Linalool Exhibits Cytotoxic Effects by Activating Antitumor Immunity
Abstract
:1. Introduction
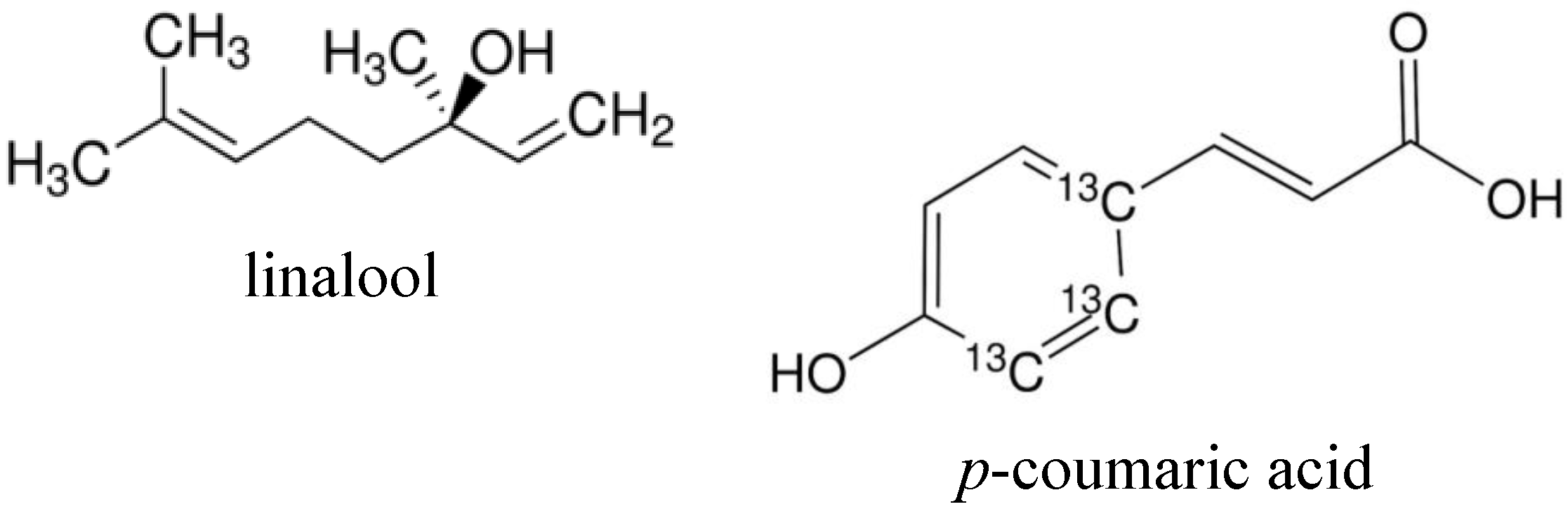
2. Results and Discussion
2.1. WST-1 Assay Analysis of the Suppression of Cancer Cell Growth with Constituents
| Cell Line | 50% Inhibitive Dose (IC50: μM) | ||
|---|---|---|---|
