Cinidium officinale and its Bioactive Compound, Butylidenephthalide, Inhibit Laser-Induced Choroidal Neovascularization in a Rat Model
Abstract
:1. Introduction
2. Results
2.1. C. officinale Extract (COE) Inhibits Laser-Induced CNV Formation and Macrophage Infiltration
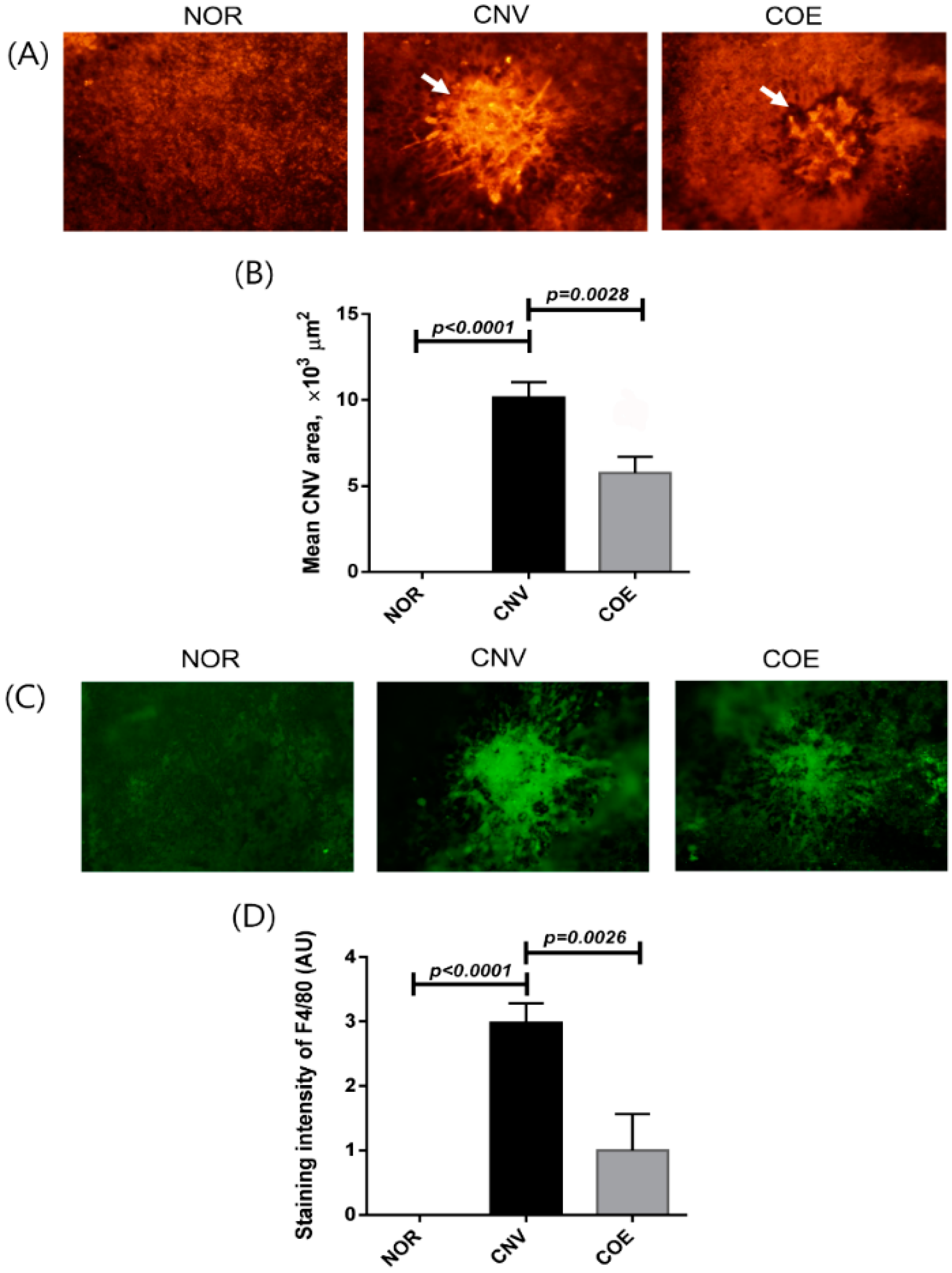
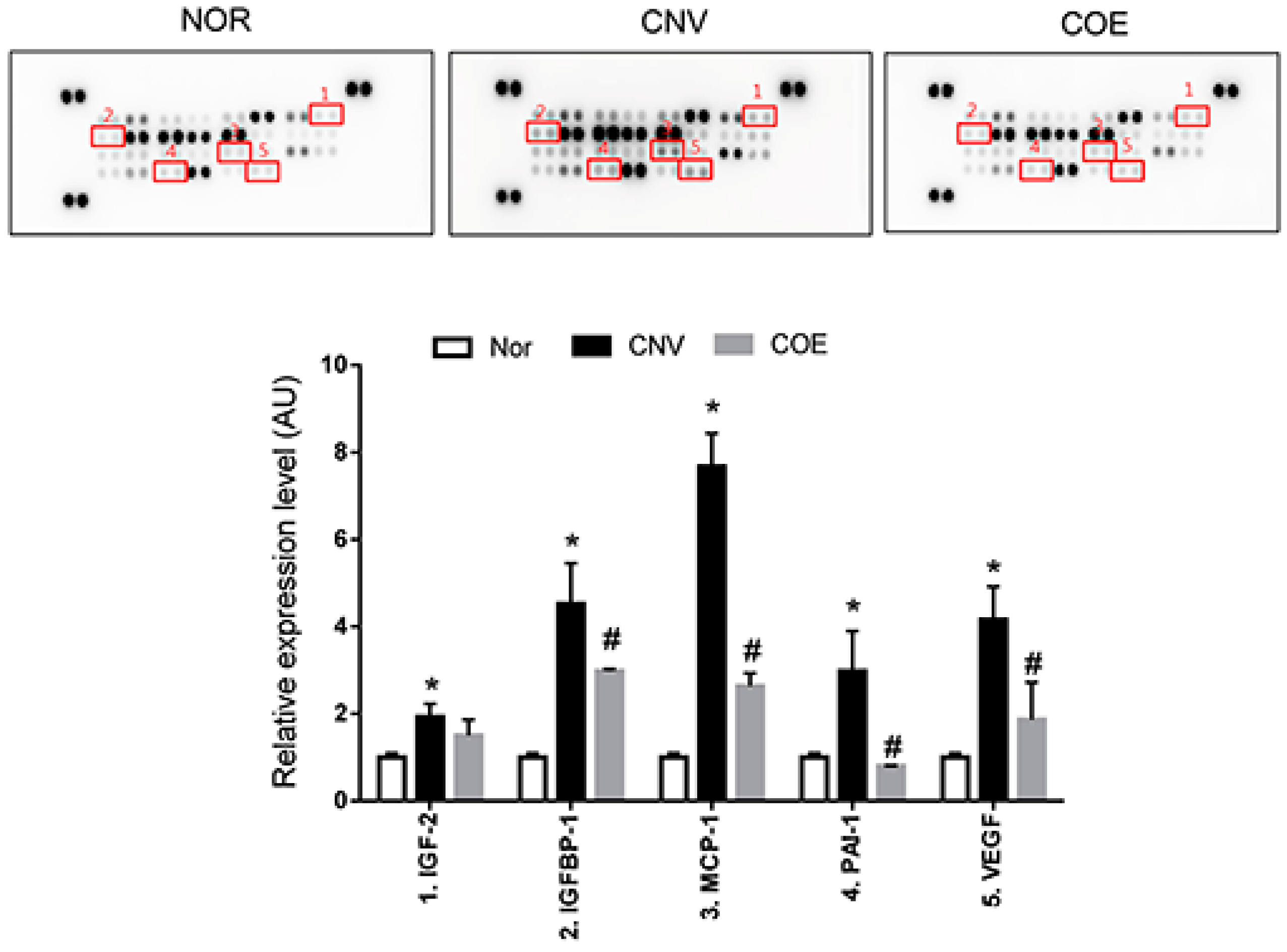
2.2. COE Regulates the Expression of Angiogenesis-Associated Factors
2.3. Butylidenephthalide Blocks Laser-Induced CNV Formation
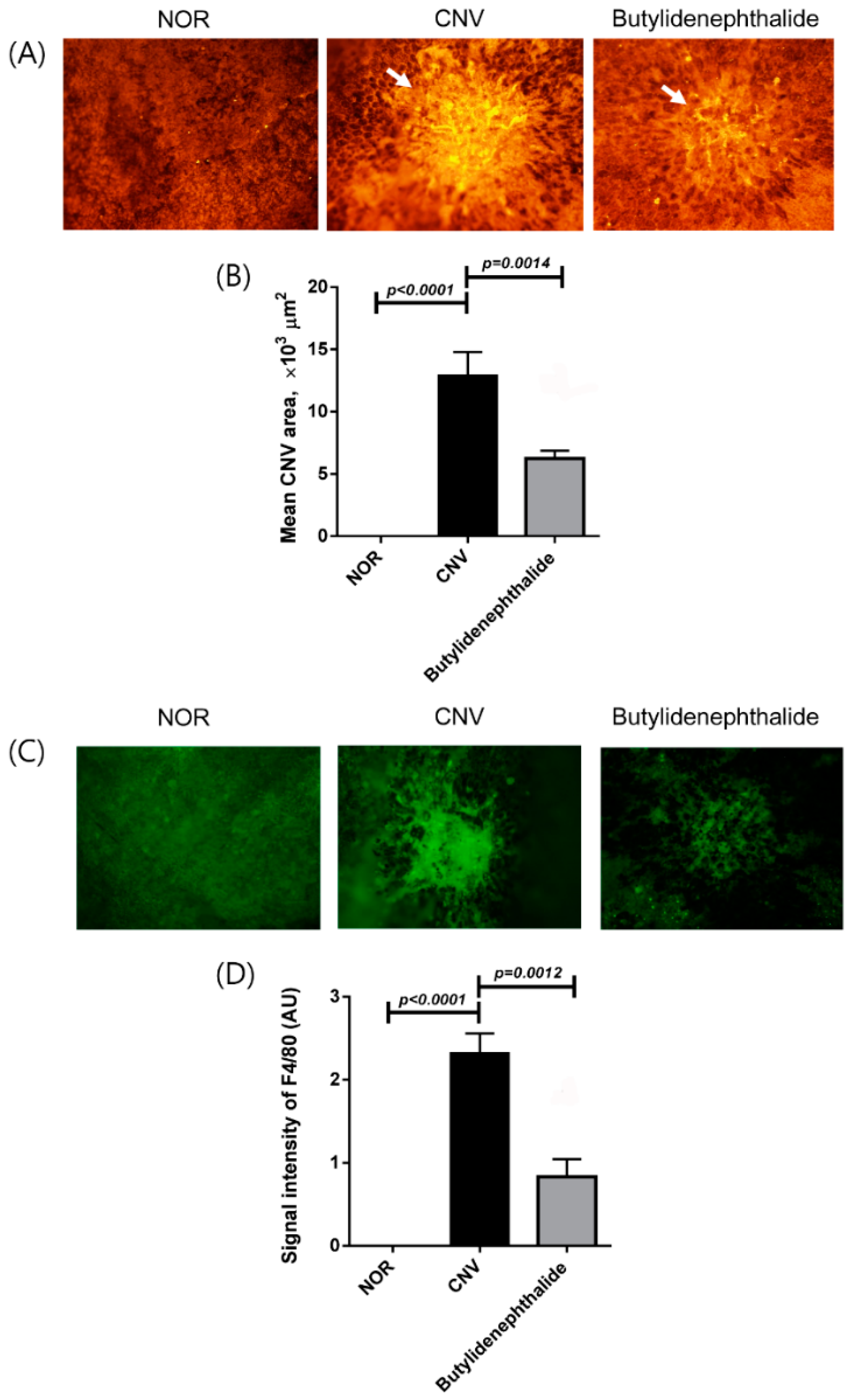
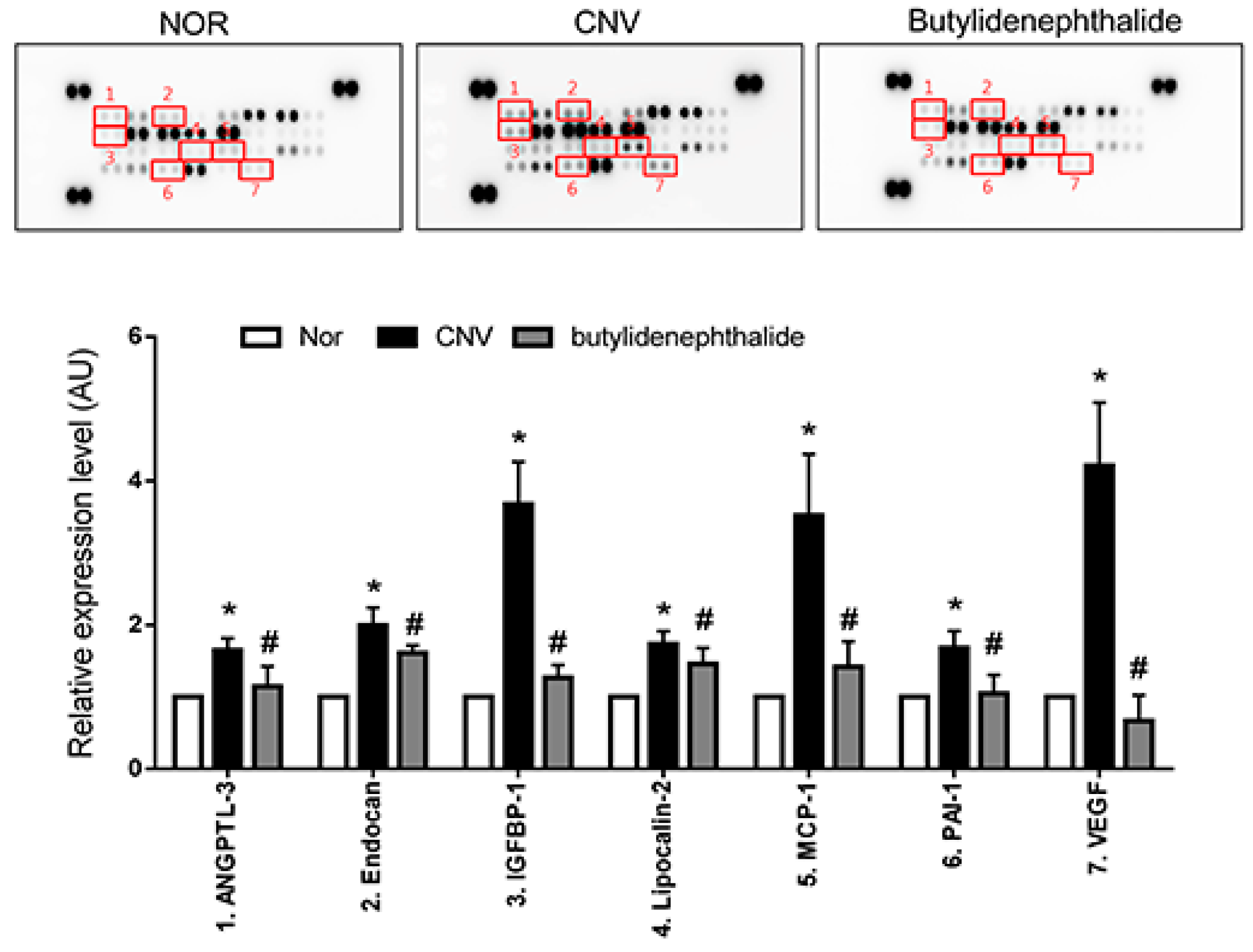
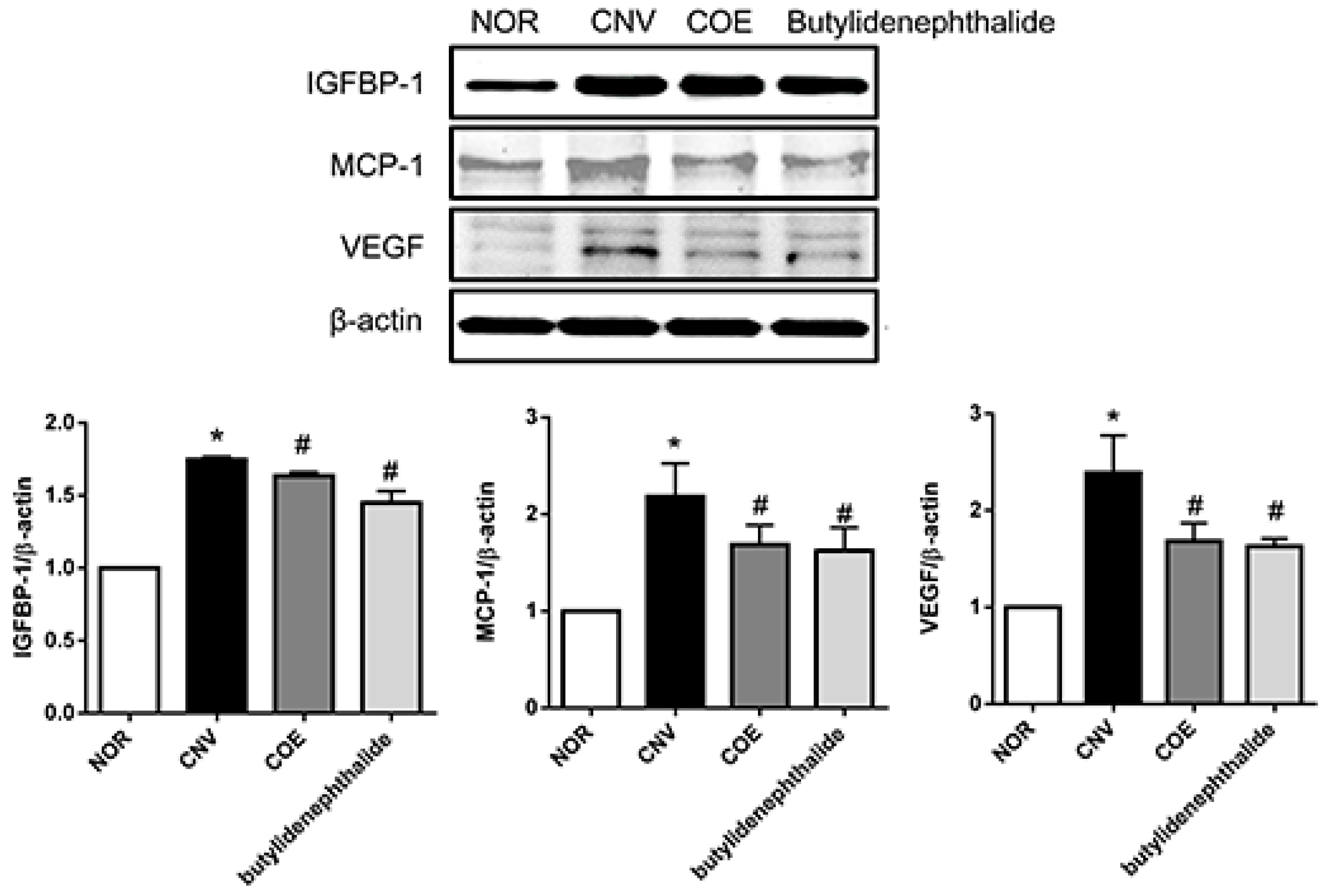
2.4. Butylidenephthalide also Regulates the Expression of Angiogenesis-Associated Factors, Similar to Those Seen in the COE-Treated Rats
3. Discussion
4. Experimental Section
4.1. Preparation of C. officinale Extract
4.2. Animals and Experimental Design
4.3. Administration of COE and Butylidenephthalide
4.4. Preparation of Choroidal Flat Mounts and Lectin Staining
4.5. Angiogenesis-Related Protein Array
4.6. Immunostaining for Infiltrating Macrophages
4.7. Western Blot Analysis
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jager, R.D.; Mieler, W.F.; Miller, J.W. Age-related macular degeneration. N. Engl. J. Med. 2008, 358, 2606–2617. [Google Scholar] [CrossRef] [PubMed]
- Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch. Ophthalmol. 2004, 122, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Aiello, L.P. Vascular endothelial growth factor and the eye: biochemical mechanisms of action and implications for novel therapies. Ophthalmic Res. 1997, 29, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Muranaka, K.; Yanagi, Y.; Tamaki, Y.; Takahashi, H.; Usui, T.; Ohashi, K.; Matsuoka, H.; Senda, T. Suppression of laser-induced choroidal neovascularization by oral administration of SA3443 in mice. FEBS Lett. 2005, 579, 6084–6088. [Google Scholar] [CrossRef] [PubMed]
- Eyetech Study Group. Anti-vascular endothelial growth factor therapy for subfoveal choroidal neovascularization secondary to age-related macular degeneration: phase II study results. Ophthalmology 2003, 110, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Diago, T.; McCannel, C.A.; Bakri, S.J.; Pulido, J.S.; Edwards, A.O.; Pach, J.M. Infectious endophthalmitis after intravitreal injection of antiangiogenic agents. Retina 2009, 29, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Fintak, D.R.; Shah, G.K.; Blinder, K.J.; Regillo, C.D.; Pollack, J.; Heier, J.S.; Hollands, H.; Sharma, S. Incidence of endophthalmitis related to intravitreal injection of bevacizumab and ranibizumab. Retina 2008, 28, 1395–1399. