Anti-Inflammatory Effect of the Blueberry Anthocyanins Malvidin-3-Glucoside and Malvidin-3-Galactoside in Endothelial Cells
Abstract
:1. Introduction
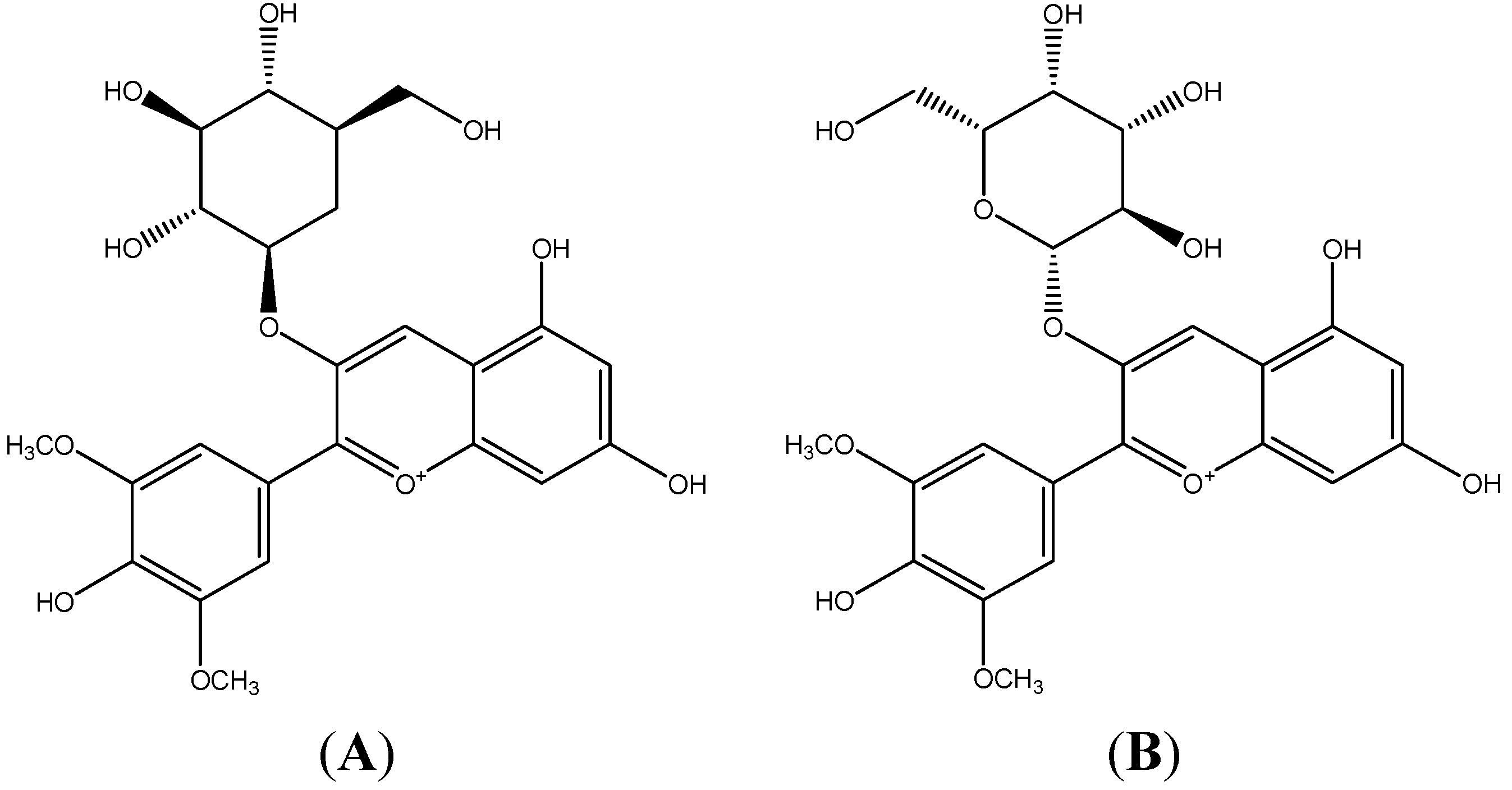
2. Results and Discussion
2.1. Effect of Mv-3-glc and Mv-3-gal on TNF-α-Induced MCP-1, ICAM-1, and VCAM-1 Production in Supernatant
| Treatment | Protein in Supernatant (μg/L) | ||
|---|---|---|---|
| MCP-1 | ICAM-1 | VCAM-1 | |
| Control | 0.023 ± 0.001 *** | 0.111 ± 0.005 *** | 0.021 ± 0.016 *** |
| 10 μg/L TNF-α | 0.519 ± 0.014 | 0.605 ± 0.020 | 0.314 ± 0.009 |
| 1 μM Mv-3-glc + TNF-α | 0.341 ± 0.009 ** | 0.336 ± 0.021 ** | 0.183 ± 0.005 ** |