| 5-Fluorouracil | Linalool | p-Coumaric Acid | |
| SW 620 | 78 ± 6.60 | 222 ± 5.44 | 87 ± 6.33 |
| Hep G2 | 278 ± 19.40 | 290 ± 5.31 | 215 ± 6.58 * |
| A549 | 45 ± 2.50 | 438 ± 6.48 | 412 ± 5.50 |
| T-47D | 648 ± 36.20 | 224 ± 4.40 ** | 474 ± 12.10 * |
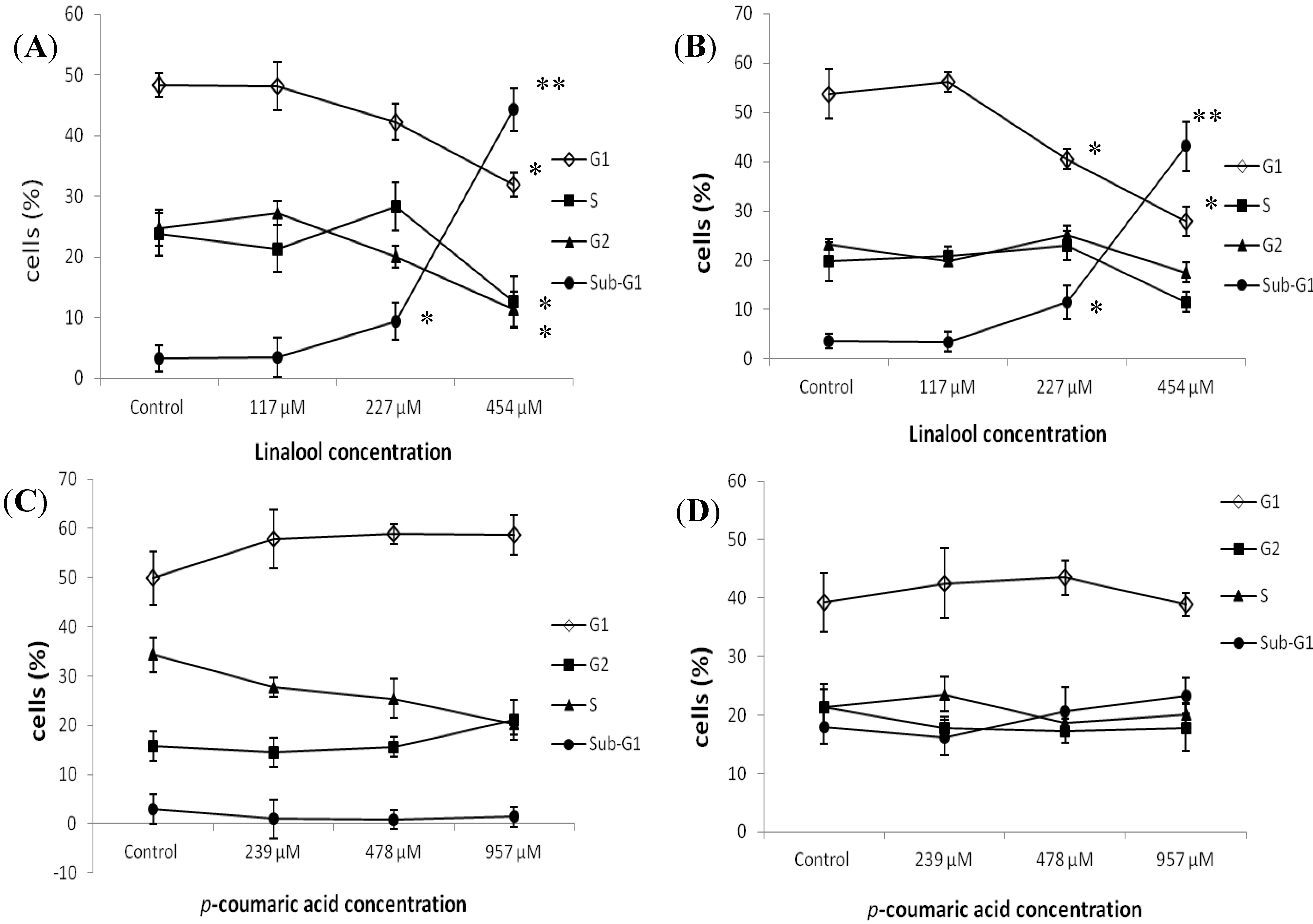
2.2. Flow Cytometry Analysis of the Effects of Constituents on Cell Cycle Distribution
