Introduction
Heterocycles are important compounds for the synthesis of anti-inflammatory pharmaceuticals and vitamins, i.e. vitamin B
6 [
1,
2,
3,
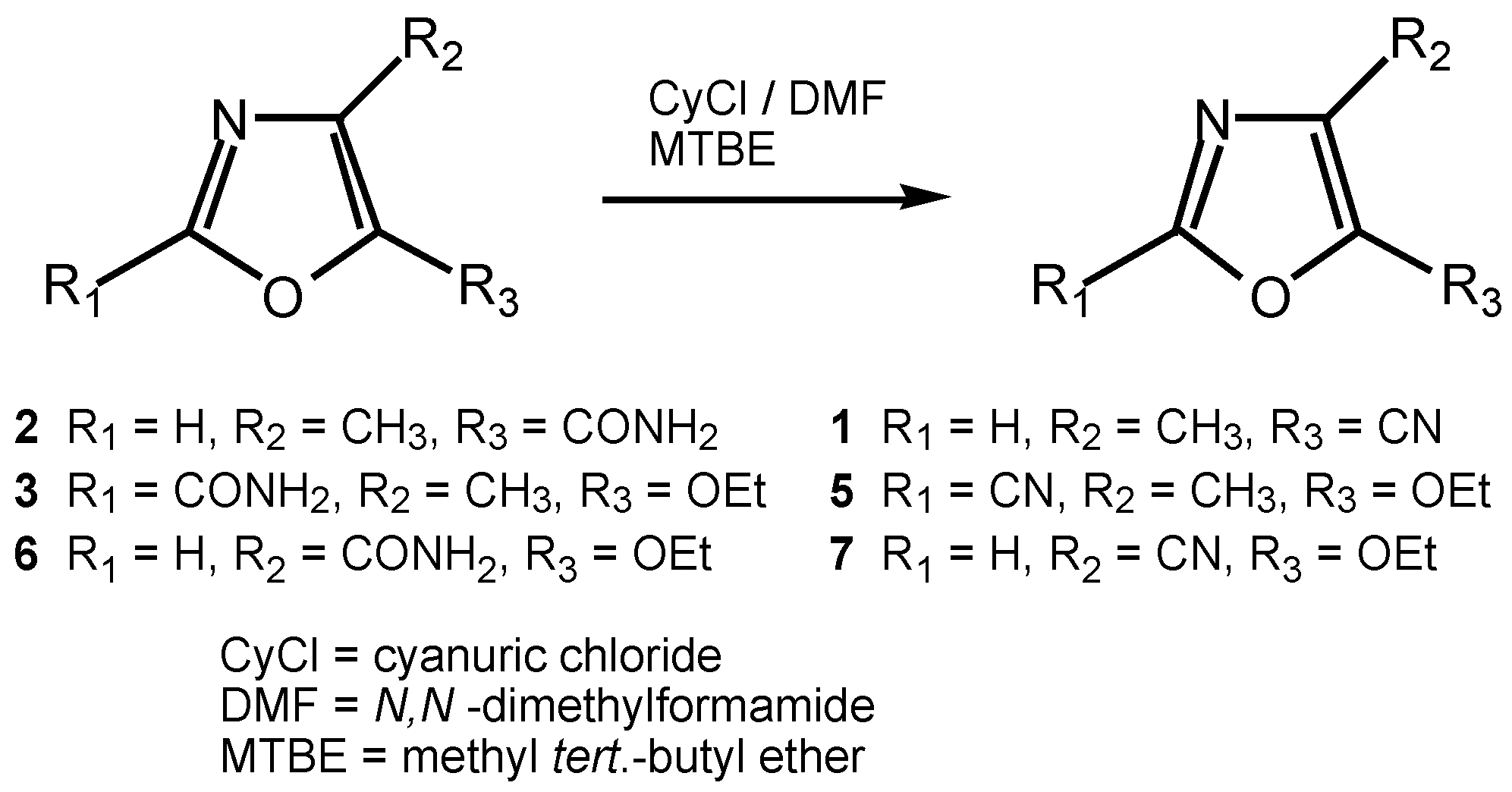
4]. 5-Cyano-4-methyl oxazole (
1) is a building block in the Vitamin B
6 synthesis [
5] and was first synthesized by Rinderspacher and Prijs by dehydrating 4-methyl-5-oxazole carboxamide (
2) using phosphorus pentoxide [
6]. This dehydration can also be accomplished with phosphorus pentoxide/quinoline, acetic anhydride, or in a catalytic gas phase reaction [
7,
8,
9]. We were interested in an alternative dehydration method of heterocyclic amides, since all the methods described so far give rise to problems when conducted on an industrial scale.
