Polyphenols with Anti-Proliferative Activities from Penthorum Chinense Pursh
Abstract
:1. Introduction
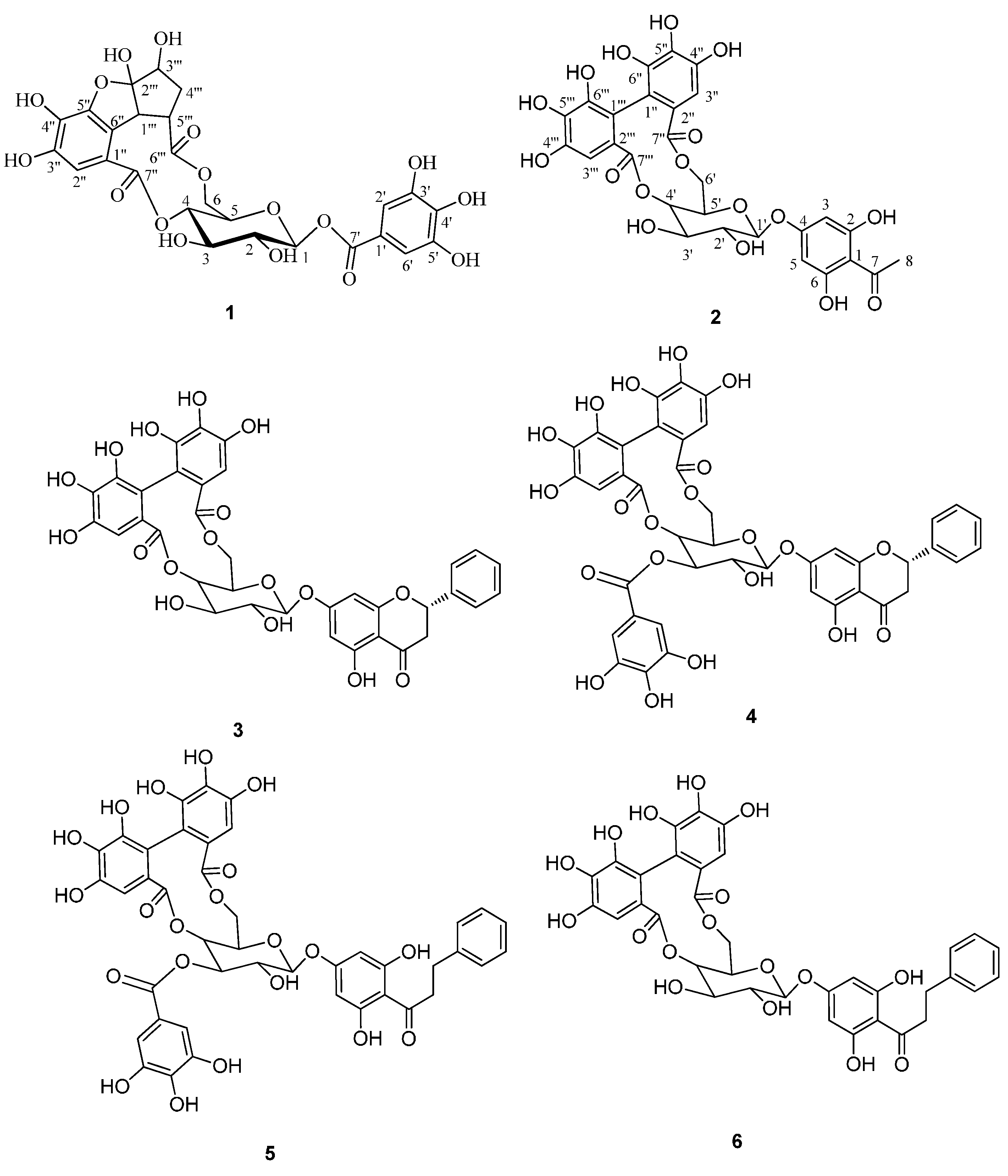
2. Results and Discussion
2.1. Structure Elucidation
| Position | δ H | δ C |
|---|---|---|
| 1 | 5.66(1H, d, 7.8) | 94.4 |
| 2 | 3.40(1H, t, 9.0) | 73.0 |
| 3 | 3.58(1H, t, 9.0) | 73.2 |
| 4 | 5.07(1H, t, 9.6) | 74.5 |
| 5 | 4.07(1H, t, 4.2) | 66.8 |
| 6 | 4.49(1H, dd, 3.6, 10.8)3.92(1H, d, 4.2) | 64.6 |
| 1ꞌ | - | 118.4 |
| 2ꞌ, 6ꞌ | 7.03(2H, s) | 109.2 |