| 10 μM Mv-3-glc + TNF-α | 0.175 ± 0.011 *** | 0.261 ± 0.017 ** | 0.127 ± 0.001 *** |
| 50 μM Mv-3-glc + TNF-α | 0.039 ± 0.001 *** | 0.098 ± 0.026 *** | 0.049 ± 0.001 *** |
| 100 μM Mv-3-glc + TNF-α | 0.033 ± 0.002 *** | 0.049 ± 0.001 *** | 0.039 ± 0.001 *** |
| Control | 0.023 ± 0.003 *** | 0.102 ± 0.003 *** | 0.013 ± 0.002 ** |
| 10 μg/L TNF-α | 0.264 ± 0.009 | 0.337 ± 0.010 | 0.124 ± 0.006 |
| 1 μM Mv-3-gal + TNF-α | 0.216 ± 0.010 * | 0.208 ± 0.009 ** | 0.106 ± 0.001 * |
| 10 μM Mv-3-gal + TNF-α | 0.109 ± 0.001 ** | 0.185 ± 0.009 ** | 0.066 ± 0.001 ** |
| 50 μM Mv-3-gal + TNF-α | 0.079 ± 0.001 ** | 0.133 ± 0.020 ** | 0.028 ± 0.003 ** |
| 100 μM Mv-3-gal + TNF-α | 0.048 ± 0.001 *** | 0.044 ± 0.022 *** | 0.017 ± 0.001 ** |
| Control | 0.015 ± 0.003 *** | 0.161 ± 0.005 *** | 0.014 ± 0.001 *** |
| 10 μg/L TNF-α | 0.507 ± 0.011 | 0.625 ± 0.018 | 0.445 ± 0.005 |
| 1 μM (Mv-3-glc + Mv-3-gal) + TNF-α | 0.421 ± 0.016 ** | 0.199 ± 0.008 *** | 0.241 ± 0.004 *** |
| 10 μM (Mv-3-glc + Mv-3-gal) + TNF-α | 0.080 ± 0.002 *** | 0.170 ± 0.001 *** | 0.143 ± 0.004 *** |
| 50 μM (Mv-3-glc + Mv-3-gal) + TNF-α | 0.023 ± 0.001 *** | 0.058 ± 0.005 *** | 0.048 ± 0.003 *** |
| 100 μM (Mv-3-glc + Mv-3-gal) + TNF-α | 0.018 ± 0.001 *** | 0.013 ± 0.002 *** | 0.037 ± 0.002 *** |