Olah and coworkers described a method for dehydrating aliphatic and aromatic amides to the corresponding nitriles by using cyanuric chloride [
10], which was added to a solution of the amide in N,N-dimethylformamide (DMF). However, the dehydration of heterocyclic amides by this method has not been reported to date. A disadvantage of the process described by Olah is the exothermic reaction of cyanuric chloride and DMF [
11,
12]. For Olah et al. this exothermic behavior presented obviously no problem, since they conducted their ex-periments on a very small scale.
Scheme 1.
Dehydration of oxazole carboxamides to oxazole nitrile.
Scheme 1.
Dehydration of oxazole carboxamides to oxazole nitrile.
Results and Discussion
We surprisingly found that, by adding an organic solvent to the reagent cyanuric chloride and an N,N-disubstituted formamide, an almost quantitative yield in the dehydration of
2 to
1 can be achieved (
Scheme 1) [
13]. On the other hand, using cyanuric chloride and
2 without DMF led to low yields (1%) of
1 [
11].
The dehydration of the other oxazole amides (see
Table 1) was carried out under similar conditions, whereby the corresponding nitriles could be synthesized in 62.5 to 77.6% yield. 5-Ethoxy-4-methyl-2-carboxamide (
3), prepared from 5-ethoxy-4-methyl-oxazole-2-carboxylic acid ethyl ester (
4) [
14] and etheral ammonia solution in 90% yield, was dehydrated to 5-ethoxy-4-methyl-2-carbonitrile (
5) in 62.6% yield. The synthesis of 5-ethoxy-oxazole-4-carbonitrile (
7) using cyanuric chloride/DMF, starting from 5-ethoxy-oxazole-4-carboxamide (
6), was carried out in 77.6% yield.
As outlined in the table, not all heterocyclic carboxamides can be dehydrated to the nitriles in excellent yield using CyCl/DMF. For example, nicotinic acid amide (
8) does not react to 3-cyano-pyridine (
9) under these conditions. Imidazoles, e.g. 4-amino-5-imidazole-carboxamide hydrochloride (
10), could be dehydrated to
11 in 63.4% yield [
15]. Indole-3-acetamide (
12), though not strictley a heterocyclic carboxamide, is dehydrated to indole-3-acetonitrile (
13) in 76% yield [
16].
The nature of the N,N-disubstituted formamide is not critical. Any conventional N,N-disubstituted formamide can be used. N,N-dimethylformamide, N,N-diethyl-formamide, N,N-di-n-propylformamide, N-formylmor-pholine, N-formylpyrrolidine and N-formylpiperidine are equally efficient. The solvent used should preferably not be soluble in water because the reaction mixture will finally be neutralized and washed with water. The preferred solvents are methyl tertiary butyl ether, ethyl tertiary butyl ether, tertiary amyl methyl ether, or hexane.
In summary, heterocyclic carboxamides, especially oxazole amides, could be dehydrated to the corresponding nitriles using cyanuric chloride/DMF. The most beneficial features of this method are the mild reaction conditions (room temperature, one hour reaction time) and good yields.
Experimental
General
1H NMR spectra (δ, in ppm; J, in Hz; relative to internal TMS in CDCl3 and DMSO-d6 solns., respectively, at 20°C) were recorded on a Bruker AC 250-E spectrometer, MS (electron impact mass spectrum; m/z in % of base peak) on a Perkin Elmer Sciex API III spectrometer. Melting points (M.p.) were observed under a microscope using a Büchi/Tottoli instrument and are not corrected.
