4-Dodecyl-6-methyl-2-morpholone
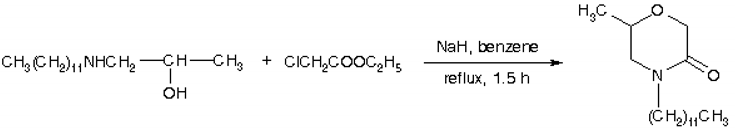
Supplementary materials
Supplementary File 1Supplementary File 2Acknowledgments
References and Notes
- Clarke, F. H. J. Org. Chem. 1962, 27, 3251.
- Sainsbury, M. Coffey, S., Ed.; Rodd's Chemistry of Carbon Compounds; Volume IV, Part H; Elsevier: Amsterdam, 1978; p. 427. [Google Scholar]
- Sample Availability: Available from the authors and MDPI, MDPI Reg. No.13732.
© 1997 MDPI. All rights reserved.
Share and Cite
Steiner, B.S.; Gajdos, J.; Koos, M.; Hassel, B. 4-Dodecyl-6-methyl-2-morpholone. Molecules 1997, 2, M42. https://doi.org/10.3390/M42
Steiner BS, Gajdos J, Koos M, Hassel B. 4-Dodecyl-6-methyl-2-morpholone. Molecules. 1997; 2(11):M42. https://doi.org/10.3390/M42
Chicago/Turabian StyleSteiner, Bohumil Steiner, Jan Gajdos, Miroslav Koos, and Bjørnar Hassel. 1997. "4-Dodecyl-6-methyl-2-morpholone" Molecules 2, no. 11: M42. https://doi.org/10.3390/M42
APA StyleSteiner, B. S., Gajdos, J., Koos, M., & Hassel, B. (1997). 4-Dodecyl-6-methyl-2-morpholone. Molecules, 2(11), M42. https://doi.org/10.3390/M42