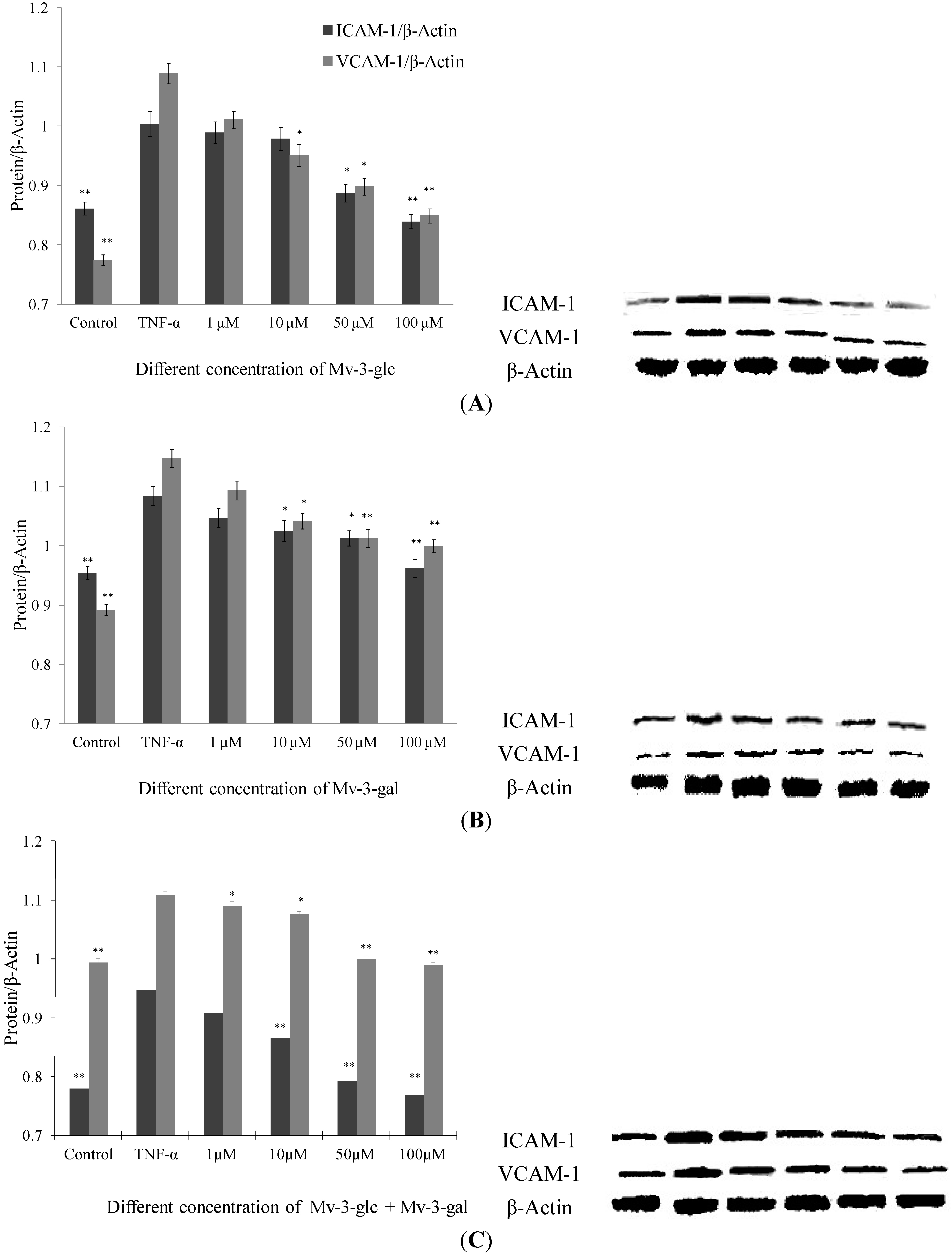
2.2. Effects of Mv-3-glc and Mv-3-gal on TNF-α-Induced ICAM-1 and VCAM-1 Proteins in Cell
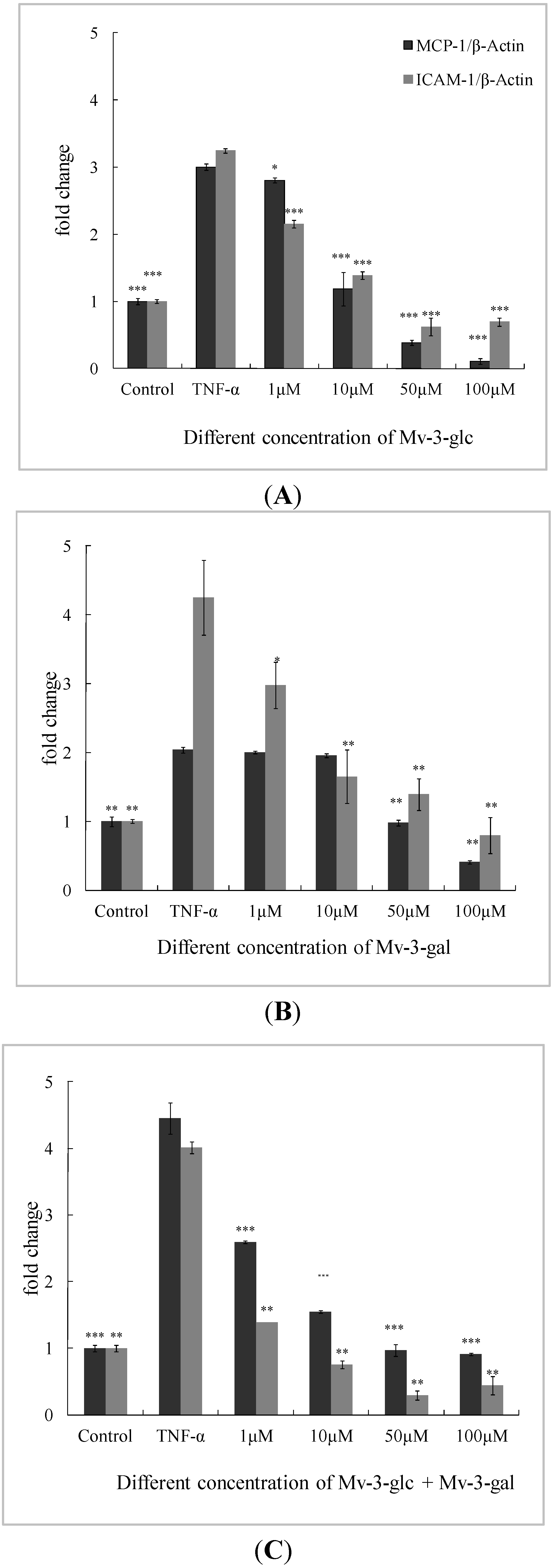
2.3. Effects of Mv-3-glc and Mv-3-gal on TNF-α-Induced MCP-1 and ICAM-1 mRNA in Cells
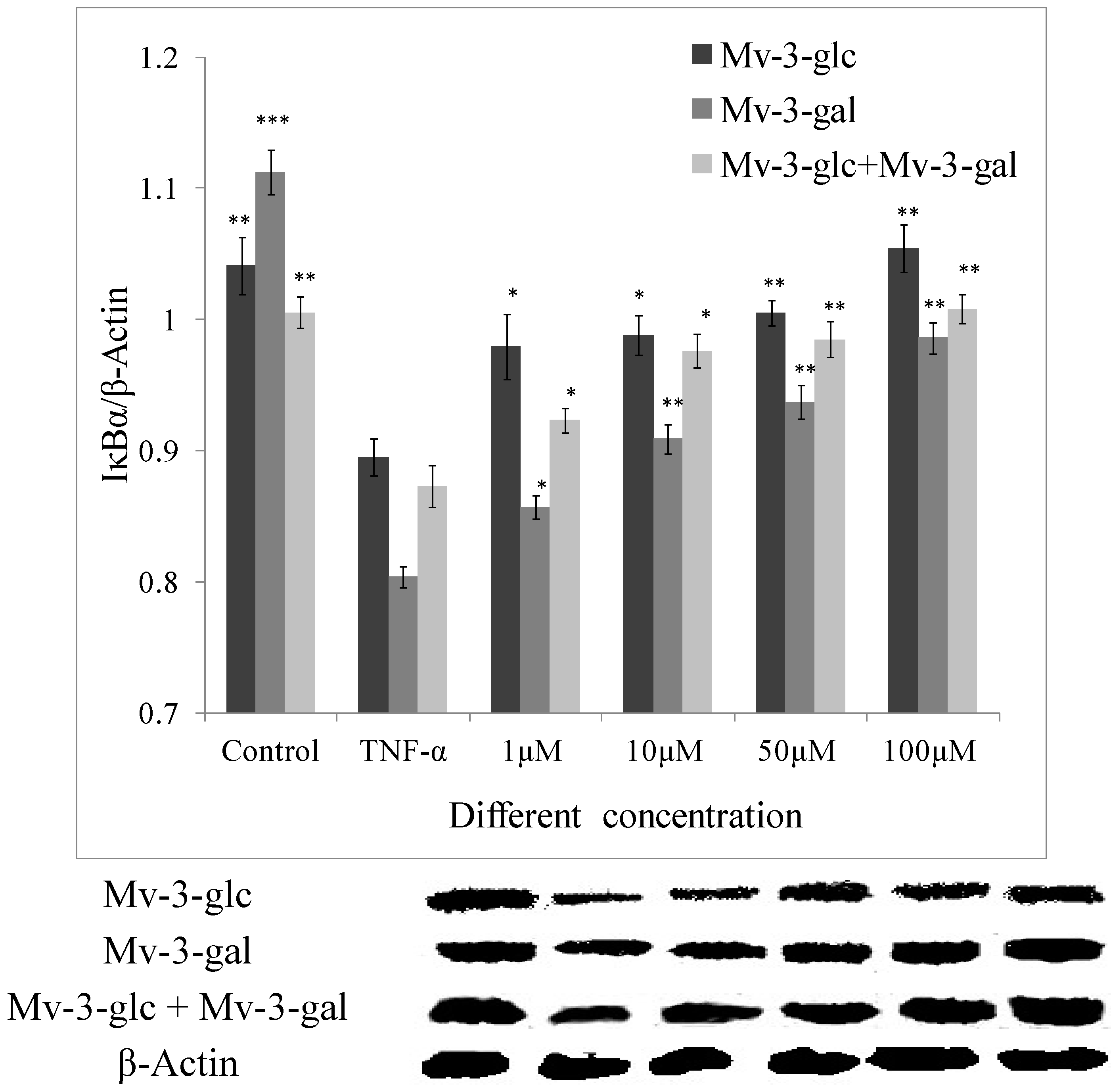
2.4. Effects of Mv-3-glc and Mv-3-gal on TNF-α-Induced IκB Degradation
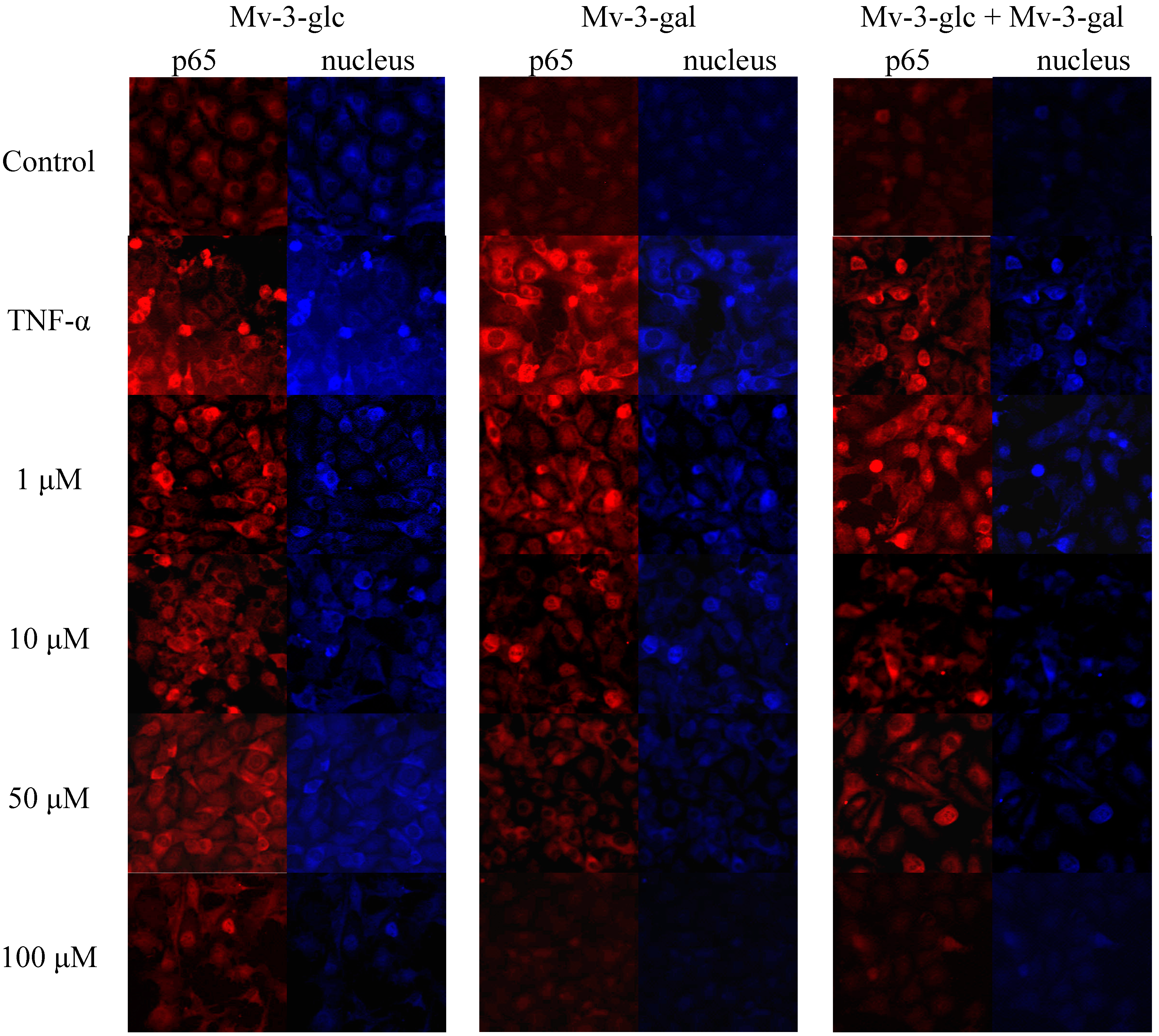
2.5. Effects of Mv-3-glc and Mv-3-gal on TNF-α-Induced NF-κB Translocation
2.6. Discussion
3. Experimental Section
3.1. Chemicals and Reagents
3.2. Antibodies
3.3. Endothelial Cell Culture and Treatment
3.4. ELISA Analysis and Western Blotting
3.5. Real-Time qRT-PCR
3.6. Immunofluorescence
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, T.S.C. Vegetables and Fruits: Nutritional and Therapeutic Values; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Tulipani, S.; Mezzetti, B.; Capocasa, F.; Bompadre, S.; Beekwilder, J.; de Vos, C.H.R.; Capanoglu, E.; Bovy, A.; Battino, M. Antioxidants, phenolic compounds, and nutritional quality of different strawberry genotypes. J. Agric. Food Chem. 2008, 56, 696–704. [Google Scholar]
- Shahidi, F.; Ho, C.T. (Eds.) Phenolic Compounds in Foods and Natural Health Products; American Chemical Society: Washington, DC, USA, 2005.
