Membrane Disintegration Caused by the Steroid Saponin Digitonin Is Related to the Presence of Cholesterol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Digitonin Ruptures Red Blood Cells (RBCs)
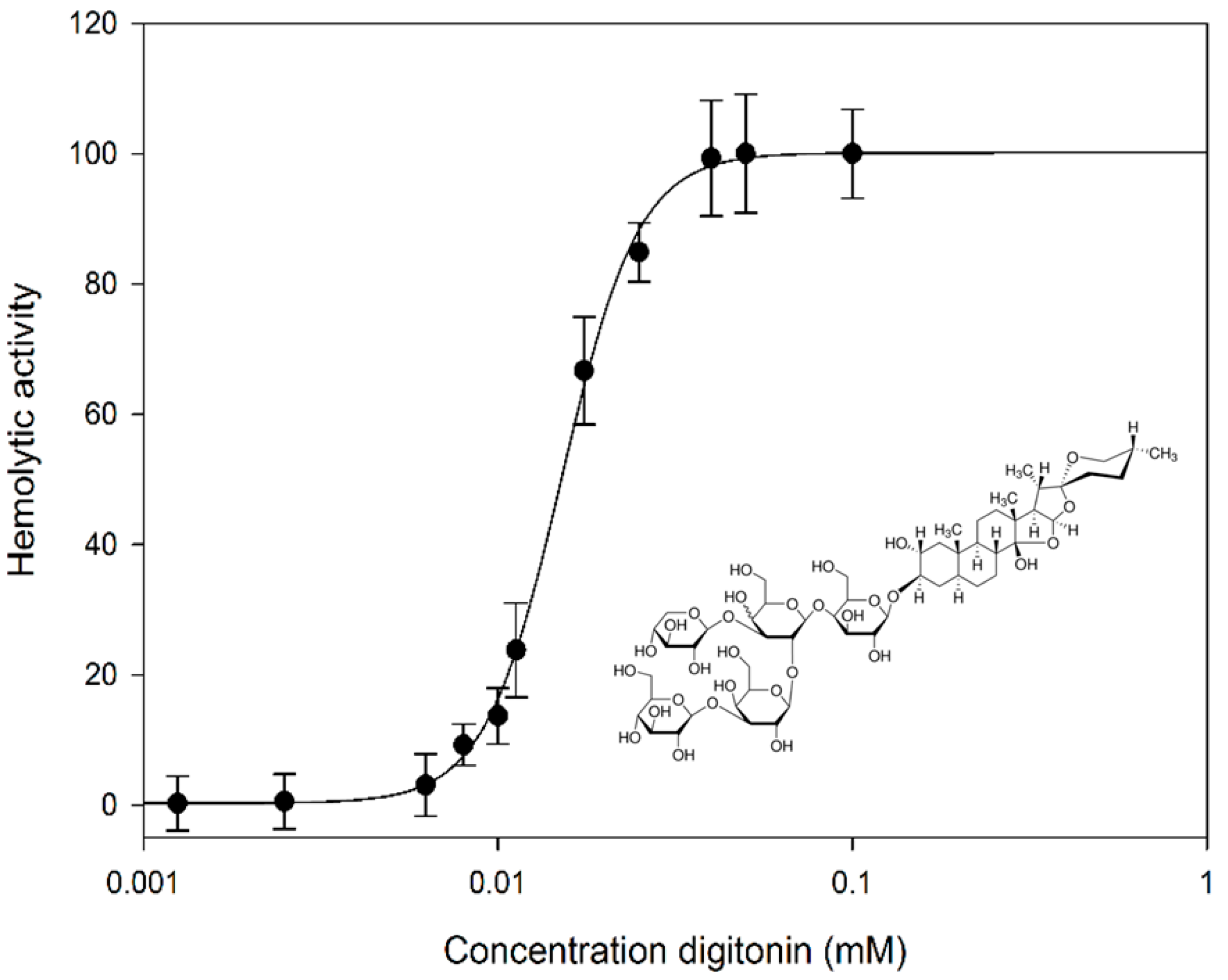
2.2. Digitonin Causes Calcein Leakage
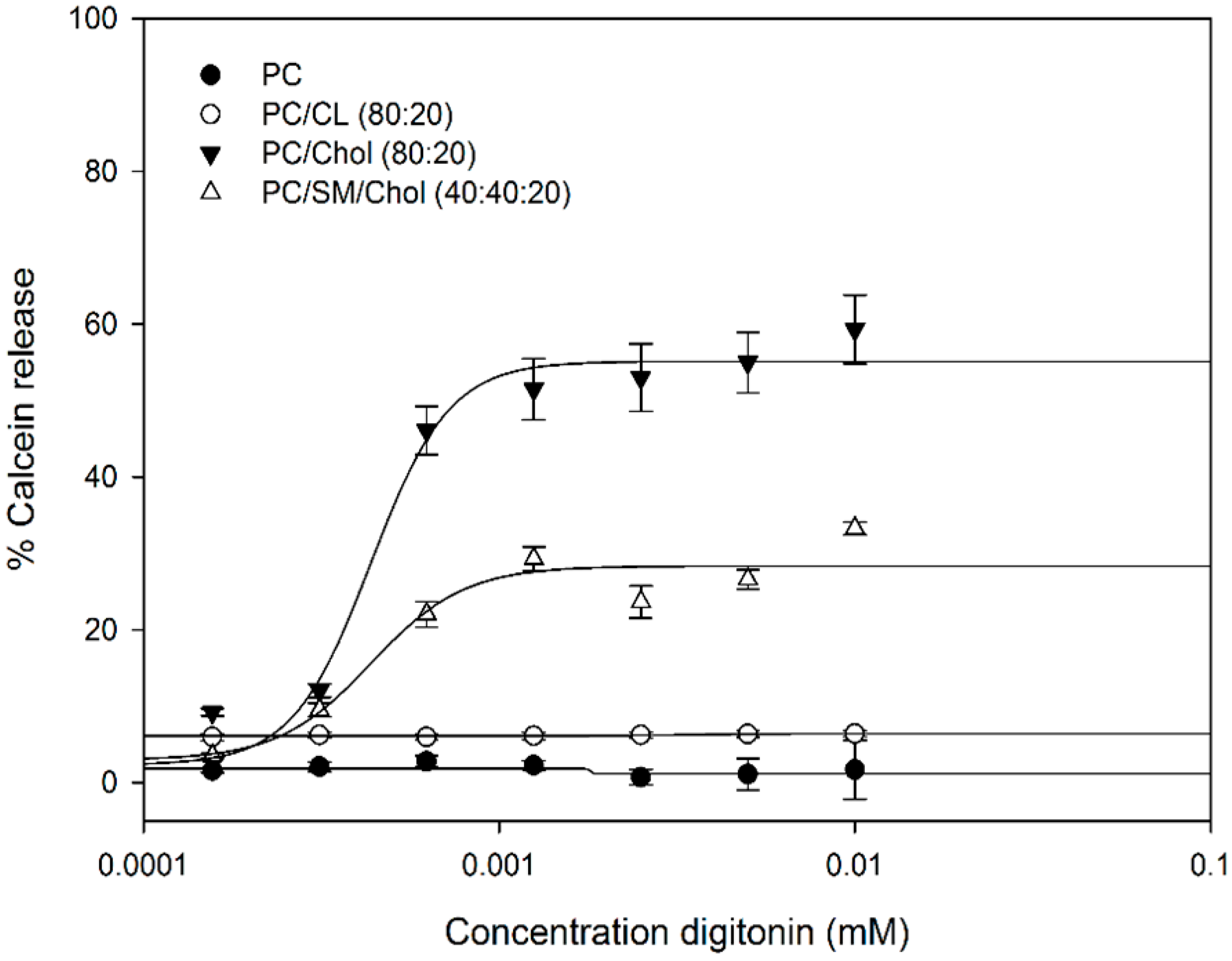
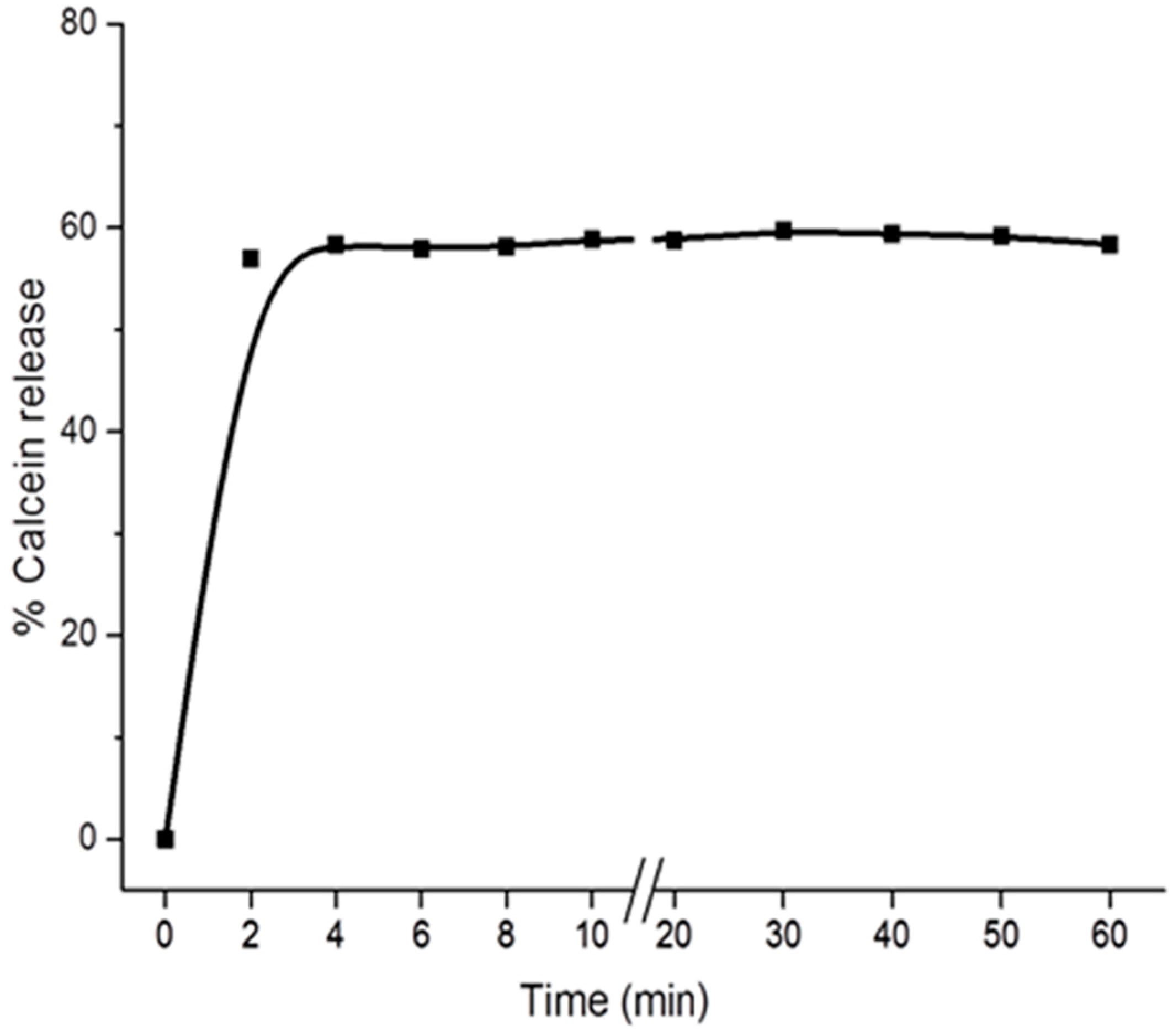
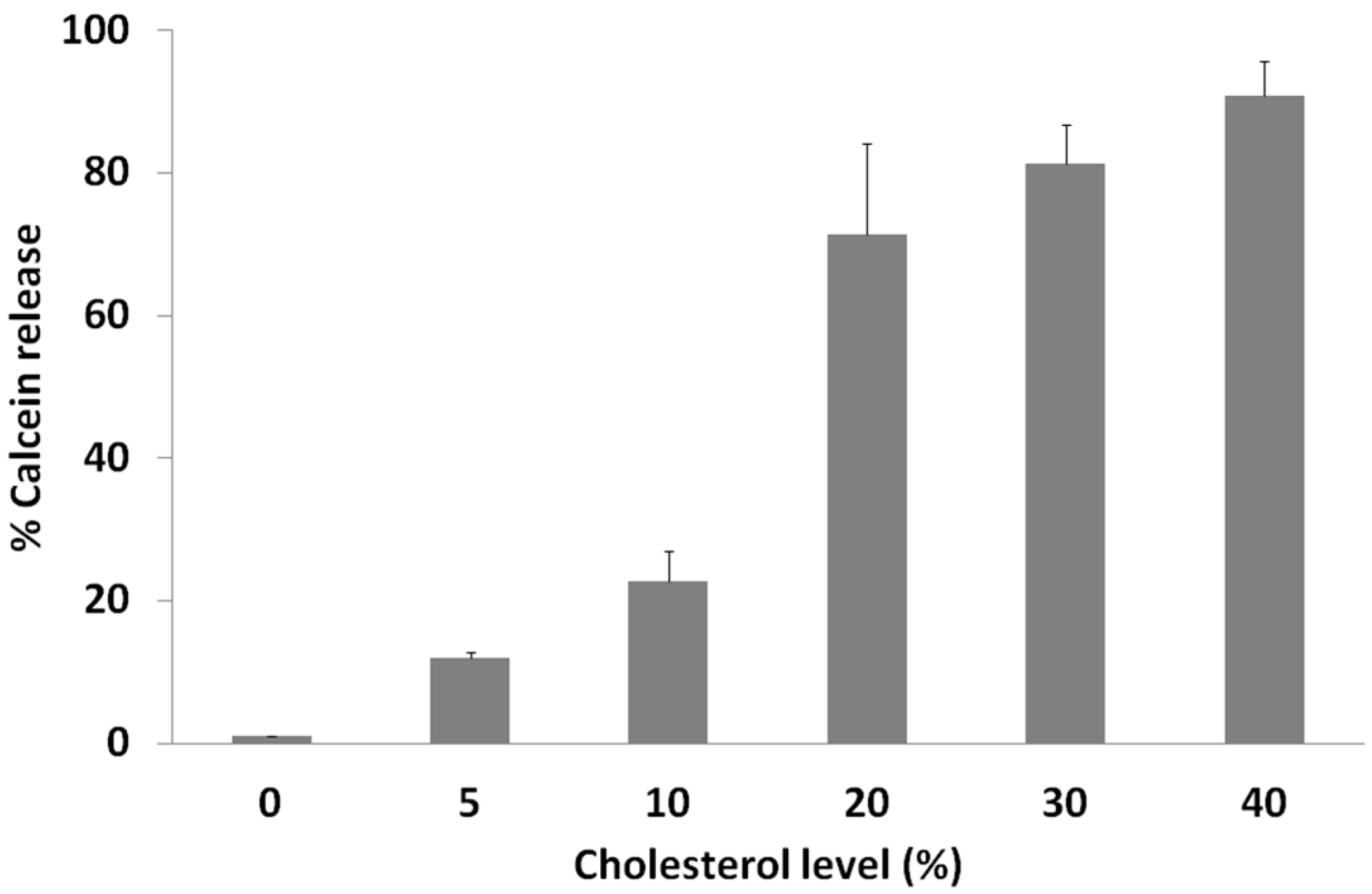
2.3. Calcein Release Depends on Membrane Cholesterol Concentration
2.4. Digitonin Affects Vesicle Size Only in the Presence of Cholesterol
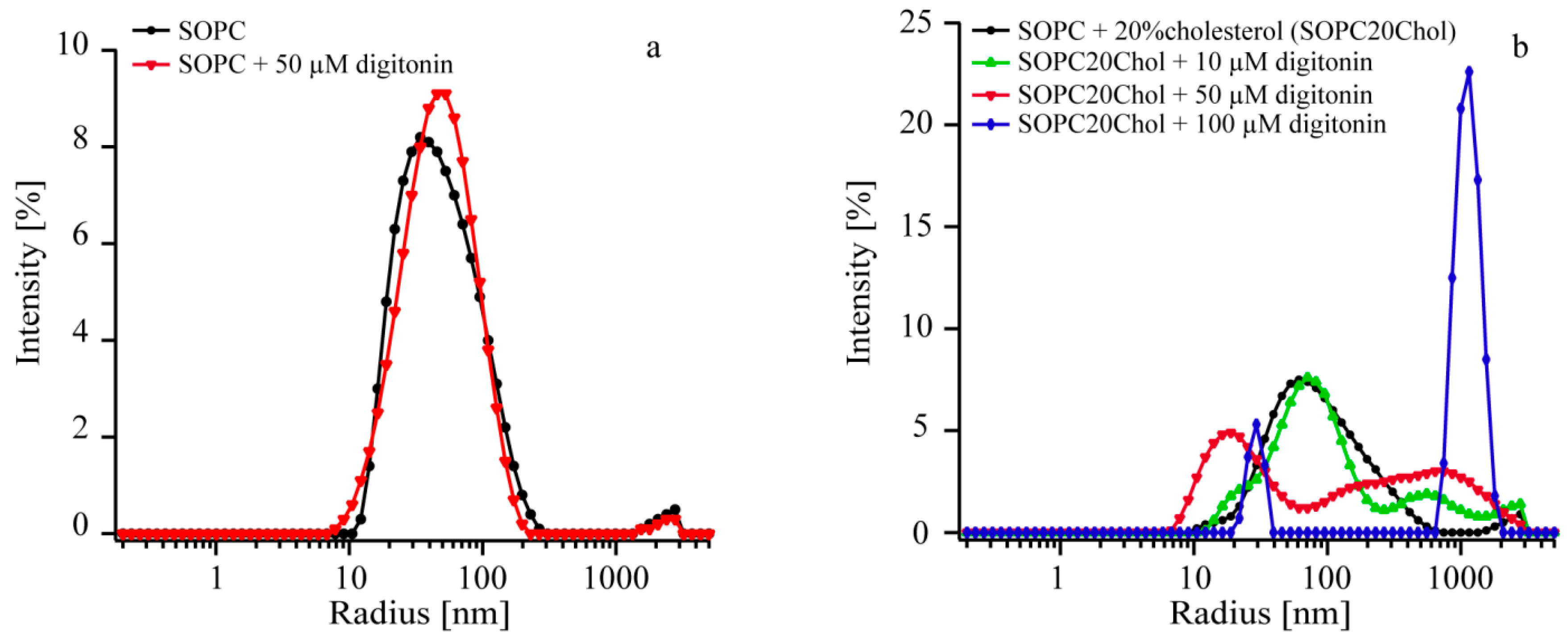
| Sample | Before Incubation | Digitonin Concentration (µM) | After Incubation | ||
|---|---|---|---|---|---|
| rmax (nm) | FWHM (nm) | rmax (nm) | FWHM (nm) | ||
| SOPC | 80 | 90 | 0 | 77 | 83 |
| SOPC + 50 | 32 | 78 | 50 | 46 | 74 |
| Sample | Before Incubation | Digitonin Concentration (µM) | After Incubation | ||||
|---|---|---|---|---|---|---|---|
| Peak 1 | Peak 1 | Peak 2 | |||||
| rmax (nm) | FWHM (nm) | rmax (nm) | FWHM (nm) | rmax (nm) | FWHM (nm) | ||
| SOPC20Chol | 77 | 93 | 0 | 63 | 65 | ND | ND |
| SOPC20Chol + 10 | 58 | 154 | 10 | 71 | 99 | 470 | 1566 |
| SOPC20Chol + 50 | 58 | 34 | 50 | 17 | 23 | 39 | 3038 |
| SOPC20Chol + 100 | 51 | 252 | 100 | 30 | 10 | 1116 | 588 |
2.5. Digitonin Affects GUV Membrane Permeability and Integrity in the Presence of Cholesterol
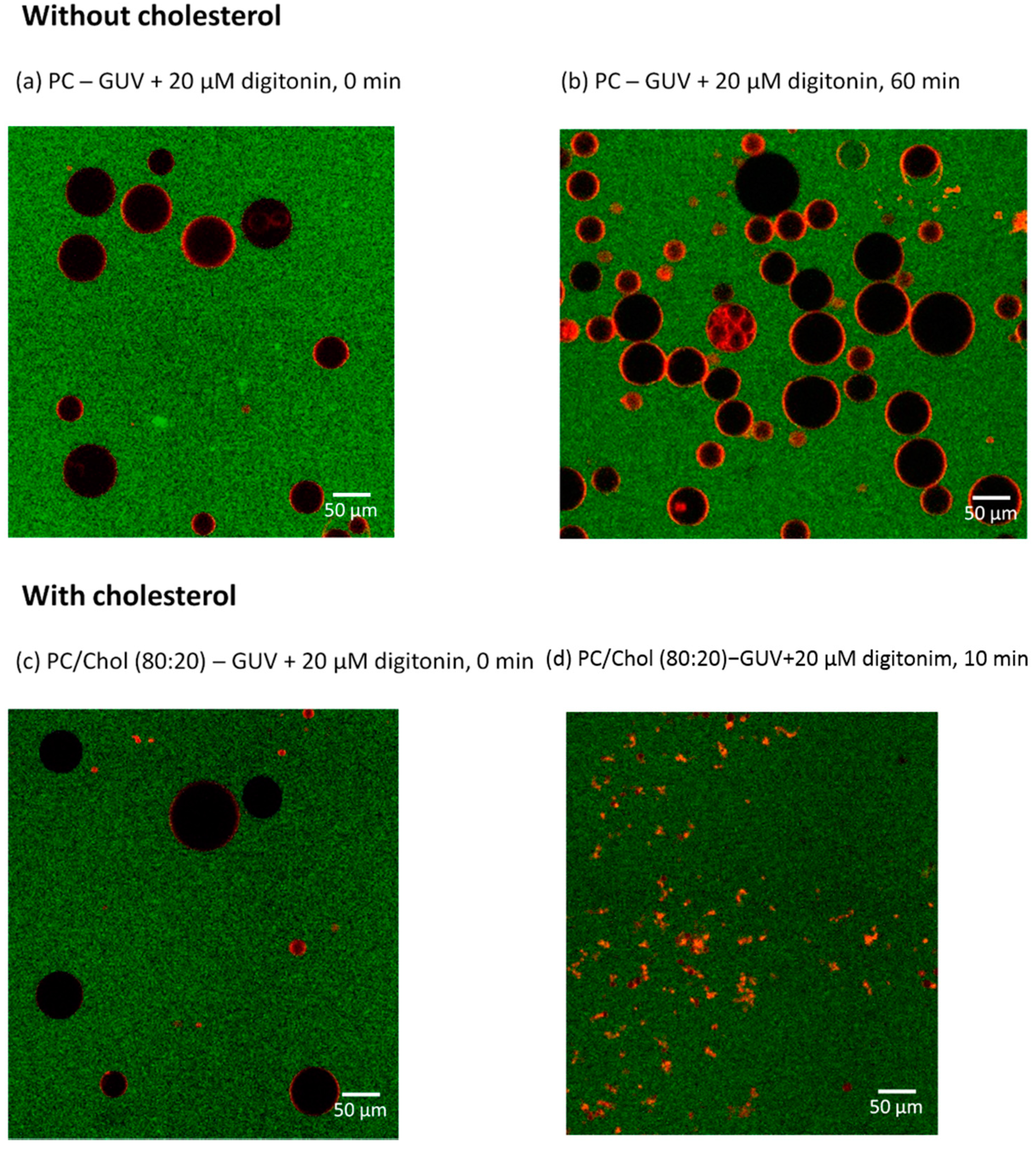
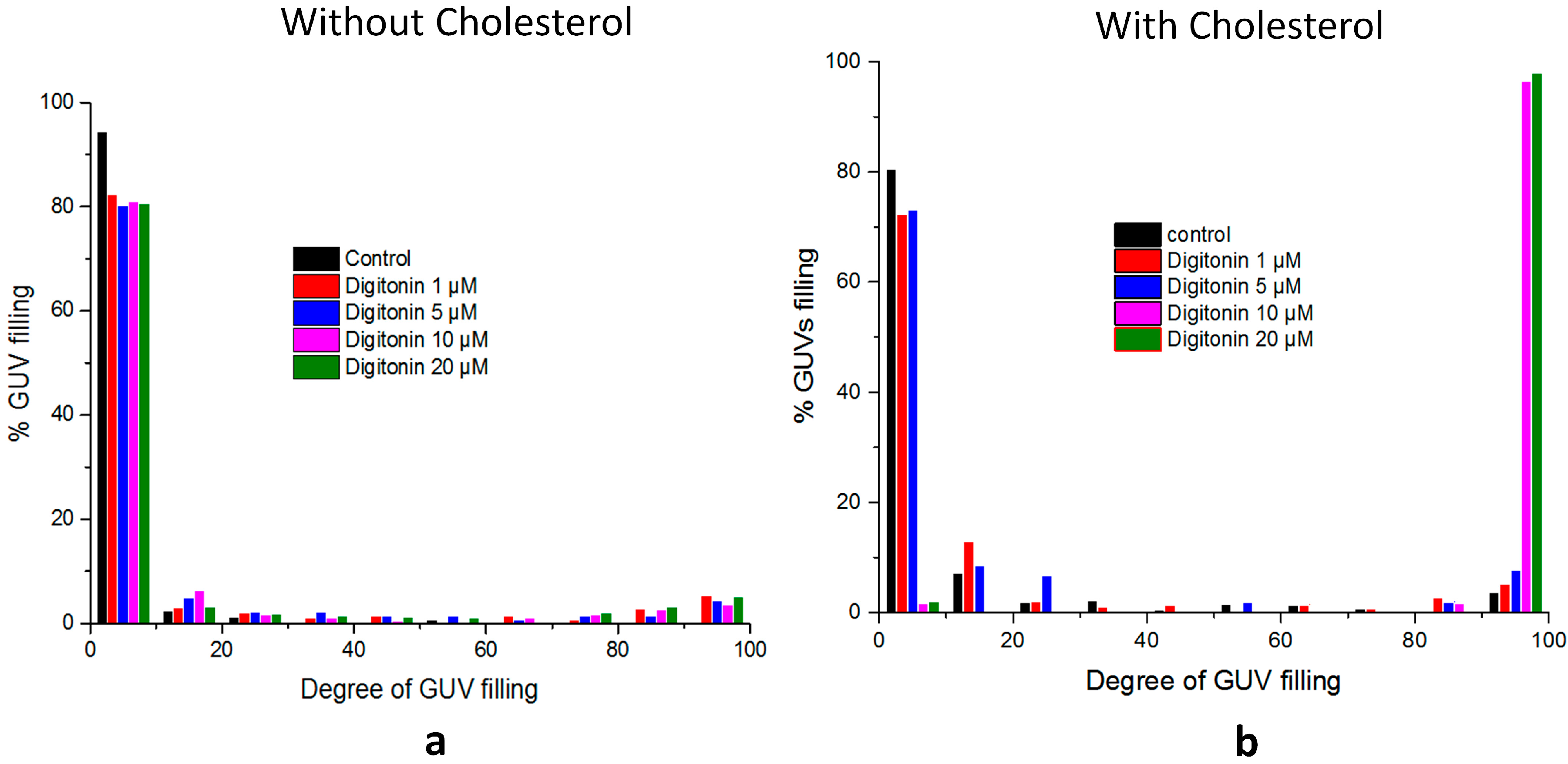
2.6. Visualizing the Disrupting Effect of Digitonin on Individual PC/Chol (80:20) GUVs
