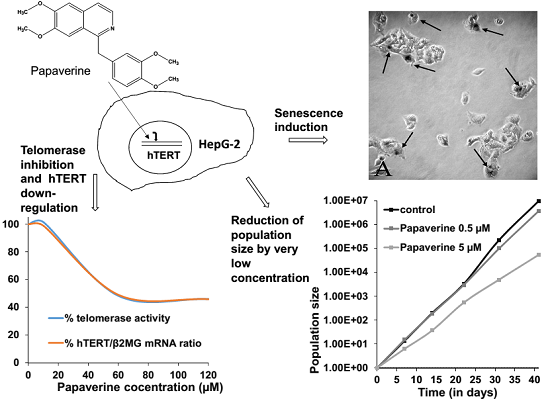
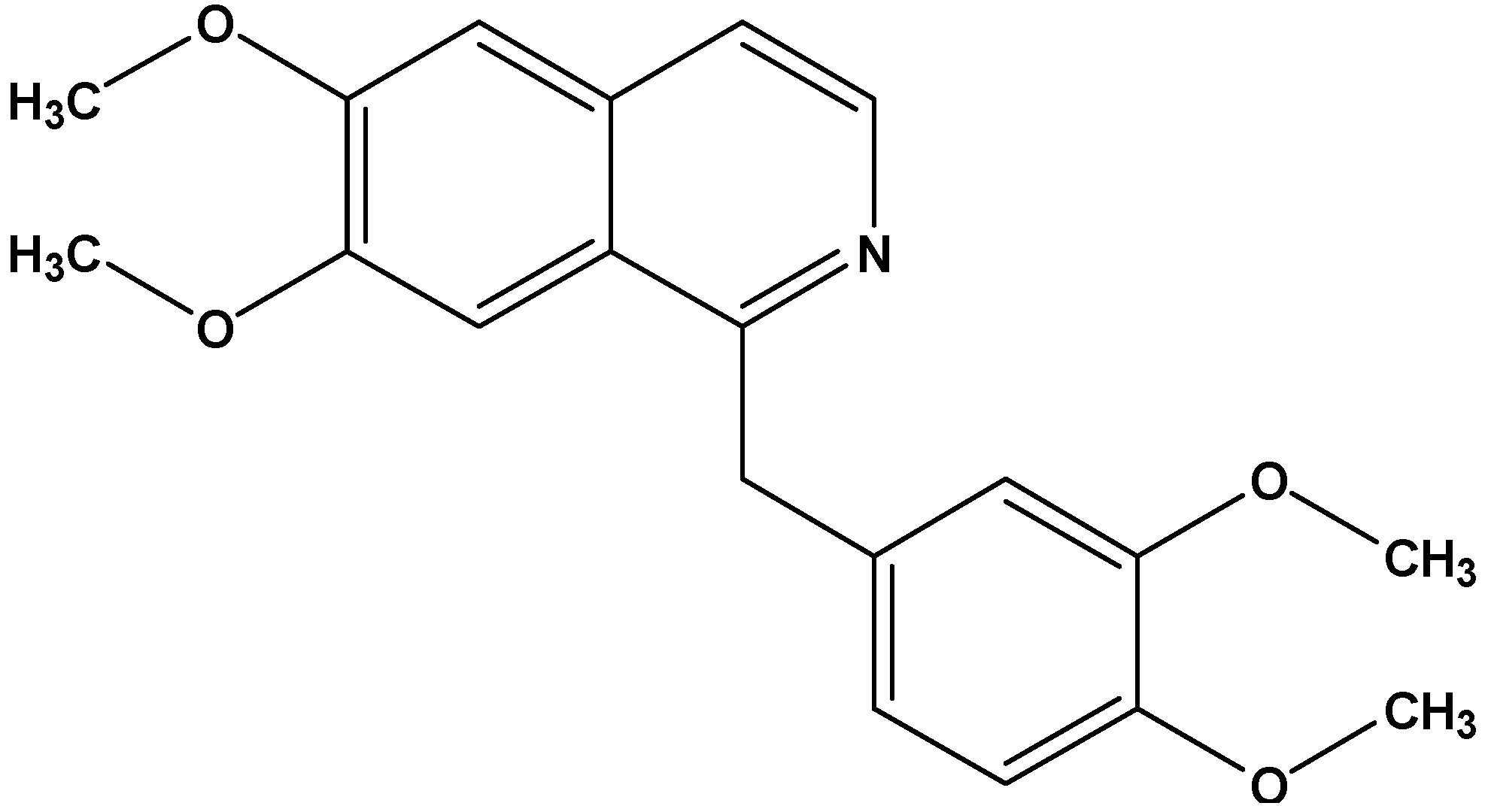
Antiproliferative Effect of the Isoquinoline Alkaloid Papaverine in Hepatocarcinoma HepG-2 Cells — Inhibition of Telomerase and Induction of Senescence
Abstract
:1. Introduction
2. Results and Discussion
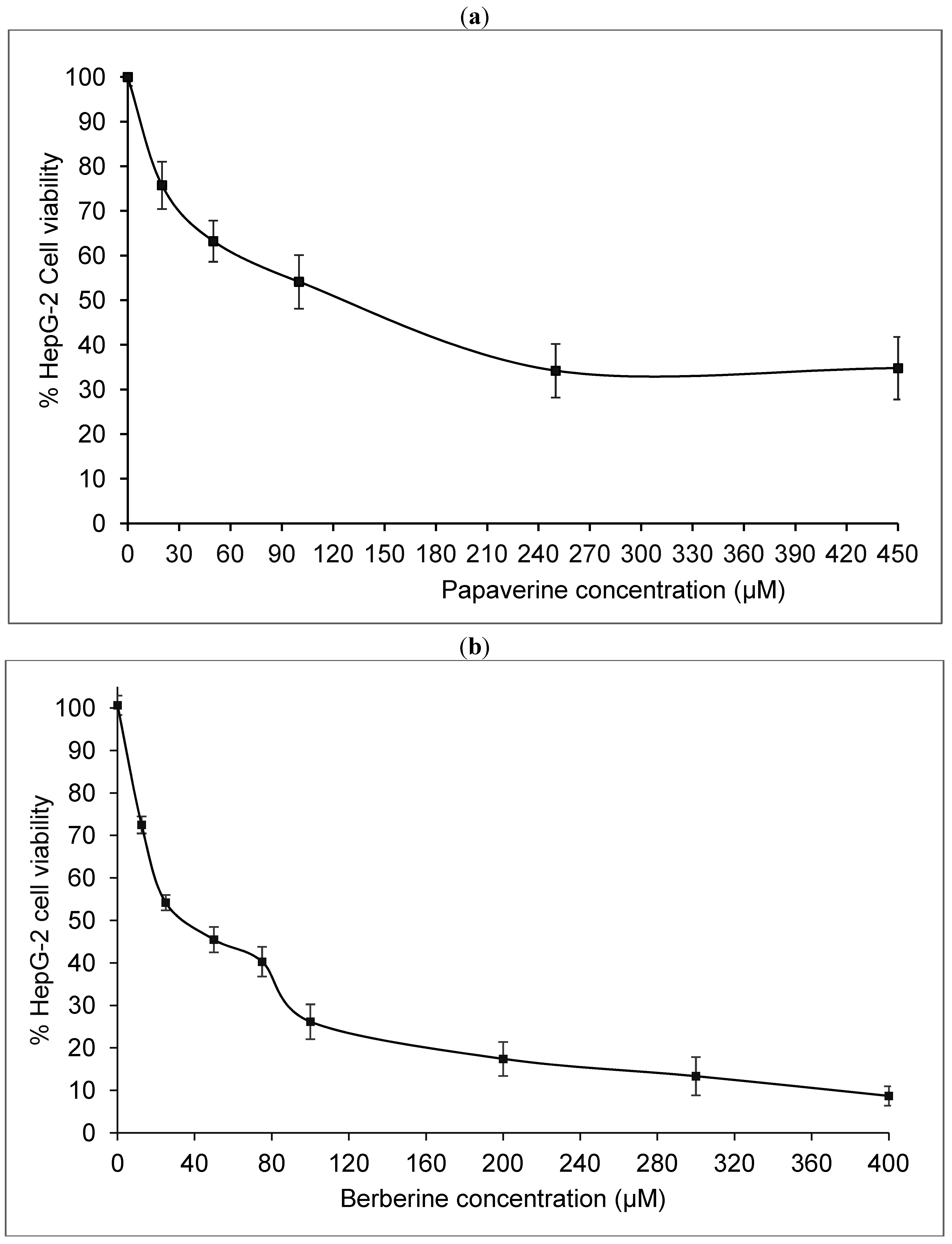
2.1. Papaverine Shows a Moderate Cytotoxicity in HepG-2
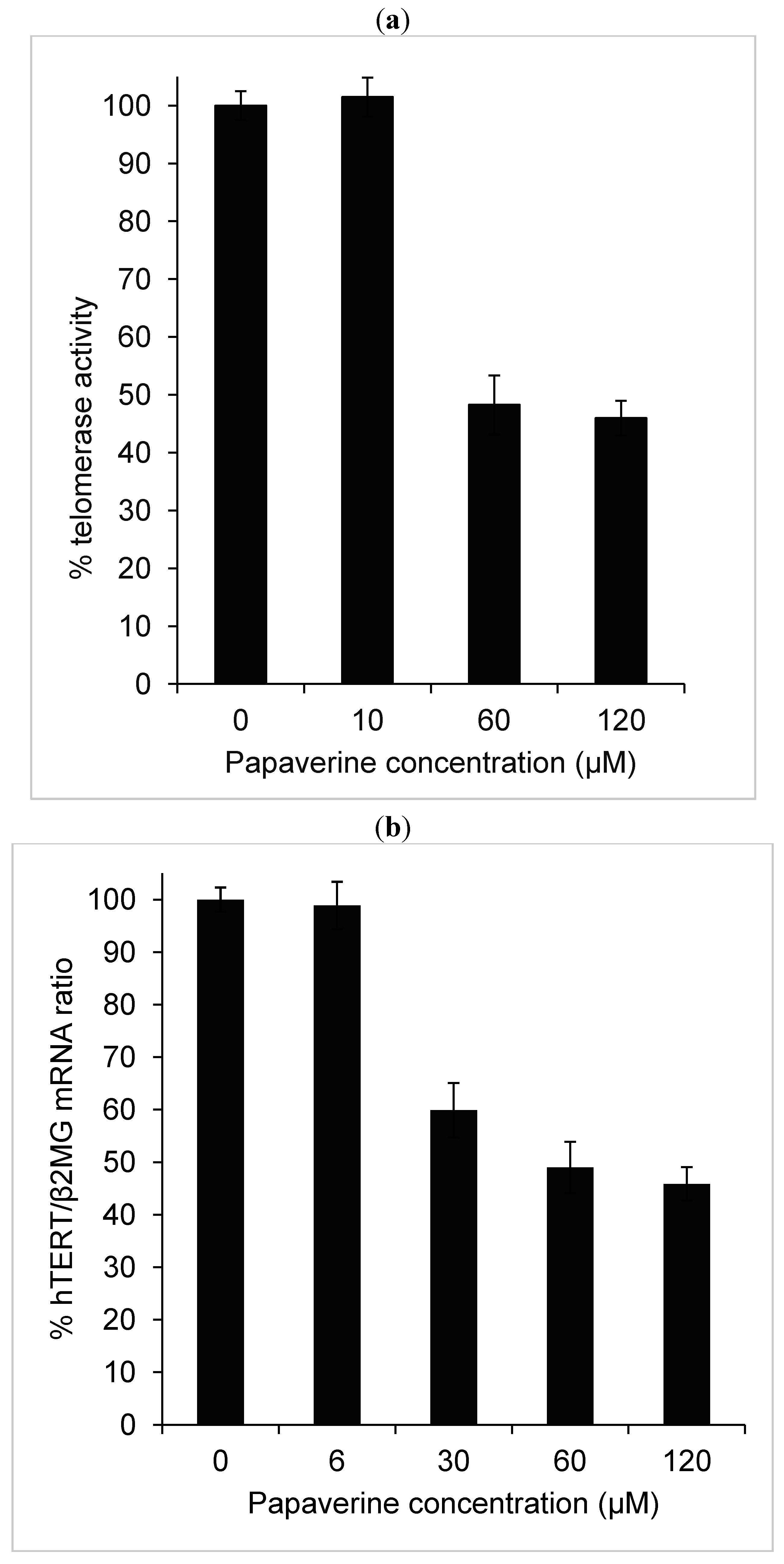
2.2. Telomerase Activity Is Reduced in Papaverine-Treated HepG-2 Cells
2.3. Papaverine does not Directly Interact with Telomeric Sequences
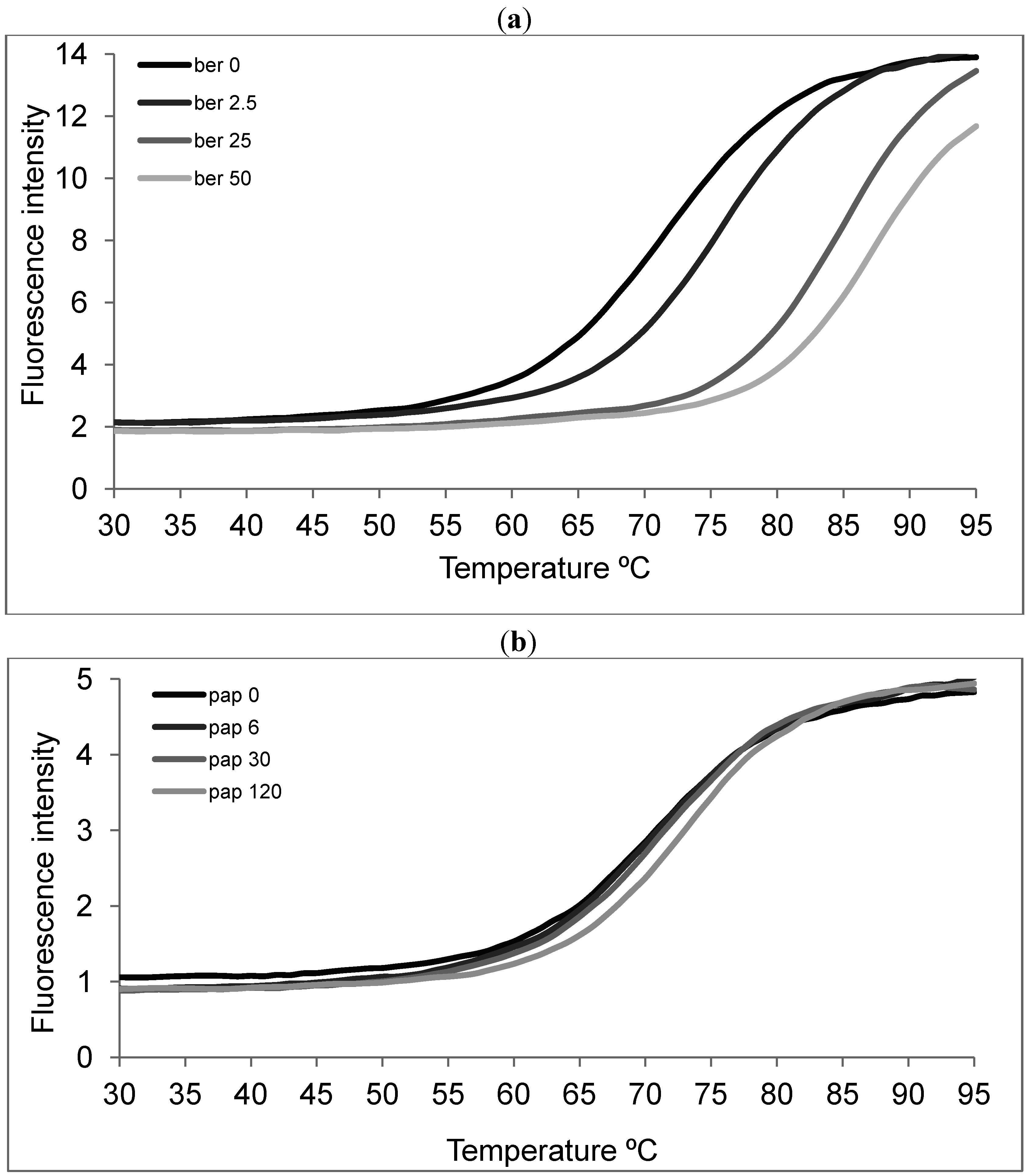
2.4. Papaverine Reduces Transcription Level of hTERT
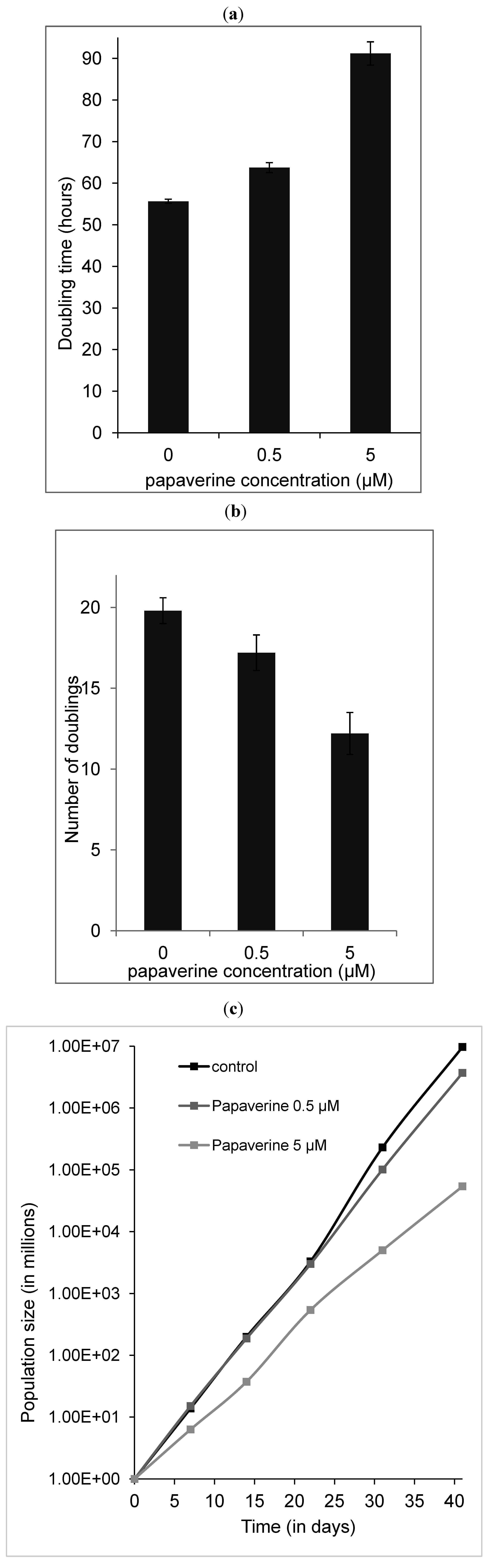
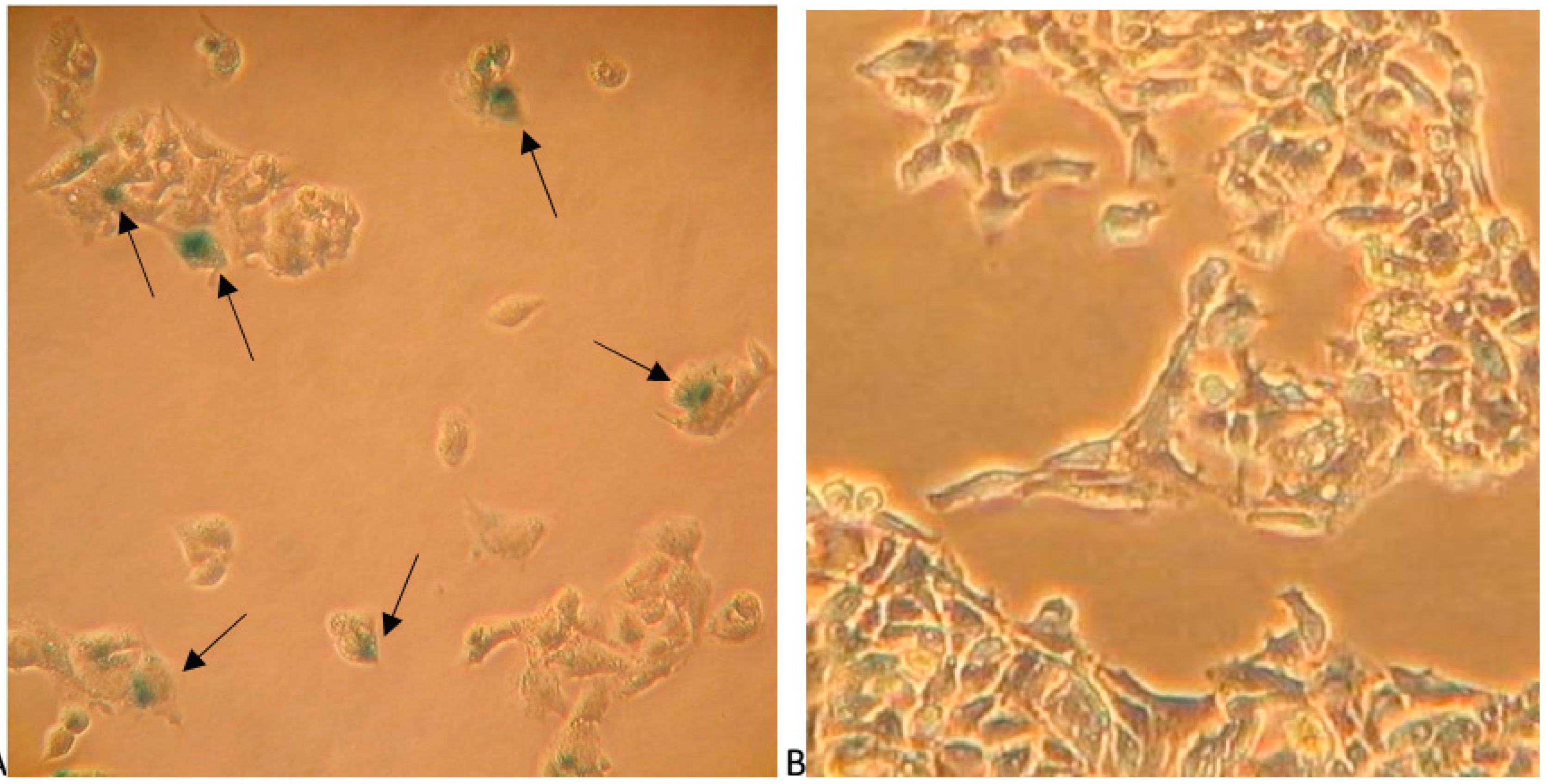
2.5. Papaverine Accelerates Senescence in HepG-2 Cells after Long Exposure to Very Low Concentrations
3. Experimental Section
3.1. Cell Culture and Cytotoxicity Test
3.2. Assay of Telomerase Activity
