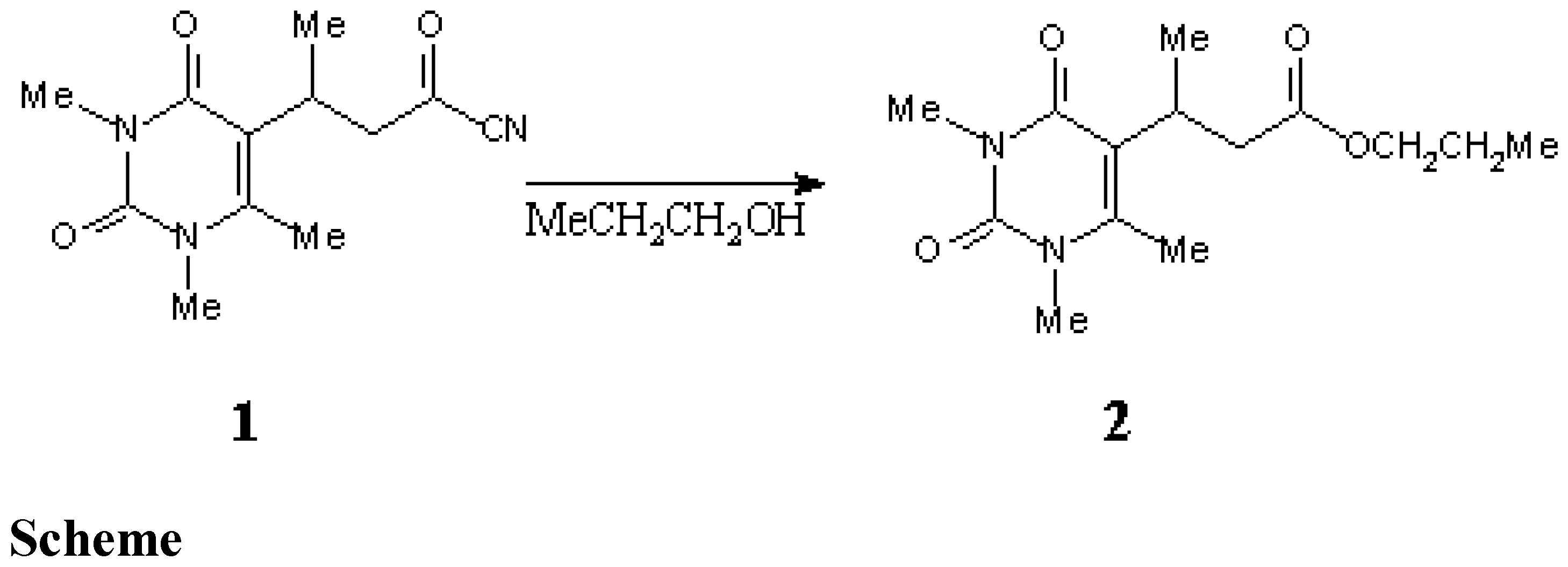
Propyl 3-(1,2,3,4-Tetrahydro-1,3,6-trimethyl-2,4-dioxopyrimidin-5-yl)butanoate
Supplementary materials
Supplementary File 1Supplementary File 2Acknowledgment:
References
- Zhuo, J.-C.; Wyler, H. Helv. Chim. Acta 1993, 76, 1916. [CrossRef]
- Sample Availability: Available from MDPI, 0.3g, MDPI 10060.
© 1997 MDPI. All rights reserved
Share and Cite
Zhuo, J.-C.; Wyler, H. Propyl 3-(1,2,3,4-Tetrahydro-1,3,6-trimethyl-2,4-dioxopyrimidin-5-yl)butanoate. Molecules 1997, 2, M10. https://doi.org/10.3390/M10
Zhuo J-C, Wyler H. Propyl 3-(1,2,3,4-Tetrahydro-1,3,6-trimethyl-2,4-dioxopyrimidin-5-yl)butanoate. Molecules. 1997; 2(4):M10. https://doi.org/10.3390/M10
Chicago/Turabian StyleZhuo, Jin-Cong, and Hugo Wyler. 1997. "Propyl 3-(1,2,3,4-Tetrahydro-1,3,6-trimethyl-2,4-dioxopyrimidin-5-yl)butanoate" Molecules 2, no. 4: M10. https://doi.org/10.3390/M10
APA StyleZhuo, J.-C., & Wyler, H. (1997). Propyl 3-(1,2,3,4-Tetrahydro-1,3,6-trimethyl-2,4-dioxopyrimidin-5-yl)butanoate. Molecules, 2(4), M10. https://doi.org/10.3390/M10