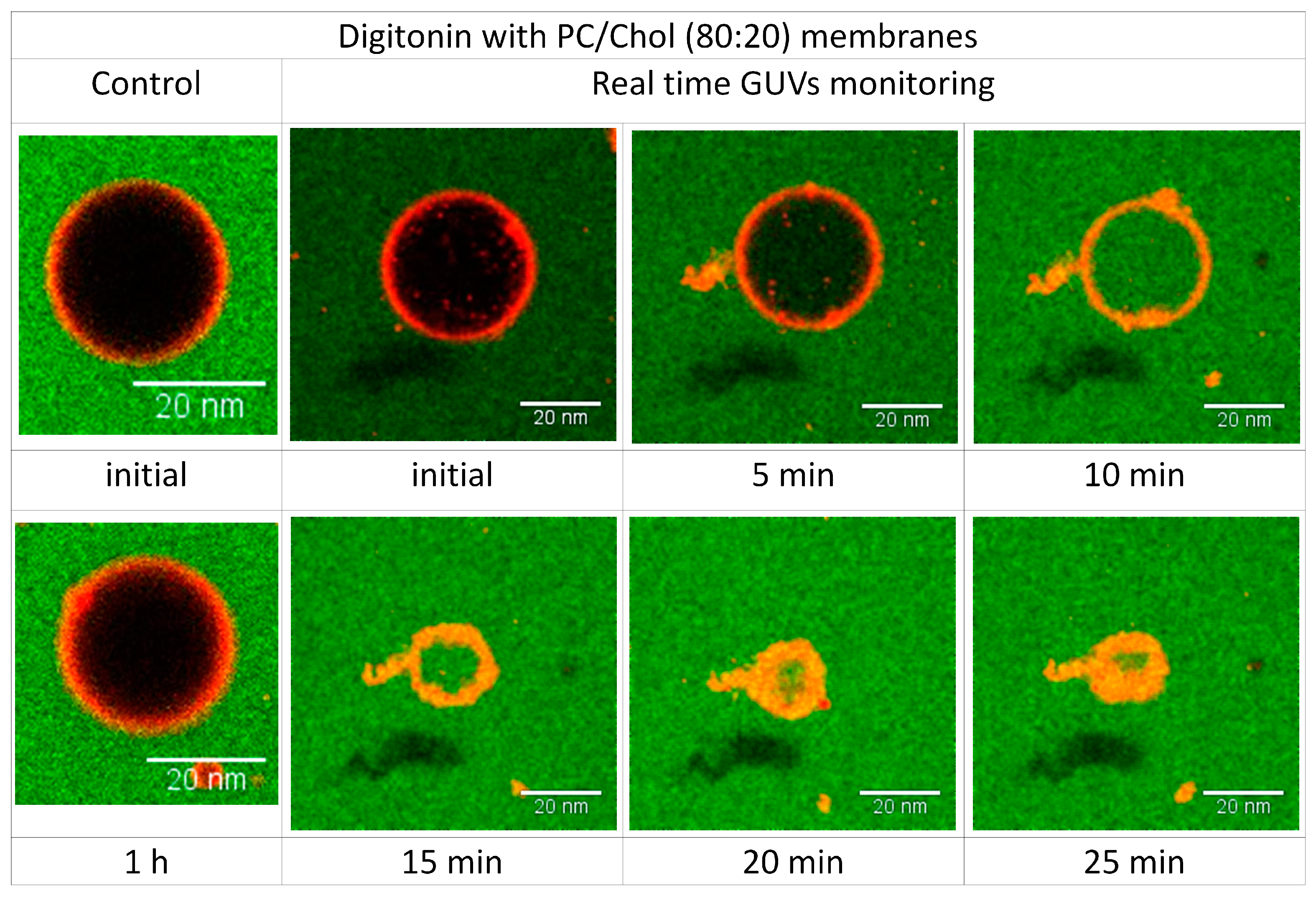
3. Experimental Section
3.1. Materials
3.2. Hemolytic Activity
3.3. Preparation of Lipid Vesicles and Entrapment of Calcein
3.4. Permeabilization of LUVs (Calcein Release Assay)
3.5. Preparation of Giant Unilamellar Vesicles (GUVs)
3.6. Membrane Permeability Assays
3.7. Confocal Microscopy (CM) and Fluorescence Correlation Spectroscopy (FCS)
3.8. GUV Image Analysis
3.9. Size Measurement by Dynamic Light Scattering (DLS)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Caulier, G.; Van Dyck, S.; Gerbaux, P.; Eeckhaut, I.; Flammang, P. Review of saponin diversity in sea cucumbers belonging to the family Holothuriidae. SPC Beche-de-mer Inf. Bull. 2011, 31, 48–54. [Google Scholar]
- Andersson, L.; Bohlin, L.; Iorizzi, M.; Riccio, R.; Minale, L.; Moreno-López, W. Biological activity of saponins and saponin-like compounds from starfish and brittle-stars. Toxicon 1989, 27, 179–188. [Google Scholar] [CrossRef]
- Hostettmann, K.; Marston, A. Saponins; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Sparg, S.G.; Light, M.E.; van Staden, J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Augustin, J.M.; Kuzina, V.; Andersen, S.B.; Bak, S. Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochemistry 2011, 72, 435–457. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, H.; Bachran, D.; Panjideh, H.; Schellmann, N.; Weng, A.; Melzig, M.; Sutherland, M.; Bachran, C. Saponins as tool for improved targeted tumor therapies. Curr. Drug Targets 2009, 10, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, J.K.; Munter, S.; Wink, M.; Frischknecht, F. Synergistic and additive effects of epigallocatechin gallate and digitonin on Plasmodium sporozoite survival and motility. PLoS ONE 2010, 5, e8682. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, F.; Wink, M. Synergistic interactions of saponins and monoterpenes in HeLa cells, COS7 cells and in erythrocytes. Phytomedicine 2011, 18, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M.; Nojima, S.; Akiyama, T.; Sankawa, U.; Inoue, K. Interaction of digitonin and its analogs with membrane cholesterol. J. Biochem. 1984, 96, 1231–1239. [Google Scholar] [PubMed]
- Jekunen, A.P.; Shalinsky, D.R.; Hom, D.K.; Albright, K.D.; Heath, D.; Howell, S.B. Modulation of cisplatin cytotoxicity by permeabilization of the plasma membrane by digitonin in vitro. Biochem. Pharmacol. 1993, 45, 2079–2085. [Google Scholar] [CrossRef]
- Gögelein, H.; Hüby, A. Interaction of saponin and digitonin with black lipid membranes and lipid monolayers. BBA-Biomembranes 1984, 773, 32–38. [Google Scholar] [CrossRef]
- Sudji, I.R.; Wink, M. Digitonin synergistically enhances the cytotoxicity of anticancer drugs in several cancer cell lines. 2015; submitted. [Google Scholar]