General procedure
A typical procedure for the dehydration of heterocyclic carboxamides to the heterocyclic carbonitriles is described in the following. 22.88 g (198 mmol) of 2 are suspended in 100 ml of DMF at room temperature, and a solution of 18.31 g (99.3 mmol) of CyCl in 250 ml of MTBE is added over a period of 15 minutes. The mixture is stirred at room temperature for one hour, whereby the initially yellow suspension becomes orange. Subsequently, it is neutralized with 50 ml of saturated aqueous Na2CO3 solution. The phases are separated and the aqueous phase is extracted twice with 150 ml MTBE each time. The combined organic phases are washed with 250 ml of dist. water, dried over Na2SO4, filtered, and finally the solvent is removed on a rotary evaporator. An orange liquid crude product re-mains. The yield of 1 is 19.49 g (99.4%).
4-Methyl-oxazole-5-carbonitrile (1)
1H-NMR (CDCl3): 2.40 (s, CH3), 7.95 (s, =CH); IR (KBr): 3136, 2234, 1604, 1495; MS (70 eV): 108 (18, M), 80 (56), 53 (100), 43 (4), 38 (17), 27 (38); Anal. cal. For C5H4N2O (108.099): C 55.56, H 3.73, N 25.91; found C 55.34, H 3.76, N 26.02.
Methyl-oxazole-5-carboxamide (2)
M.p. 195-202 °C; 1H-NMR (DMSO-d6): 2.36 (s, CH3), 7.57 and 7.79 (s, CONH2), 8.39 (s, =CH); IR (KBr): 3368, 3305, 3150, 3122, 2955, 2927, 2855, 1680, 1620, 1494, 1443, 1311, 1265, 1103, 937, 865; MS (70 eV): 126 (100, M), 109 (10), 82 (30), 71 (15), 55 (20), Anal. cal. For C5H6N2O2 (126.115): C 47.62, H 4.80, N 22.21; found C 47.65, H4.80, N 22.21.
Ethoxy-4-methyl-oxazole-2-carboxamide (3)
M.p. 163 °C; 1H-NMR (DMSO-d6): 1.31 (t, CH3), 2.05 (s, CH3), 4.27 (q, CH2), 7.66 and 8.00 (s, CONH2); IR (KBr): 2990, 2928, 2872, 1702, 1671, 1646, 1530, 1418, 1231, 1084; MS (70 eV): 170 (6, M+), 154 (1), 142 (4), 127 (4), 99 (20), 71 (52), 42 (100); Anal. cal. For C7H10N2O3 (170.168): C 49.21, H 5.92, N 16.46; found C 49.33, H 5.76, N 16.41.
5-Ethoxy-4-methyl-oxazole-2-carbonitrile (5)
1H-NMR (CDCl3): 1.43 (t, CH3), 2.10 (s, CH3), 4.36 (q, CH2); IR (KBr): 2987, 2933, 2237, 1641, 1515; MS (70 eV): 152 (40), 124 (60), 96 (60), 69 (35), 54 (38), 42 (58), 29 (100); Anal.cal. for C7H8N2O2 (152.153): C 55.26, H 5.30, N 18.41; found C 55.22, H 5.52, N 18.53.
5-Ethoxy-oxazole-4-carboxamide (6)
M.p. 99 °C; 1H-NMR (DMSO-d6): 1.47 (t, CH3), 4.52 (q, CH2), 6.06 and 6.60 (s, CONH2), 7.35 (s, =CH); IR (KBr): 3389, 3133, 2987, 1661, 1624, 1604, 1527; MS (70 eV): 156 (8), 141 (8), 128 (80), 72 (78), 44 (86), 29 (100); Anal. cal. for C6H8N2O3 (156.141): C 46.15, H 5.16, N 17.94; found C 49.09, H 5.29, N 17.97.
5-Ethoxy-oxazole-4-carbonitrile (7)
1H-NMR (CDCl3): 1.52 (t, CH3), 4.58 (q, CH2), 7.38 (s, =CH); IR (KBr): 3142, 2992, 2234, 1636, 1616, 1531; MS (70 eV): 138 (20), 110 (60), 54 (62), 29 (100); Anal. cal. for C6H6N2O2 (138.126): C 52.17, H 4.38, N 20.28; found C 52.08, H 4.15, N 20.31.