- Liu, R.H. Health benefits of fruits and vegetables are from additive and synergistic combination of phytochemicals. Am. J. Clin. Nutr. 2003, 78, S517S–S520. [Google Scholar]
- Huang, W.Y.; Cai, Y.Z.; Zhang, Y.B. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr. Cancer 2010, 62, 1–20. [Google Scholar]
- Zhou, Z.; Liu, Y.; Miao, A.D.; Wang, S. Protocatechuic aldehyde suppresses TNF-a-induced ICAM-1 and VCAM-1 expression in human umbilical vein endothelial cells. Eur. J. Pharmacol. 2005, 513, 1–8. [Google Scholar]
- Kanterman1, J.; Sade-Feldman1, M.; Baniyash, M. New insights into chronic inflammation-induced immunosuppression. Semin. Cancer Biol. 2012, 22, 307–308. [Google Scholar]
- Krikorian, R.; Shidler, M.D.; Nash, T.A.; Kalt, W.; Vinqvist-Tymchuk, M.R.; Shukitt-Hale, B.; Joseph, J.A. Blueberry supplementation improves memory in older adults. J. Agric. Food Chem. 2010, 58, 3996–4000. [Google Scholar]
- Shaughnessy, K.S.; Boswall, I.A.; Scanlan, A.P.; Gottschall-Pass, K.T.; Sweeney, M.I. Diets containing blueberry extract lower blood pressure in spontaneously hypertensive stroke-prone rats. Nutr. Res. 2009, 29, 130–138. [Google Scholar]
- Kalt, W.; Foote, K.; Fillmore, S.A.; Lyon, M.; van Lunen, T.A.; Mc Rae, K.B. Effect of blueberry feeding on plasma lipids in pigs. Br. J. Nutr. 2008, 100, 70–78. [Google Scholar]
- Castrejon, A.D.R.; Eichholz, I.; Rohn, S.; Krohn, L.W.; Huykens-Keil, S. Phenolic profile and antioxidant activity of highbush blueberry (Vaccinium corymbosum L.) during fruit maturation and ripening. Food Chem. 2008, 109, 564–572. [Google Scholar] [CrossRef]
- Li, H.Y.; Deng, Z.Y.; Zhu, H.H.; Hu, C.L.; Liu, R.H.; Young, J.C.; Tsao, R. Highly pigmented vegetables: Anthocyanin compositions and their role in antioxidant activities. Food Res. Int. 2012, 46, 250–259. [Google Scholar]
- Srivastava, A.; Akoh, C.C.; Fischer, J.; Krewer, G. Effect of anthocyanin fractions from selected cultivars of Georgia-grown blueberries on apoptosis and phase II enzymes. J. Agric. Food Chem. 2007, 55, 3180–3185. [Google Scholar]
- Moyer, R.A.; Hummer, K.E.; Finn, C.E.; Frei, B.; Wrolstad, R. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, rubus, and ribes. J. Agric. Food Chem. 2002, 50, 519–525. [Google Scholar]
- Huang, W.Y.; Zhang, H.C.; Liu, W.X.; Li, C.Y. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J. Zhejiang Univ. Sci. B 2012, 13, 94–102. [Google Scholar]
- Huang, W.Y.; Wang, J.; Liu, Y.M.; Zheng, Q.S.; Li, C.Y. Inhibitory effect of Malvidin on TNF-α-induced inflammatory response in endothelial cells. Eur. J. Pharmacol. 2014, 723, 67–72. [Google Scholar]
- Esposito, D.; Chen, A.; Grace, M.H.; Komarnytsky, S.; Lila, M.A. Inhibitory effects of wild blueberry anthocyanins and other flavonoids on biomarkers of acute and chronic inflammation in vitro. J. Agric. Food Chem. 2014, 62, 7022–7028. [Google Scholar]
- Jia, Z.S.; Zhou, B.; Yang, L.; Wu, L.M.; Liu, Z.L. Synergistic antioxidant effect of green tea polyphenols with a-tocopherol on free radical initiated peroxidation of linoleic acid in micelles. J. Chem. Soc. Perkin Trans. 2 2000, 785–791. [Google Scholar]
- Rossetto, M.; Vanzani, P.; Mattivi, F.; Lunelli, M.; Scarpa, M.; Rigo, A. Synergistic antioxidant effect of catechin and malvidin-3-glucoside on free radical-initiated peroxidation of linoleic acid in micelles. Arch. Biochem. Biophys. 2002, 408, 239–245. [Google Scholar]