- Nazari, M.; Kurdi, M.; Heerklotz, H. Classifying surfactants with respect to their effect on lipid membrane order. Biophys. J. 2012, 102, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Eid, S.Y.; El-Readi, M.Z.; Wink, M. Digitonin synergistically enhances the cytotoxicity of plant secondary metabolites in cancer cells. Phytomedicine 2012, 19, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, N.; Makky, A.; Sudji, I.R.; Wink, M.; Tanaka, M. Mechanistic investigation of interactions between steroidal saponin digitonin and cell membrane models. J. Phys. Chem. B 2014, 50, 14632–14639. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Inoue, K.; Nojima, S.; Sankawa, U.; Shoji, J.; Kawasaki, T.; Shibata, S. Interaction of saponins with red blood cells as well as with the phosphatidylcholine liposomal membranes. J. Pharmacobio-Dynam. 1979, 2, 374–382. [Google Scholar] [CrossRef]
- Woldemichael, G.M.; Wink, M. Identification and biological activities of triterpenoid saponins from Chenopodium quinoa. J. Agric. Food Chem. 2001, 49, 2327–2332. [Google Scholar] [CrossRef] [PubMed]
- Böttger, S.; Melzig, M.F. The influence of saponins on cell membrane cholesterol. Bioorg. Med. Chem. 2013, 21, 7118–7124. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Konoki, K.; Tachibana, K. Cholesterol-independent membrane disruption caused by triterpenoid saponins. BBA-Lipids Lipid Met. 1996, 1299, 252–258. [Google Scholar] [CrossRef]
- Yu, B.S.; Chung, H.H.; Kim, A. Effects of saponins on the osmotic behavior of multilamellar liposomes. Arch. Pharm. Res. 1984, 7, 17–22. [Google Scholar] [CrossRef]
- Li, X.X.; Davis, B.; Haridas, V.; Gutterman, J.U.; Colombini, M. Proapoptotic triterpene electrophiles (avicins) form channels in membranes: Cholesterol dependence. Biophys. J. 2005, 88, 2577–2584. [Google Scholar] [CrossRef] [PubMed]