3.3. RNA Isolation, Reverse Transcription and Real-Time RT-PCR
3.4. FRET Analysis of Synthetic Double Labelled Telomeric Oligonucleotide
3.5. β-Galactosidase Staining as a Senescence Marker
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Greider, C.W.; Blackburn, E.H. Telomeres, telomerase and cancer. Sci. Am. 1996, 274, 92–97. [Google Scholar] [CrossRef]
- Smith, L.L.; Coller, H.A. Telomerase modulates expression of growth-controlling genes and enhances cell proliferation. Nat. Cell Biol. 2003, 5, 474–479. [Google Scholar]
- Zheng, Y.L.; Zhang, F. Telomeraseenzymatic component hTERT shortens long telomeres in human cells. Cell Cycle 2014, 11, 1765–1776. [Google Scholar]
- Jiang, H.; Ju, Z. Telomere shortening and ageing. Z. Gerontol. Geriatr. 2007, 40, 314–324. [Google Scholar]
- Stadtfeld, M.; Nagaya, M. Induced pluripotent stem cells generated without viral integration. Science 2008, 322, 945–949. [Google Scholar]
- Reig-Viader, R.; Vila-Cejudo, M. Telomeric repeat-containing RNA (TERRA) and telomerase are components of telomeres during mammalian gametogenesis. Biol. Reprod. 2014. [Google Scholar] [CrossRef]
- Armanios, M. Telomeres and age-related disease: How telomere biology informs clinical paradigms. J. Clin. Invest. 2013, 123, 996–1002. [Google Scholar]
- Günes, C.; Rudolph, K.L. The role of telomeres in stem cells and cancer. Cell 2013, 152, 390–393. [Google Scholar]
- Shay, J.W.; Wright, W.E. Telomerase: A target for cancer therapeutics. Cancer Cell 2002, 2, 257–265. [Google Scholar]
- Hsu, Y.; Lin, J.J. Telomere and telomerase as targets for anti-cancer and regeneration therapies. Acta Pharmacol. Sin. 2005, 26, 513–518. [Google Scholar]
- Turano, A.; Scura, G. Inhibitory effect of papaverine on HIV replication in vitro. AIDS Res Hum Retroviruses. AIDS Res. Hum. Retrovir. 1989, 5, 183–192. [Google Scholar] [CrossRef]