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Saishin, Y.; Saishin, Y.; Mori, K.; Ando, A.; Yamamoto, S.; Oshima, Y.; Nambu, H.; Melia, M.B.; Bingaman, D.P.; et al. Topical nepafenac inhibits ocular neovascularization. Investig. Ophthalmol. Vis. Sci. 2003, 44, 409–415. [Google Scholar] [CrossRef]
- Kim, J.; Kim, C.S.; Jo, K.; Cho, Y.S.; Kim, H.G.; Lee, G.H.; Lee, Y.M.; Sohn, E.; Kim, J.S. HL-217, a new topical anti-angiogenic agent, inhibits retinal vascular leakage and pathogenic subretinal neovascularization in Vldlr(−)/(−) mice. Biochem. Biophys. Res. Commun. 2015, 456, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Higashi, K. The therapeutic effect of Unsei-in on facial redness (inflammatory congestion) in atopic dermatitis. Jpn. J. Oriental. Med. 1996, 46, 753–760. [Google Scholar]
- Wang, J.D.; Narui, T.; Kurata, H.; Takeuchi, K.; Hashimoto, T.; Okuyama, T. Hematological studies on naturally occurring substances. II. Effects of animal crude drugs on blood coagulation and fibrinolysis systems. Chem. Pharm. Bull. 1989, 37, 2236–2238. [Google Scholar] [CrossRef] [PubMed]
- Haranaka, K.; Satomi, N.; Sakurai, A.; Haranaka, R.; Okada, N.; Kobayashi, M. Antitumor activities and tumor necrosis factor producibility of traditional Chinese medicines and crude drugs. Cancer Immunol. Immunother. 