| 3ꞌ, 5ꞌ | - | 145.7 |
| 4ꞌ | - | 139.4 |
| 7ꞌ | - | 164.7 |
| 1ꞌꞌ | - | 117.7 |
| 2ꞌꞌ | 6.82(1H, s) | 110.6 |
| 3ꞌꞌ | - | 146.1 |
| 4ꞌꞌ | - | 133.6 |
| 5ꞌꞌ | - | 146.8 |
| 6ꞌꞌ | - | 119.7 |
| 7ꞌꞌ | - | 167.1 |
| 1ꞌꞌꞌ | 3.68(1H, d, 9.0) | 55.7 |
| 2ꞌꞌꞌ | - | 116.8 |
| 3ꞌꞌꞌ | 3.96(1H, dd, 6.6, 12.0) | 75.4 |
| 4ꞌꞌꞌ | 2.04(1H, d, 6.0)1.93(1H, d, 12.0) | 35.0 |
| 5ꞌꞌꞌ | 2.42(1H, d, 6.0) | 45.3 |
| 6ꞌꞌꞌ | - | 173.6 |
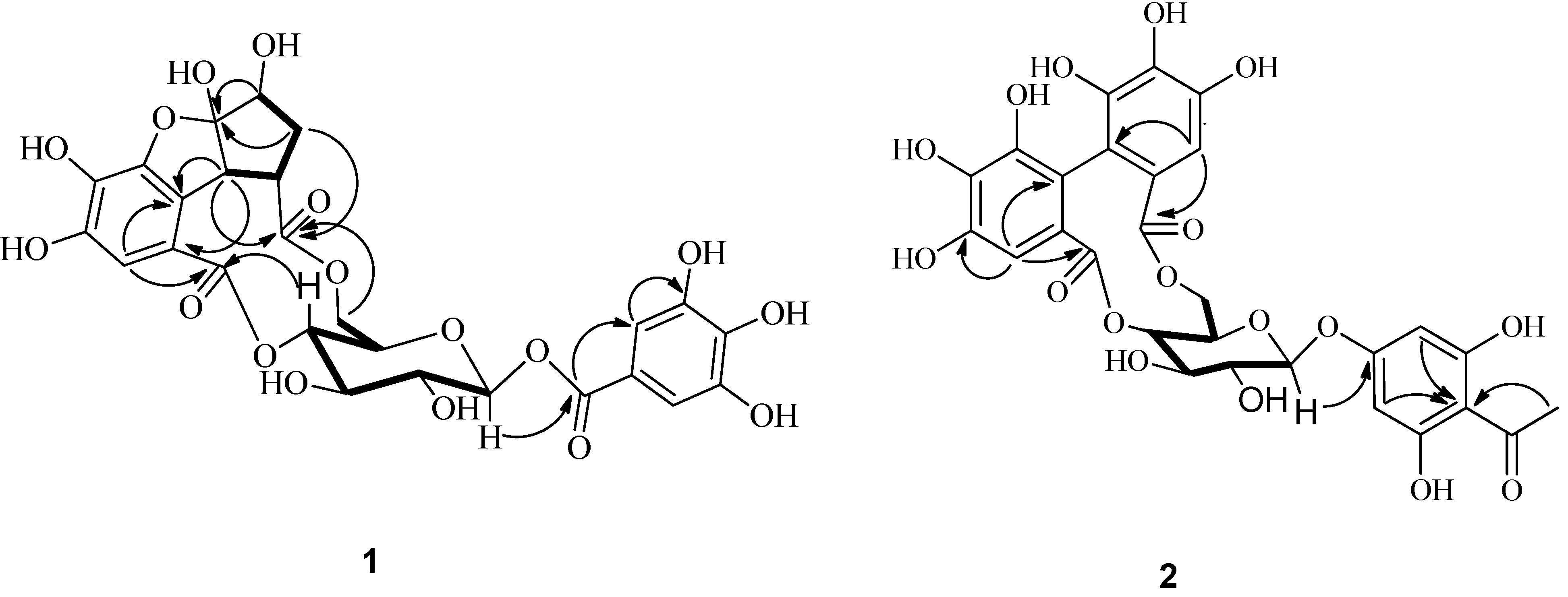
 ) of compound 1.