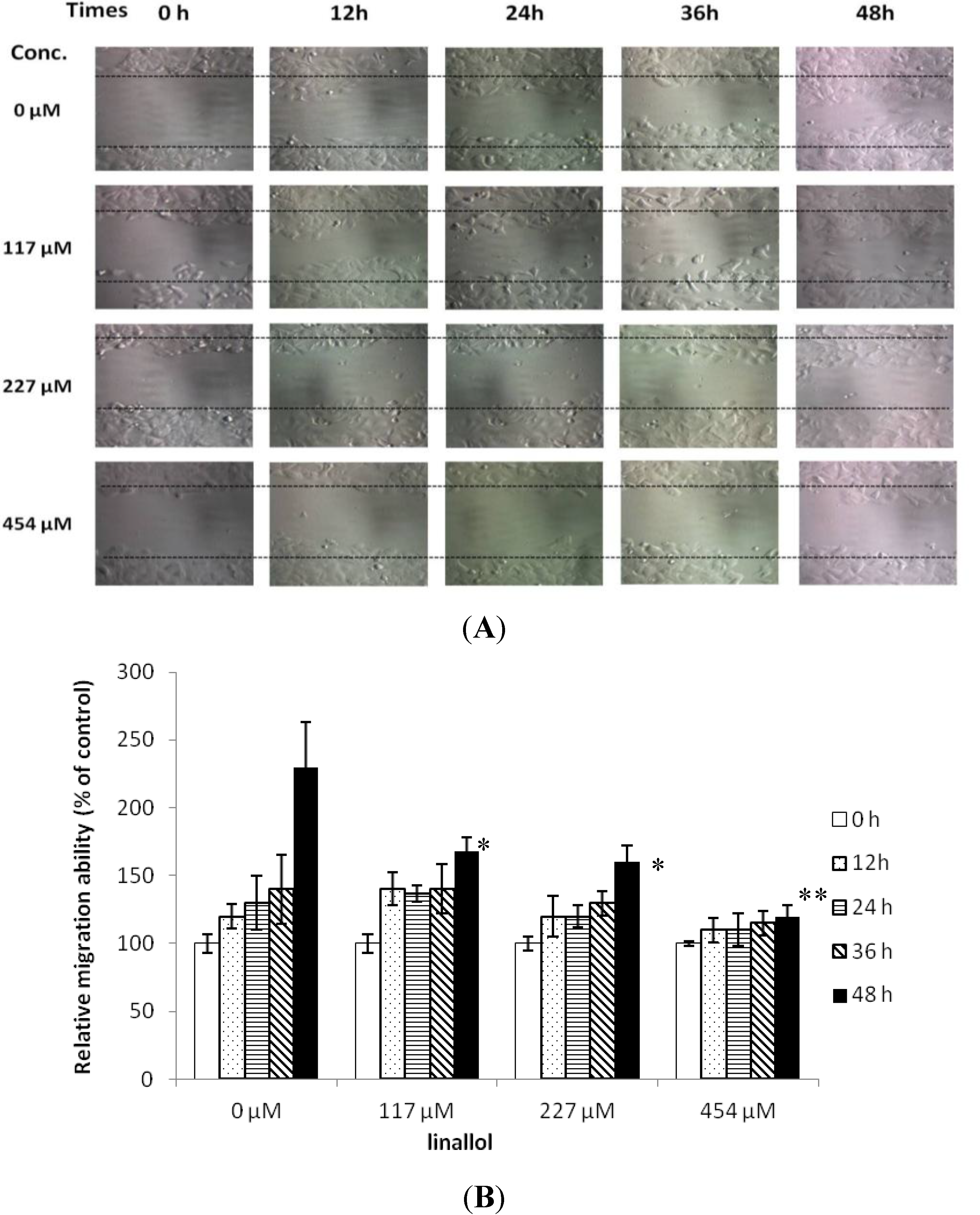
2.3. Cell Migrations of Ductal Breast Epithelial Tumor by Constituents
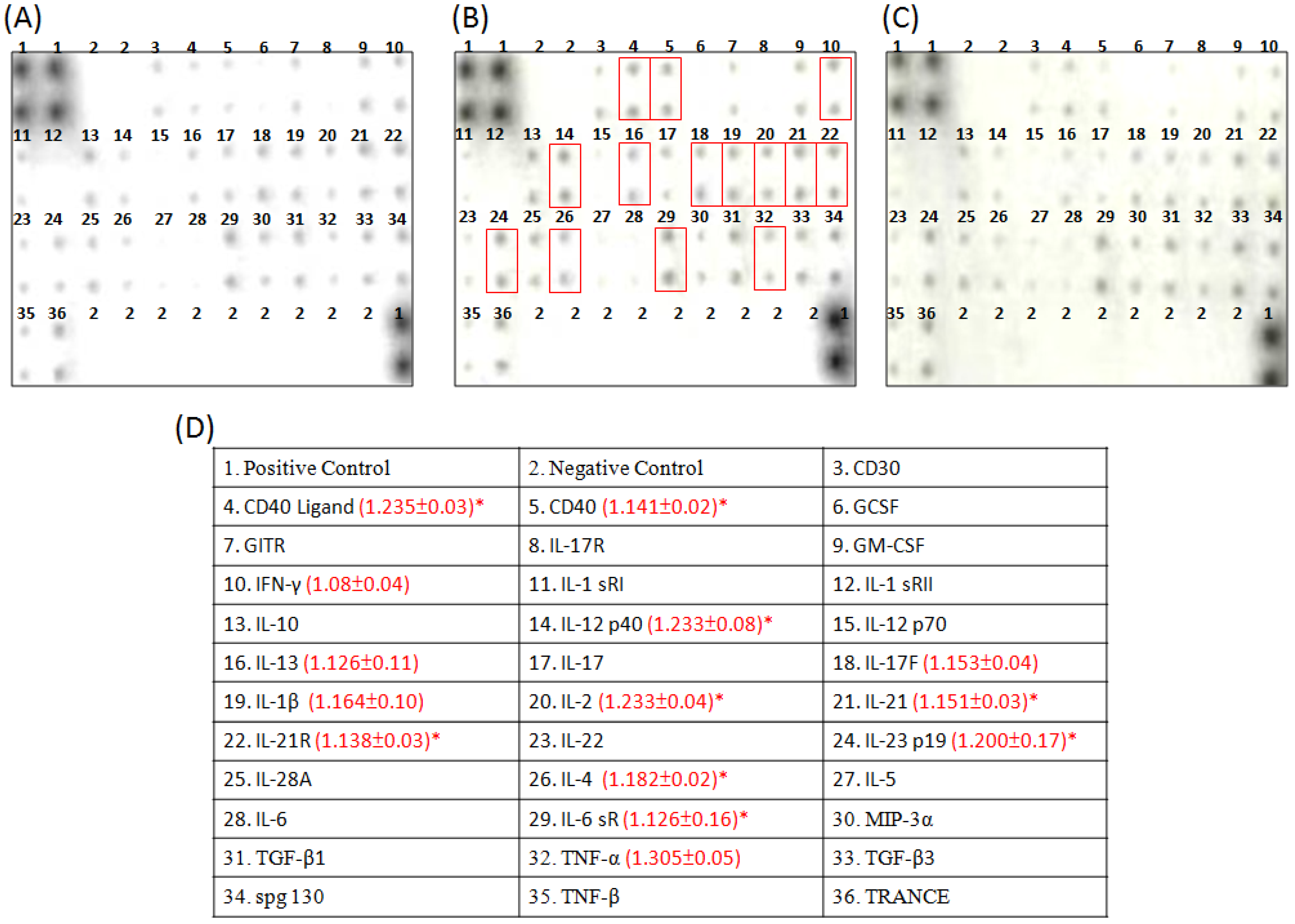
2.4. Cytokine Array Analysis of the Effects of Constituents on the Cytokine Secretion
3. Experimental Section
3.1. Cytotoxic Effects of Constituents
- ODC: The light absorption rate of the control group without test drug
- ODT: The light absorption rate of the various experimental groups administered with varying concentrations of the test drug
- IC50: Concentrations were compared and tested to achieve the optimal concentration for a 50.0% cell death rate.