1985, 20, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kwak, D.H.; Kim, J.K.; Kim, J.Y.; Jeong, H.Y.; Keum, K.S.; Han, S.H.; Rho, Y.I.; Woo, W.H.; Jung, K.Y.; Choi, B.K.; et al. Anti-angiogenic activities of Cnidium officinale Makino and Tabanus bovinus. J. Ethnopharmacol. 2002, 81, 373–379. [Google Scholar] [CrossRef]
- Kwon, J.H.; Ahn, Y.J. Acaricidal activity of butylidenephthalide identified in Cnidium officinale rhizome against dermatophagoides farinae and dermatophagoides pteronyssinus (Acari: Pyroglyphidae). J. Agric. Food Chem. 2002, 50, 4479–4483. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.C.; Cindrova-Davies, T.; Belleri, M.; Morbidelli, L.; Miller, N.; Cho, C.W.; Chan, K.; Wang, Y.T.; Luo, G.A.; Ziche, M.; et al. The natural compound n-butylidenephthalide derived from the volatile oil of Radix angelica sinensis inhibits angiogenesis in vitro and in vivo. Angiogenesis 2011, 14, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Hicklin, D.J.; Ellis, L.M. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 2005, 23, 1011–1027. [Google Scholar] [CrossRef] [PubMed]
- Hoeben, A.; Landuyt, B.; Highley, M.S.; Wildiers, H.; van Oosterom, A.T.; de Bruijn, E.A. Vascular endothelial growth factor and angiogenesis. Pharmacol. Rev. 2004, 56, 549–580. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Kamei, M.; Suzuki, M.; Matsumura, N.; Nishida, K.; Sakimoto, S.; Sakaguchi, H.; Nishida, K. Suppression and regression of choroidal neovascularization in mice by a novel CCR2 antagonist, INCB3344. PLoS ONE 2011, 6, e28933. