3-Dodecyl-5-methyl-2-oxazolidinthione
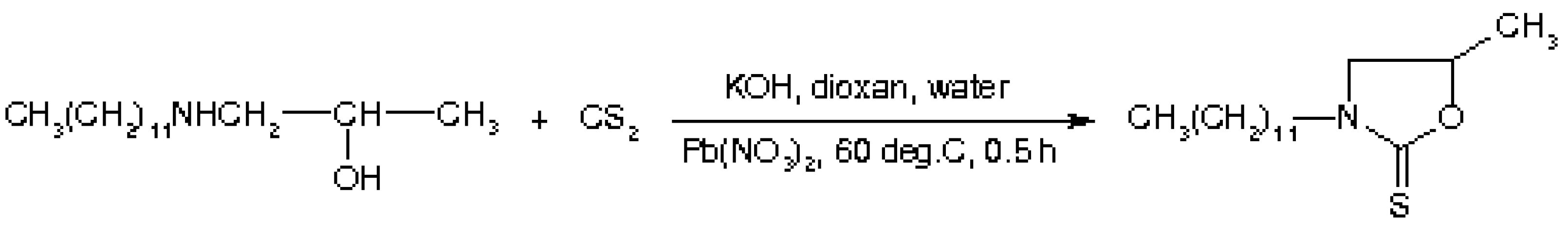
Supplementary materials
Supplementary File 1Supplementary File 2References and Notes
- Ettlinger, M. G. J. Am. Chem. Soc. 1950, 72, 4792. [CrossRef]
- Sainsbury, M. Ansell, M. F., Ed.; Rodd’s Chemistry of Carbon Compounds, 2nd edn.; Vol. IV, Part C; Elsevier: Amsterdam, 1986; p. 303. [Google Scholar]
- Sample availability: Available from the authors and MDPI, MDPI Reg. No.13741.
© 1997 MDPI. All rights reserved
Share and Cite
Steiner, B.; Gajdos, J.; Koos, M. 3-Dodecyl-5-methyl-2-oxazolidinthione. Molecules 1997, 2, M40. https://doi.org/10.3390/M40
Steiner B, Gajdos J, Koos M. 3-Dodecyl-5-methyl-2-oxazolidinthione. Molecules. 1997; 2(11):M40. https://doi.org/10.3390/M40
Chicago/Turabian StyleSteiner, Bohumil, Jan Gajdos, and Miroslav Koos. 1997. "3-Dodecyl-5-methyl-2-oxazolidinthione" Molecules 2, no. 11: M40. https://doi.org/10.3390/M40
APA StyleSteiner, B., Gajdos, J., & Koos, M. (1997). 3-Dodecyl-5-methyl-2-oxazolidinthione. Molecules, 2(11), M40. https://doi.org/10.3390/M40