- Saucier, C.T.; Waterhouse, A.L. Antioxidant activity of green tea polyphenols against lipid peroxidation initiated by lipid-soluble radicals in micelles. J. Agric. Food Chem. 1999, 47, 4491–4494. [Google Scholar]
- Lentsch, A.B.; Jordan, J.A.; Czermak, B.J.; Diehl, K.M.; Younkin, E.M.; Sarma, V.; Ward, P.A. Inhibition of NF-kappaB activation and augmentation of IkappaBbeta by secretory leukocyte protease inhibitor during lung inflammation. Am. J. Pathol. 1999, 154, 239–247. [Google Scholar]
- Aqil, F.; Gupta, A.; Munagala, R.; Jeyabalan, J.; Kausar, H.; Sharma, R.J.; Singh, I.P.; Gupta, R.C. Antioxidant and antiproliferative activities of anthocyanin/ellagitannin-enriched extracts from Syzygium cumini L. (‘Jamun’, the Indian blackberry). Nutr. Cancer Int. J. 2012, 64, 428–438. [Google Scholar] [CrossRef]
- Nie; Zhao, Z.P.; Chen, G.P.; Zhang, B.; Ye, M.; Hu, Z.L. Brassica napus possesses enhanced antioxidant capacity via heterologous expression of anthocyanin pathway gene transcription factors. J. Plant Physiol. 2013, 60, 108–115. [Google Scholar]
- Pop, R.; Stefanut, M.N.; Cata, A.; Tanasie, C.; Medeleanu, M. Ab initio study regarding the evaluation of the antioxidant character of cyanidin, delphinidin and malvidin. Cent. Eur. J. Chem. 2012, 10, 180–186. [Google Scholar]
- Hosseinian, F.S.; Beta, T. Saskatoon and wild blueberries have higher anthocyanin contents than other Manitoban berries. J. Agric. Food Chem. 2007, 55, 10832–10838. [Google Scholar]
- Hyun, J.W.; Chung, H.S. Cyanidin and malvidin from Oryza sativa cv. Heugjinjubyeo mediate cytotoxicity against human monocytic leukemia cells by arrest of G(2)/M phase and induction of apoptosis. J. Agric. Food Chem. 2004, 52, 2213–2217. [Google Scholar] [CrossRef]
- Patterson, S.J.; Fischer, J.G.; Dulebohn, R.V. DNA damage in HT-29 colon cancer cells is enhanced by high concentrations of the anthocyanin malvidin. FASEB J. 2008, 22, 890. [Google Scholar]
- Lee, C.; Han, D.; Kim, B.; Baek, N.; Baik, B.K. Antioxidant and anti-hypertensive activity of anthocyanin-rich extracts from hulless pigmented barley cultivars. Int. J. Food Sci. Technol. 2013, 48, 984–991. [Google Scholar]
- Chen, P.-N.; Chu, S.-C.; Chiou, H.-L.; Kuo, W.-H.; Chiang, C.-L.; Hsieh, Y.-S. Mulberry anthocyanins, cyanidin 3-rutinoside and cyanidin 3-glucoside, exhibited an inhibitory effect on the migration and invasion of a human lung cancer cell line. Cancer Lett. 2006, 235, 248–259. [Google Scholar]
- Yun, J.W.; Lee, W.S.; Kim, M.J.; Lu, J.N.; Kang, M.H.; Kim, H.G.; Kim, D.C.; Choi, E.J.; Choi, J.Y.; Kim, H.G.; et al. Characterization of a profile of the anthocyanins isolated from Vitis coignetiae Pulliat and their anti-invasive activity on HT-29 human colon cancer cells. Food Chem. Toxicol. 2010, 48, 903–909. [Google Scholar]
- Lee, S.G.; Kim, B.; Yang, Y.; Pham, T.X.; Park, Y.-K.; Manatou, J.; Koo, S.I.; Chun, O.K.; Lee, J.-Y. Berry anthocyanins nsuppress the expression and secretion of proinflammatory mediators in macrophages by inhibiting nuclear translocation of NF-κB independent of NRF2-mediated mechanism. J. Nutr. Biochem. 2014, 25, 404–411. [Google Scholar]
- Joana, P.; Teresa, C.P.D.; Leonor, M.A. Malvidin-3-glucoside protects endothelial cells up-regulating endothelial NO synthase and inhibiting peroxynitrite-induced NF-κB activation. Chem. Biol. Interact. 2012, 199, 192–200. [Google Scholar]