) of compound 1.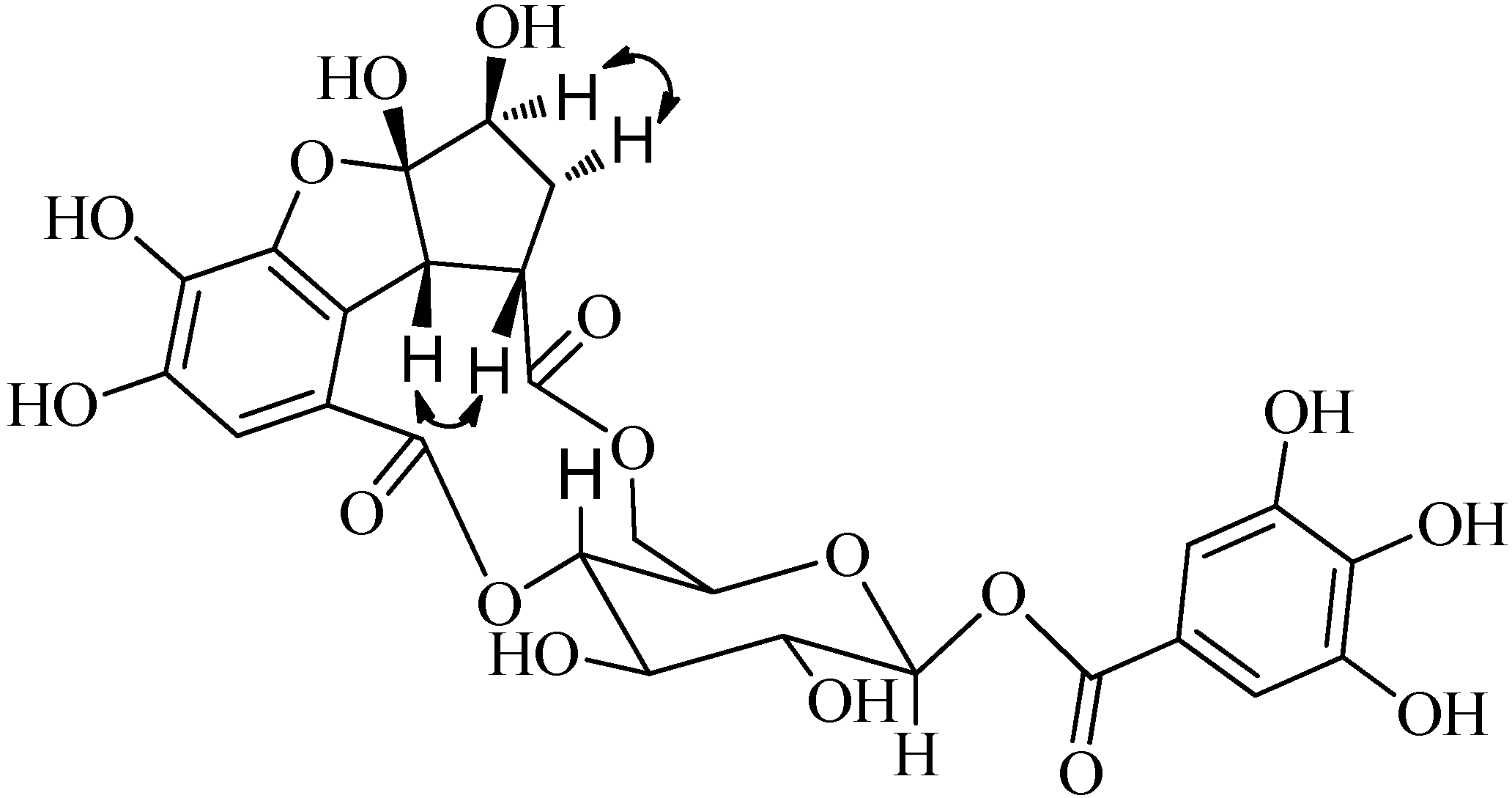
| Position | δ H | δ C |
|---|---|---|
| 1 | - | 105.8 |
| 2, 6 | - | 164.0 |
| 3, 5 | 6.08(2H, s) | 95.0 |
| 4 | - | 163.3 |
| 7 | - | 203.6 |
| 8 | 2.61(3H, s) | 32.8 |
| 1ꞌ | 5.04(1H, d, 7.8) | 99.8 |
| 2ꞌ | 3.57(1H, t, 9.6) | 73.8 |
| 3ꞌ | 3.36(1H, t, 9.0) | 73.8 |
| 4ꞌ | 4.61(1H, t, 9.6) | 71.7 |
| 5ꞌ | 4.13(1H, m) | 71.1 |
| 6ꞌ | 4.98(1H, dd, 6.0, 13.2) 3.74(1H, d, 13.2) | 62.9 |
| 1ꞌꞌ | - | 115.3 |
| 2ꞌꞌ | - | 124.5 |
| 3ꞌꞌ | 6.35(1H, s) | 105.3 |
| 4ꞌꞌ | - | 144.3 |
| 5'' | - | 135.1 |
| 6'' | - | 144.6 |
| 7ꞌꞌ | - | 167.9 |
| 1ꞌꞌꞌ | - | 115.6 |
