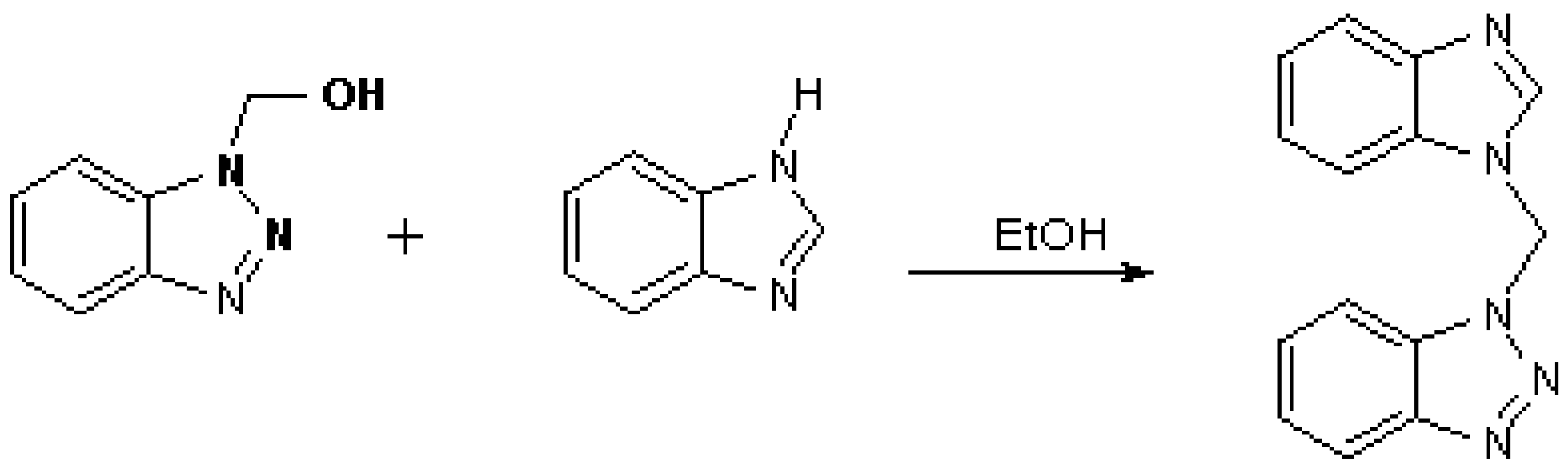
1-Benzimidazolyl-1-benztriazolylmethane
Supplementary materials
Supplementary File 1Supplementary File 2Acknowledgements
References and Notes
- Julia, S.; Sala, P.; del Mazo, J.; Sancho, M.; Ochoa, C.; Elguero, J.; Fayet, J.P.; Vertut, M.C. N-Polyazo- lylmethanes. 1. Synthesis and NMR study of N,N-diazolylmethanes. J. Heterocycl. Chem. 1982, 19, 1141. [Google Scholar] [CrossRef]
- Fries, K.; Guterbock, H.; Kuhn, H. Untersuchungen in der Reihe des Azimidobenzols und des N-Methyl-Azimidobenzols. Ann. 1934, 511, 213. [Google Scholar] [CrossRef]
- Sample Availability: Available from the author, 2g.
© 1997 MDPI. All rights reserved
Share and Cite
Viktor, M.; Rudolf, K. 1-Benzimidazolyl-1-benztriazolylmethane. Molecules 1997, 2, M12. https://doi.org/10.3390/M12
Viktor M, Rudolf K. 1-Benzimidazolyl-1-benztriazolylmethane. Molecules. 1997; 2(5):M12. https://doi.org/10.3390/M12
Chicago/Turabian StyleViktor, Milata, and Kada Rudolf. 1997. "1-Benzimidazolyl-1-benztriazolylmethane" Molecules 2, no. 5: M12. https://doi.org/10.3390/M12
APA StyleViktor, M., & Rudolf, K. (1997). 1-Benzimidazolyl-1-benztriazolylmethane. Molecules, 2(5), M12. https://doi.org/10.3390/M12