- Seeman, P.; Cheng, D.; Iles, G. Structure of membrane holes in osmotic and saponin hemolysis. J. Cell Biol. 1973, 56, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Shany, S.; Bernheimer, A.W.; Grushoff, P.S.; Kim, K.S. Evidence for membrane cholesterol as the common binding site for cereolysin, streptolysin O and saponin. Mol. Cell. Biochem. 1974, 3, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Armah, C.; Mackie, A.; Roy, C.; Price, K.; Osbourn, A.; Bowyer, P.; Ladha, S. The membrane-permeabilizing effect of avenacin A-1 involves the reorganization of bilayer cholesterol. Biophys. J. 1999, 76, 281–290. [Google Scholar] [CrossRef]
- Ohvo-Rekilä, H.; Ramstedt, B.; Leppimäki, P.; Slotte, J.P. Cholesterol interactions with phospholipids in membranes. Progr. Lipid Res. 2002, 41, 66–97. [Google Scholar] [CrossRef]
- Yeagle, P.L. The roles of cholesterol in the biology of cells. In The Structure of Biological Membranes, 3rd ed.; Yeagle, P.L., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011; pp. 119–132. [Google Scholar]
- Mitra, S.; Dungan, S.R. Cholesterol solubilization in aqueous micellar solutions of quillaja saponin, bile salts, or nonionic surfactants. J. Agric. Food Chem. 2001, 49, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Takagi, S.; Sankawa, U.; Inari, S.; Saito, H. Saponin-cholesterol interaction in the multibilayers of egg yolk lecithin