3.2. Flow Cytometry Analysis
3.3. Wound Healing Migration Assays
3.4. Separate Lymphocytes in Peripheral Blood in Humans
3.5. Cytokine Antibody Array Assay
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interests
References
- Ministry of Health and Welfare. Available online: http://www.mohw.gov.tw (accessed on 9 December 2013).
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA: Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- Viel, S.; Charrier, E.; Marcais, A.; Rouzaire, P.; Bienvenu, J.; Karlin, L.; Salles, G.; Walzer, T. Monitoring NK cell activity in patients with hematological malignancies. Oncoimmunology 2013, 2, e26011. [Google Scholar] [CrossRef]
- Moll, M.; Kuemmerle-Deschner, J.B. Inflammasome and cytokine blocking strategies in autoinflammatory disorders. Clin. Immunol. 2013, 147, 242–275. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Wijeratne, E.K.; Liang, J.Y.; Gunatilaka, A.L.; Wu, J.M. Differential control of growth, cell cycle progression, and expression of NF-kappaB in human breast cancer cells MCF-7, MCF-10A, and MDA-MB-231 by ponicidin and oridonin, diterpenoids from the chinese herb Rabdosia rubescens. Biochem. Biophy. Res. Commun. 2005, 337, 224–231. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Chen, C.Y.; Lin, I.P.; Tsai, E.M.; Kuo, P.L.; Hou, M.F. 4-Shogaol, an active constituent of dietary ginger, inhibits metastasis of MDA-MB-231 human breast adenocarcinoma cells by decreasing the repression of NF-kappaB/Snail on RKIP. J. Agric. Food Chem. 2012, 60, 852–861. [Google Scholar]
- Sakurai, M.H.; Matsumoto, T.; Kiyohara, H.; Yamada, H. B-cell proliferation activity of pectic polysaccharide from a medicinal herb, the roots of Bupleurum falcatum L. and its structural requirement. Immunology 1999, 97, 540–547. [Google Scholar] [CrossRef]
- Sultana, N.; Saify, Z.S. Naturally occurring and synthetic agents as potential anti-inflammatory and immunomodulants. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2012, 11, 3–19. [Google Scholar] [CrossRef]
- Liu, J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar] [CrossRef]
- Inada, A.; Nakanishi, T.; Tokuda, H.; Nishino, H.; Iwashima, A.; Sharma, O.P. Inhibitory effects of lantadenes and related triterpenoids on Epstein-Barr virus activation. Planta Med. 1995, 61, 558–559. [Google Scholar] [CrossRef]
- Constantinou, A.; Mehta, R.; Runyan, C.; Rao, K.; Vaughan, A.; Moon, R. Flavonoids as DNA topoisomerase antagonists and poisons: Structure-activity relationships. J. Nat. Prod. 1995, 58, 217–225. [Google Scholar]
- Yu, C.; Zhang, Z.; Zhang, H.; Zhen, Z.; Calway, T.; Wang, Y.; Yuan, C.S.; Wang, C.Z. Pretreatment of baicalin and wogonoside with glycoside hydrolase: A promising approach to enhance anticancer potential. Oncol. Rep. 2013, 30, 2411–2418. [Google Scholar]
- Nishioka, T.; Kawabata, J.; Aoyama, Y. Baicalein, an alpha-glucosidase inhibitor from Scutellaria baicalensis. J. Nat. Prod. 1998, 61, 1413–1415. [Google Scholar] [CrossRef]
- Ortiz de Urbina, A.V.; Martin, M.L.; Fernandez, B.; San Roman, L.; Cubillo, L. In vitro antispasmodic activity of peracetylated penstemonoside, aucubin and catalpol. Planta Med. 1994, 60, 512–515. [Google Scholar] [CrossRef]