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.; Amaral, J.; Becerra, S.P.; Fariss, R.N. A novel imaging technique for experimental choroidal neovascularization. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5163–5170. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A. Ocular neovascularization. J. Mol. Med. 2013, 91, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Ambati, J.; Anand, A.; Fernandez, S.; Sakurai, E.; Lynn, B.C.; Kuziel, W.A.; Rollins, B.J.; Ambati, B.K. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat. Med. 2003, 9, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Funatsu, H.; Yamashita, H.; Sakata, K.; Noma, H.; Mimura, T.; Suzuki, M.; Eguchi, S.; Hori, S. Vitreous levels of vascular endothelial growth factor and intercellular adhesion molecule 1 are related to diabetic macular edema. Ophthalmology 2005, 112, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Elner, S.G.; Chen, X.; Field, M.G.; Petty, H.R.; Elner, V.M. MCP-1-activated monocytes induce apoptosis in human retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6026–6034. [Google Scholar] [CrossRef] [PubMed]
- Ehlken, C.; Grundel, B.; Michels, D.; Junker, B.; Stahl, A.; Schlunck, G.; Hansen, L.L.; Feltgen, N.; Martin, G.; Agostini, H.T.; et al. Increased expression of angiogenic and inflammatory proteins in the vitreous of patients with ischemic central retinal vein occlusion. PLoS ONE 2015, 10, e0126859. [Google Scholar] [CrossRef] [PubMed]
- Lofqvist, C.; Willett, K.L.; Aspegren, O.; Smith, A.C.; Aderman, C.M.; Connor, K.M.; Chen, J.; Hellstrom, A.; Smith, L.E. Quantification and localization of the IGF/insulin system expression in retinal blood vessels and neurons during oxygen-induced retinopathy in mice. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1831–1837. [Google Scholar] [CrossRef] [PubMed]
- Onishi, Y.; Yamaura, T.; Tauchi, K.; Sakamoto, T.; Tsukada, K.; Nunome, S.; Komatsu, Y.; Saiki, I. Expression of the anti-metastatic effect induced by Juzen-taiho-to is based on the content of Shimotsu-to constituents. Biol. Pharm. Bull. 1998, 21, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Kvanta, A.; Algvere, P.V.; Berglin, L.; Seregard, S. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1929–1934. [Google Scholar] [CrossRef]
- Fu, R.H.; Hran, H.J.; Chu, C.L.; Huang, C.M.; Liu, S.P.; Wang, Y.C.; Lin, Y.H.; Shyu, W.C.; Lin, S.Z. Lipopolysaccharide-stimulated activation of murine DC2.4 cells is attenuated by n-butylidenephthalide through suppression of the NF-kappaB pathway. Biotechnol. Lett. 2011, 33, 903–910. [Google Scholar] [CrossRef] [PubMed]