as studied by deuterium nuclear magnetic resonance: Digitonin and its analogs. Biochemistry 1980, 19, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.S.; Choi, H.O. The effects of digitonin and glycyrrhizin on liposomes. Arch. Pharma. Res. 1986, 9, 119–125. [Google Scholar] [CrossRef]
- Meyer, H.W.; Richter, W. Freeze-fracture studies on lipids and membranes. Micron 2001, 32, 615–644. [Google Scholar] [CrossRef]
- Meyer, H.W.; Winkelmann, H.; Richter, W. Digitonin induced alterations of the erythrocyte membrane as visible by freeze-fracturing. Exp. Pathol. 1978, 16, 60–68. [Google Scholar] [CrossRef]
- Tanaka, T.; Kaneda, Y.; Li, T.S.; Matsuoka, T.; Zempo, N.; Esato, K. Digitonin enhances the antitumor effect of cisplatin during isolated lung perfusion. Ann. Thorac. Surg. 2001, 72, 1173–1178. [Google Scholar] [CrossRef]
- Walde, P.; Cosentino, K.; Engel, H.; Stano, P. Giant vesicles: Preparations and applications. ChemBioChem 2010, 11, 848–865. [Google Scholar] [CrossRef] [PubMed]
- Tamba, Y.; Ohba, S.; Kubota, M.; Yoshioka, H.; Yoshioka, H.; Yamazaki, M. Single GUV method reveals interaction of tea catechin (−)-epigallocatechin gallate with lipid membranes. Biophys. J. 2007, 92, 3178–3194. [Google Scholar] [CrossRef] [PubMed]
- Tamba, Y.; Yamazaki, M. Single giant unilamellar vesicle method reveals effect of antimicrobial peptide magainin 2 on membrane permeability. Biochemistry 2005, 44, 15823–15833. [Google Scholar] [CrossRef] [PubMed]