| 2''' | - | 124.8 |
| 3''' | 6.54(1H, s) | 106.3 |
| 4''' | - | 144.4 |
| 5''' | - | 135.4 |
| 6''' | - | 144.6 |
| 7''' | - | 167.1 |
2.2. Cytotoxicity of Penthorum Chinense Extract
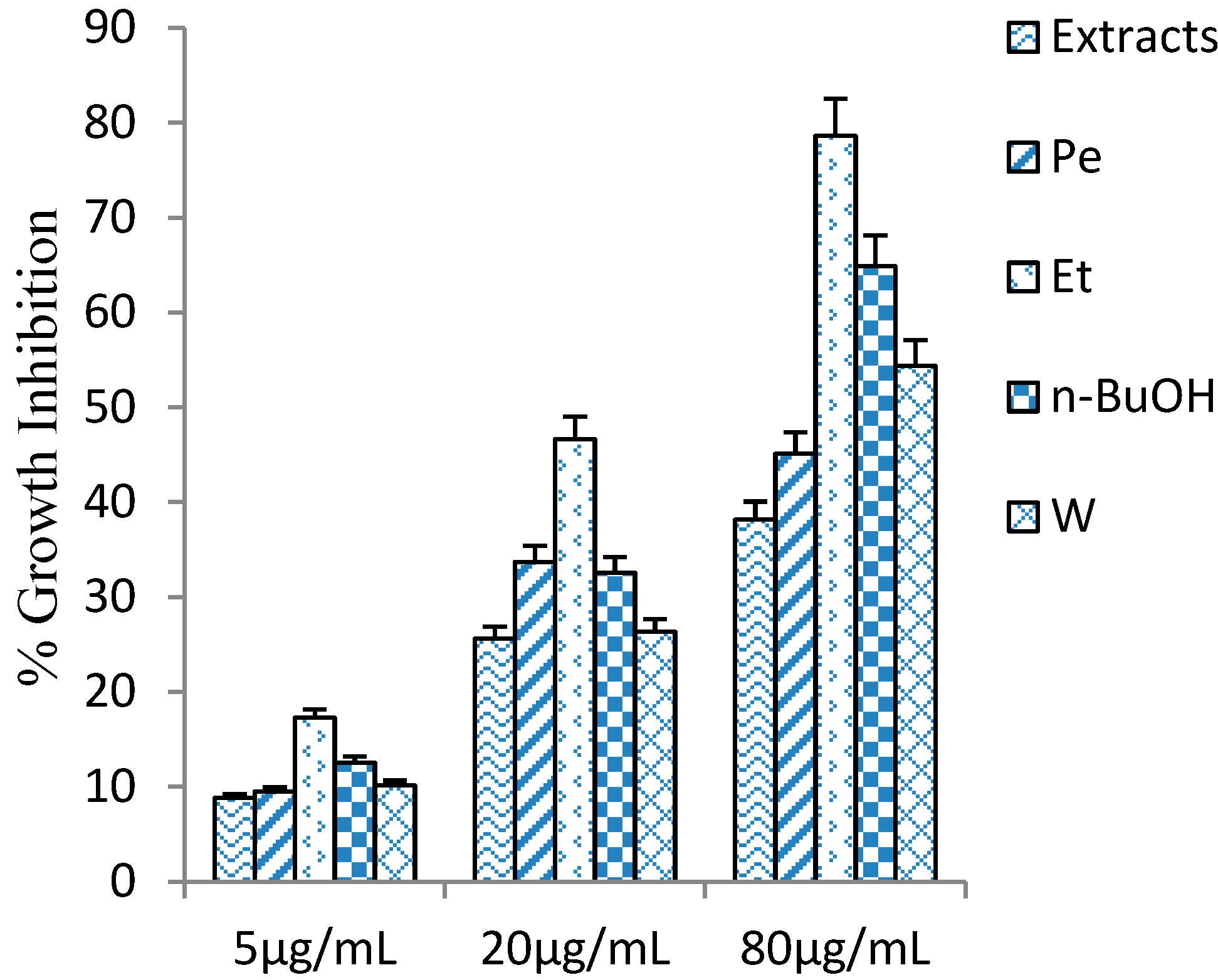
2.3. Effect on PDGF Induced Proliferation
3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Characterization of Compound 1 and Compound 2
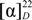 +34.7 (c 0.19, CH3OH); IR (KBr) vmax 3407.6, 1724.0, 1619.9, 1454.0, 1214.9, 1033.6, 763.7, 547.7 cm−1; 1H-NMR and 13C-NMR data, see Table 1; HRESIMS m/z 631.0901 [M+Na]+ (calcd for C22H24O17Na, 631.0906).
