 2015. "Isoeugenin, a Novel Nitric Oxide Synthase Inhibitor Isolated from the Rhizomes of Imperata cylindrica" Molecules 20, no. 12: 21336-21345. https://doi.org/10.3390/molecules201219767
APA StyleAn, H.-J., Nugroho, A., Song, B.-M., & Park, H.-J. (2015). Isoeugenin, a Novel Nitric Oxide Synthase Inhibitor Isolated from the Rhizomes of Imperata cylindrica. Molecules, 20(12), 21336-21345. https://doi.org/10.3390/molecules201219767