- Tsoyi, K.; Park, H.B.; Kim, Y.M.; Chung, J.I.; Shin, S.C.; Lee, W.S.; Seo, H.G.; Lee, J.H.; Chang, K.C.; Kim, H.J. Anthocyanins from black soybean coats inhibit UVB-induced inflammatory cyclooxygenase-2 gene expression and PGE2 production through regulation of the nuclear factor-κB and phosphatidylinositol 3-kinase/AKt pathway. J. Agric. Food Chem. 2008, 56, 8969–8974. [Google Scholar]
- Paixao, J.; Dinis, T.C.P.; Almeida, L.M. Dietary anthocyanins protect endothelial cells against peroxynitrite-induced mitochondrial apoptosis pathway and Bax nuclear translocation: An in vitro approach. Apoptosis 2011, 16, 976–989. [Google Scholar]
- Cao, L.H.; Lee, Y.J.; Kang, D.G.; Kim, J.S.; Lee, H.S. Effect of Zanthoxylum schinifolium on TNF-α-induced vascular inflammation in human umbilical vein endothelial cells. Vasc. Pharmacol. 2009, 50, 200–207. [Google Scholar]
- Umetani, M.; Nakao, H.; Doi, T.; Lwaski, A.; Ohtaka, M.; Nagoya, T. A novel cell adhesion inhibitor, K-7174, reduces the endothelial VCAM -1induction by inflammatory cytokines, acting through the regulation of GATA. Biochem. Bioph. Res. Commun. 2000, 272, 270–274. [Google Scholar]
- Hatada, E.N.; Do, R.K.; Orlofsky, A. NF-kappa B1 p50 is required for BLyS attenuation of apoptosis but dispensable for processing of NF-kappa B2 p100 to p52 in quiescent mature B cells. J. Immunol. 2003, 171, 761–768. [Google Scholar]
- Yang, L.; Froio, R.M.; Sciuto, T.E.; Dvorak, A.M.; Alon, R.; Luscinskas, F.W. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood 2005, 106, 584–592. [Google Scholar]
- Sarvesh, K.; Brajendra, K.S.; Anil, K.P.; Ajit, K.; Sunil, K. A chromone analog inhibits TNF-α induced expression of cell adhesion molecules on human endothelial cells via blocking NF-κB activation. Bioorg. Med. Chem. 2007, 15, 2952–2962. [Google Scholar]
- Finkenzeller, G.; Graner, S.; Kirkpatrick, C.J.; Fuchs, S.; Stark, G.B. Impaired in vivo vasculogenic potential of endothelial progenitor cells in comparison to human umbilical vein endothelial cells in a spheroid-based implantation model. Cell Prolif. 2009, 42, 498–505. [Google Scholar]
- Stewart, K.G.; Zhang, Y.; Davidge, S.T. Estrogen decreases prostaglandin H synthase products from endothelial cells. J. Soc. Gynecol. Investig. 1999, 6, 322–327. [Google Scholar] [CrossRef]
- Sample Availability: Samples are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Huang, W.-Y.; Liu, Y.-M.; Wang, J.; Wang, X.-N.; Li, C.-Y. Anti-Inflammatory Effect of the Blueberry Anthocyanins Malvidin-3-Glucoside and Malvidin-3-Galactoside in Endothelial Cells. Molecules 2014, 19, 12827-12841. https://doi.org/10.3390/molecules190812827
Huang W-Y, Liu Y-M, Wang J, Wang X-N, Li C-Y. Anti-Inflammatory Effect of the Blueberry Anthocyanins Malvidin-3-Glucoside and Malvidin-3-Galactoside in Endothelial Cells. Molecules. 2014; 19(8):12827-12841. https://doi.org/10.3390/molecules190812827
Chicago/Turabian StyleHuang, Wu-Yang, Ya-Mei Liu, Jian Wang, Xing-Na Wang, and Chun-Yang Li. 2014. "Anti-Inflammatory Effect of the Blueberry Anthocyanins Malvidin-3-Glucoside and Malvidin-3-Galactoside in Endothelial Cells" Molecules 19, no. 8: 12827-12841. https://doi.org/10.3390/molecules190812827
APA StyleHuang, W.-Y., Liu, Y.-M., Wang, J., Wang, X.-N., & Li, C.-Y. (2014). Anti-Inflammatory Effect of the Blueberry Anthocyanins Malvidin-3-Glucoside and Malvidin-3-Galactoside in Endothelial Cells. Molecules, 19(8), 12827-12841. https://doi.org/10.3390/molecules190812827