- Pott, T.; Bouvrais, H.; Méléard, P. Giant unilamellar vesicle formation under physiologically relevant conditions. Chem. Phys. Lipids 2008, 154, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Bleicken, S.; Wagner, C.; García-Sáez, A.J. Mechanistic differences in the membrane activity of Bax and Bcl-xL correlate with their opposing roles in apoptosis. Biophys. J. 2013, 104, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Weber, P.A.; Chang, H.C.; Spaeth, K.E.; Nitsche, J.M.; Nicholson, B.J. The permeability of gap junction channels to probes of different size is dependent on connexin composition and permeant-pore affinities. Biophys. J. 2004, 87, 958–973. [Google Scholar] [CrossRef] [PubMed]
- Geddes, C.D.; Lakowicz, J.R. Advanced Concepts in Fluorescence Sensing: Part A: Small Molecule Sensing; Springer: Heidelberg, Germany, 2007; Volume 9. [Google Scholar]
- Menger, F.M.; Keiper, J.S. Digitonin as a chemical trigger for the selective transformation of giant vesicles. Angew. Chem. Int. Edit. 1998, 37, 3433–3435. [Google Scholar] [CrossRef]
- García-Sáez, A.J.; Coraiola, M.; Serra, M.D.; Mingarro, I.; Müller, P.; Salgado, J. Peptides corresponding to helices 5 and 6 of Bax can independently form large lipid pores. FEBS J. 2006, 273, 971–981. [Google Scholar] [CrossRef] [PubMed]
- García-Sáez, A.J.; Ries, J.; Orzáez, M.; Pérez-Payà, E.; Schwille, P. Membrane promotes tBID interaction with BCLXL. Nat. Struct. Mol. Biol. 2009, 16, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Apellániz, B.; Nieva, J.L.; Schwille, P.; García-Sáez, A.J. All-or-none versus graded: Single-vesicle analysis reveals lipid composition effects on membrane permeabilization. Biophys. J. 2010, 99, 3619–3628. [Google Scholar] [CrossRef] [PubMed]