- Nohl, H.; Rohr-Udilova, N. Ubiquinol and the papaverine derivative caroverine prevent the expression of tumour- promoting factors in adenoma and carcinoma colon cancer cells induced by dietary fat. BioFactors 2005, 25, 87–95. [Google Scholar]
- Pöch, G.; Kukovetz, W.R. Papaverine-induced inhibition of phosphodiesterase activity in various mammalian tissues. Life Sci. 1971, 10, 133–144. [Google Scholar]
- Friedman, Z.; Hackett, S.F. Human retinal pigment epithelial cells in culture possess A2-adenosine receptors. Brain Res. 1989, 492, 29–35. [Google Scholar]
- Hultman, K.A.; Scott, A.W. Small molecule modifier screen for kit-dependent functions in zebrafish embryonic melanocytes. Zebrafish 2008, 5, 279–287. [Google Scholar] [CrossRef]
- Ahmad, N.; Gupta, S. Differential antiproliferative and apoptotic response of sanguinarineforcancercells versus normal cells. Clin. Cancer Res. 2000, 6, 1524–1528. [Google Scholar]
- Noureini, S.K.; Wink, M. Transcriptional down regulation of hTERT and induction of senescence in HepG2 cells by the benzophenanthridine alkaloid chelidonine. World J. Gastroenterol. 2009, 15, 3603–3610. [Google Scholar] [CrossRef]
- Huang, Y.H.; Wu, J.C. Survival benefit of transcatheter arterial chemoembolization in patients with hepatocellular carcinoma larger than 10 cm in diameter. Aliment. Pharmacol. Ther. 2006, 23, 129–135. [Google Scholar]
- Mizukoshi, E.; Nakamoto, Y. Cytotoxic T cell responses to human telomerase reverse transcriptase in patients with hepatocellular carcinoma. Hepatology 2006, 43, 1284–1294. [Google Scholar]
- Ko, E.; Jung, G. Positive association of long telomeres with the invasive capacity of hepatocellular carcinoma cells. Biochem. Biophys. Res. Commun. 2014, 447, 358–363. [Google Scholar]
- Rubis, B.; Kaczmarek, M. The biological activity of G-quadruplex DNA binding papaverine-derived ligand in breast cancer cells. Invest. New Drugs 2009, 27, 289–296. [Google Scholar]