+34.7 (c 0.19, CH3OH); IR (KBr) vmax 3407.6, 1724.0, 1619.9, 1454.0, 1214.9, 1033.6, 763.7, 547.7 cm−1; 1H-NMR and 13C-NMR data, see Table 1; HRESIMS m/z 631.0901 [M+Na]+ (calcd for C22H24O17Na, 631.0906).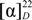 −21.7 (c 0.23, CH3OH); IR (KBr) vmax 3407.6, 1731.7, 1627.6, 1596.8, 1517.7, 1442.5, 1363.4, 1286.3, 1232.3, 1174.4, 1018.2, 962.3, 831.2, 742.5, 566.9 cm−1; 1H-NMR and 13C-NMR data, see Table 2; HRESIMS m/z 633.1083 [M+H]+ (calcd for C28H25O17H, 633.1019).
−21.7 (c 0.23, CH3OH); IR (KBr) vmax 3407.6, 1731.7, 1627.6, 1596.8, 1517.7, 1442.5, 1363.4, 1286.3, 1232.3, 1174.4, 1018.2, 962.3, 831.2, 742.5, 566.9 cm−1; 1H-NMR and 13C-NMR data, see Table 2; HRESIMS m/z 633.1083 [M+H]+ (calcd for C28H25O17H, 633.1019).3.5. Cell Culture
3.6. Cell Viability
3.7. Activation of HSC-T6 Cells Induced by PDGF
3.8. Anti-Proliferative Activity Assay
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Escarpa, A.; González, M.C. Approach to the content of total extractable phenolic compounds from different food samples by comparison of chromatographic and spectrophotometric methods. Anal. Chim. Aata 1974, 427, 119–127. [Google Scholar] [CrossRef]
- Sanli, N.; Fonrodona, G.; Barròn, D.; Özkan, G.; Barbosa, J. Prediction of chromatographic retention, pKa values and optimization of the separation of polyphenolic acids in strawberries. J. Chromatogr. A 2002, 975, 299–309. [Google Scholar] [CrossRef]
- Beltrán, J.L.; Sanli, N.; Fonrodona, G.; Barròn, D.; Özkan, G.; Barbosa, J. Spectrophotometric, potentiometric and chromatographic pKa values of polyphenolic acids in water and acetonitrile–water media. Anal. Chim. Aata 2003, 484, 253–264. [Google Scholar] [CrossRef]
- Ikeda, H.; Itoh, K. Germination and water dispersal of seeds from a threatened plant species Penthorum chinense. Ecol. Res. 2001, 16, 99–106. [Google Scholar] [CrossRef]
- Zhang, T.T.; Xu, X.L.; Jiang, M.H.; Jiang, J.G. Hepatoprotective function of Penthorum chinense pursh. Food Funct. 2013, 4, 1581–1585. [Google Scholar] [CrossRef]
- Mahesh, T.; Menon, V.P. Quercetin allievates oxidative stress in streptozotocin-induced diabetic rats. Phytother. Res. 2004, 18, 123–127. [Google Scholar] [CrossRef]
- Sigurdsson, S.; Ogmundsdottir, H.M.; Hallgrimsson, J.; Gudbjarnason, S. Antitumour activity of Angelica archangelica leaf extract. In Vivo 2005, 19, 191–194. [Google Scholar]
- Moon, Y.J.; Wang, X.D.; Morris, M.E. Dietary flavonoids: Effects on xenobiotic and carcinogen metabolism. Toxicol. In Vitro 2006, 20, 187–210. [Google Scholar] [CrossRef]
- Wang, L.Q.; Yang, J.; Deng, E.; Wang, G.B.; Peng, Z.S. Optimizing the shoot proliferation protocol of Penthorum chinense by axillary buds. Biotechnol. Lett. 2008, 30, 2199–2203. [Google Scholar] [CrossRef]