- Hermann, E.; Bleicken, S.; Subburaj, Y.; García-Sáez, A.J. Automated analysis of giant unilamellar vesicles using circular Hough transformation. Bioinformatics 2014, 30, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sudji, I.R.; Subburaj, Y.; Frenkel, N.; García-Sáez, A.J.; Wink, M. Membrane Disintegration Caused by the Steroid Saponin Digitonin Is Related to the Presence of Cholesterol. Molecules 2015, 20, 20146-20160. https://doi.org/10.3390/molecules201119682
Sudji IR, Subburaj Y, Frenkel N, García-Sáez AJ, Wink M. Membrane Disintegration Caused by the Steroid Saponin Digitonin Is Related to the Presence of Cholesterol. Molecules. 2015; 20(11):20146-20160. https://doi.org/10.3390/molecules201119682
Chicago/Turabian StyleSudji, Ikhwan Resmala, Yamunadevi Subburaj, Nataliya Frenkel, Ana J. García-Sáez, and Michael Wink. 2015. "Membrane Disintegration Caused by the Steroid Saponin Digitonin Is Related to the Presence of Cholesterol" Molecules 20, no. 11: 20146-20160. https://doi.org/10.3390/molecules201119682
APA StyleSudji, I. R., Subburaj, Y., Frenkel, N., García-Sáez, A. J., & Wink, M. (2015). Membrane Disintegration Caused by the Steroid Saponin Digitonin Is Related to the Presence of Cholesterol. Molecules, 20(11), 20146-20160. https://doi.org/10.3390/molecules201119682