- R & D systems. Available online: http://www.rndsystems.com/index.aspx (accessed on 30 September 2015).
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.M.; Lee, Y.R.; Kim, J.S.; Kim, Y.H.; Kim, J. Cinidium officinale and its Bioactive Compound, Butylidenephthalide, Inhibit Laser-Induced Choroidal Neovascularization in a Rat Model. Molecules 2015, 20, 20699-20708. https://doi.org/10.3390/molecules201119728
Lee YM, Lee YR, Kim JS, Kim YH, Kim J. Cinidium officinale and its Bioactive Compound, Butylidenephthalide, Inhibit Laser-Induced Choroidal Neovascularization in a Rat Model. Molecules. 2015; 20(11):20699-20708. https://doi.org/10.3390/molecules201119728
Chicago/Turabian StyleLee, Yun Mi, Yu Ri Lee, Jin Sook Kim, Young Ho Kim, and Junghyun Kim. 2015. "Cinidium officinale and its Bioactive Compound, Butylidenephthalide, Inhibit Laser-Induced Choroidal Neovascularization in a Rat Model" Molecules 20, no. 11: 20699-20708. https://doi.org/10.3390/molecules201119728
APA StyleLee, Y. M., Lee, Y. R., Kim, J. S., Kim, Y. H., & Kim, J. (2015). Cinidium officinale and its Bioactive Compound, Butylidenephthalide, Inhibit Laser-Induced Choroidal Neovascularization in a Rat Model. Molecules, 20(11), 20699-20708. https://doi.org/10.3390/molecules201119728