- Lu, Q.; Jiang, M.H.; Jiang, J.G.; Zhang, R.; Zhang, M.W. Isolation and identification of compounds from Penthorum chinense Pursh with antioxidant and antihepatocarcinoma properties. J. Agric. Food Chem. 2012, 60, 11097–11103. [Google Scholar] [CrossRef]
- Hideyuki, I.; Tsutomu, H.; Osamu, N.; Tadashi, S.; Takuo, O.; Takashi, Y. Modified dehydroellagitannins, geraniinic acids B and C, and phyllanthusiin F. Chem. Pharm. Bull. 1999, 47, 1148–1151. [Google Scholar]
- Huang, Y.L.; Chen, C.C.; Hsu, F.L.; Chen, C.F. Two tannins from Phyllanthus tenellus. J. Nat. Prod. 1998, 61, 523–524. [Google Scholar] [CrossRef]
- Kazunori, H.; Takao, K.; Kazuaki, N.; Yukinobu, I.; Minoru, O.; Hiroshi, M. Two glucosides from roots of Asiasarum Sieboldi. Phytochemistry 1992, 31, 2477–2480. [Google Scholar] [CrossRef]
- Ikuko, I.O.; Naomi, G.; Junichi, T.; Tatsuo, H.; Maxwell, A.G.; Yoko, A. Thonningianins A and B, new antioxidants from the African medicinal herb Thonningia sanguinea. J. Nat. Prod. 2000, 63, 676–679. [Google Scholar] [CrossRef]
- Hegde, V.R.; Pu, H.Y.; Patel, M.; Das, P.R.; Butkiewicz, N.; Arreaze, G.; Gullo, V.P.; Chan, T.M. Two antiviral compounds from the plant Stylogne cauliflora as inhibitors of HCV NS3 protease. Bioorg. Med. Chem. Lett. 2003, 13, 2925–2928. [Google Scholar]
- Wang, H.W.; Liu, Y.Q.; Feng, C.G. Isolation and identification of a novel flavonoid from Penthorum chinense P. J. Asian Nat. Prod. Res. 2010, 8, 757–761. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar]
- Bartalis, J.; Halaweish, F.T. In vitro and QSAR studies of cucurbitacins on HepG2 and HSC-T6 liver cell lines. Bioorg. Med. Chem. Lett. 2011, 19, 2757–2766. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Wang, Z.; Kwong, S.Q.; Lui, E.L.H.; Friedman, S.L.; Li, F.R.; Lam, R.W.C.; Zhang, G.C.; Zhang, H.; Ye, T. Inhibition of PDGF, TGF-β , and Abl signaling and reduction of liver fibrosis by the small molecule Bcr-Abl tyrosine kinase antagonist Nilotinb. J. Hepatol. 2011, 55, 612–625. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–8 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Huang, D.; Jiang, Y.; Chen, W.; Yao, F.; Sun, L. Polyphenols with Anti-Proliferative Activities from Penthorum Chinense Pursh. Molecules 2014, 19, 11045-11055. https://doi.org/10.3390/molecules190811045
Huang D, Jiang Y, Chen W, Yao F, Sun L. Polyphenols with Anti-Proliferative Activities from Penthorum Chinense Pursh. Molecules. 2014; 19(8):11045-11055. https://doi.org/10.3390/molecules190811045
Chicago/Turabian StyleHuang, Doudou, Yun Jiang, Wansheng Chen, Fengyan Yao, and Lianna Sun. 2014. "Polyphenols with Anti-Proliferative Activities from Penthorum Chinense Pursh" Molecules 19, no. 8: 11045-11055. https://doi.org/10.3390/molecules190811045
APA StyleHuang, D., Jiang, Y., Chen, W., Yao, F., & Sun, L. (2014). Polyphenols with Anti-Proliferative Activities from Penthorum Chinense Pursh. Molecules, 19(8), 11045-11055. https://doi.org/10.3390/molecules190811045