- Counter, C.M.; Meyerson, M. Telomerase activity is restored in human cells by ectopic expression of hTERT (hEST2). Oncogene 1998, 16, 1217–1222. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival, application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar]
- Kim, N.W.; Piatyszek, M.A. Specific association of human telomerase activity with immortal cells and cancer. Science 1994, 266, 2011–2050. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar]
- Hou, M.; Dawei, X. Real-time quantitative telomeric repeat amplification protocol assay for the detection of telomerase activity. Clin. Chem. 2001, 47, 519–524. [Google Scholar]
- Noureini, S.K.; Wink, M. Antiproliferative effects of crocin in HepG-2 cells by telomerase inhibition and hTERT down-regulation. Asian Pac. J. Cancer Prev. 2012, 13, 2305–2309. [Google Scholar]
- Sriwilaijareon, N.; Petmitr, S.; Mutirangura, A.; Ponglikitmongkol, M.; Wilairat, P. Stage specificity of Plasmodium falciparum telomerase and its inhibition by berberine. Parasitol. Int. 2002, 51, 99–103. [Google Scholar]
- Mergny, J.L.; Lacroix, L. Telomerase inhibitors based on quadruplex ligands selected by a fluorescent assay. Proc. Natl. Acad. Sci. USA 2001, 98, 3062–3067. [Google Scholar]
- Stewart, C.R. Broadening by acridine orange of the thermal transition of DNA. Biopolymers 1968, 6, 1737–1743. [Google Scholar]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Q.; Liu, Z.; Li, B.; Sun, Z.; Zhou, H.; Zhang, X.; Gong, Y.; Shao, C. Berberine, agenotoxicalkaloid, induces ATM-Chk1 mediated G2 arrest in prostate cancer cells. Mutat. Res. 2012, 734, 20–29. [Google Scholar] [CrossRef]
- Samples Availability: Samples of berberine and papaverine are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Noureini, S.K.; Wink, M. Antiproliferative Effect of the Isoquinoline Alkaloid Papaverine in Hepatocarcinoma HepG-2 Cells — Inhibition of Telomerase and Induction of Senescence. Molecules 2014, 19, 11846-11859. https://doi.org/10.3390/molecules190811846
Noureini SK, Wink M. Antiproliferative Effect of the Isoquinoline Alkaloid Papaverine in Hepatocarcinoma HepG-2 Cells — Inhibition of Telomerase and Induction of Senescence. Molecules. 2014; 19(8):11846-11859. https://doi.org/10.3390/molecules190811846
Chicago/Turabian StyleNoureini, Sakineh Kazemi, and Michael Wink. 2014. "Antiproliferative Effect of the Isoquinoline Alkaloid Papaverine in Hepatocarcinoma HepG-2 Cells — Inhibition of Telomerase and Induction of Senescence" Molecules 19, no. 8: 11846-11859. https://doi.org/10.3390/molecules190811846
APA StyleNoureini, S. K., & Wink, M. (2014). Antiproliferative Effect of the Isoquinoline Alkaloid Papaverine in Hepatocarcinoma HepG-2 Cells — Inhibition of Telomerase and Induction of Senescence. Molecules, 19(8), 11846-11859. https://doi.org/10.3390/molecules190811846