- Li, W.J.; Lin, Y.C.; Wu, P.F.; Wen, Z.H.; Liu, P.L.; Chen, C.Y.; Wang, H.M. Biofunctional Constituents from Liriodendron tulipifera with Antioxidants and Anti-Melanogenic Properties. Int. J. Mol. Sci. 2013, 14, 1698–1712. [Google Scholar] [CrossRef]
- Wang, H.M.; Chiu, C.C.; Wu, P.F.; Chen, C.Y. Subamolide E from Cinnamomum subavenium induces sub-G1 cell-cycle arrest and caspase-dependent apoptosis and reduces the migration ability of human melanoma cells. J. Agric. Food Chem. 2011, 59, 8187–8192. [Google Scholar] [CrossRef]
- Cassady, K.A.; Whitley, R.J. New therapeutic approaches to the alphaherpesvirus infections. J. Antimicrob. Chemother. 1997, 39, 119–128. [Google Scholar] [CrossRef]
- Shu, Y.Z. Recent natural products based drug development: A pharmaceutical industry perspective. J. Nat. Prod. 1998, 61, 1053–1071. [Google Scholar] [CrossRef]
- Chiang, L.C.; Chiang, W.; Chang, M.Y.; Ng, L.T.; Lin, C.C. Antiviral activity of Plantago major extracts and related compounds in vitro. Antivir. Res. 2002, 55, 53–62. [Google Scholar] [CrossRef]
- Chiang, L.C.; Chiang, W.; Chang, M.Y.; Lin, C.C. In vitro cytotoxic, antiviral and immunomodulatory effects of Plantago major and Plantago asiatica. Am. J. Chin. Med. 2003, 31, 225–234. [Google Scholar]
- Chiang, L.C.; Chiang, W.; Chang, M.Y.; Ng, L.T.; Lin, C.C. Antileukemic activity of selected natural products in Taiwan. Am. J. Chin. Med. 2003, 31, 37–46. [Google Scholar] [CrossRef]
- Chiang, L.C.; Ng, L.T.; Chiang, W.; Chang, M.Y.; Lin, C.C. Immunomodulatory activities of flavonoids, monoterpenoids, triterpenoids, iridoid glycosides and phenolic compounds of Plantago species. Planta Med. 2003, 69, 600–604. [Google Scholar] [CrossRef]
- Ju, J.; Qi, Z.; Cai, X.; Cao, P.; Liu, N.; Wang, S.; Chen, Y. Toosendanin induces apoptosis through suppression of JNK signaling pathway in HL-60 cells. Toxicol. In Vitro 2013, 27, 232–238. [Google Scholar] [CrossRef]
- Statz, D.; Coon, F.B. Preparation of plant extracts for antitumor screening. Cancer Treat. Rep. 1976, 60, 999–1005. [Google Scholar]
- Vinson, J.A.; Jang, J. In vitro and in vivo lipoprotein antioxidant effect of a citrus extract and ascorbic acid on normal and hypercholesterolemic human subjects. J. Med. Food 2001, 4, 187–192. [Google Scholar] [CrossRef]
- Wu, Y.B.; Zheng, L.J.; Wu, J.G.; Chen, T.Q.; Yi, J.; Wu, J.Z. Antioxidant activities of extract and fractions from receptaculum nelumbinis and related flavonol glycosides. Int. J. Mol. Sci. 2012, 13, 7163–7173. [Google Scholar] [CrossRef]
- Yiu, C.Y.; Chen, S.Y.; Yang, T.H.; Chang, C.J.; Yeh, D.B.; Chen, Y.J.; Lin, T.P. Inhibition of Epstein-Barr Virus Lytic Cycle by an Ethyl Acetate Subfraction Separated from Polygonum cuspidatum Root and Its Major Component, Emodin. Molecules 2014, 19, 1258–1272. [Google Scholar] [CrossRef]
- Lapczynski, A.; Letizia, C.S.; Api, A.M. Addendum to Fragrance material review on linalool. Food Chem. Toxicol. 2008, 46, S190–S192. [Google Scholar] [CrossRef]
- Carson, C.F.; Riley, T.V. Antimicrobial activity of the major components of the essential oil of Melaleuca alternifolia. J. Appl. Bacteriol. 1995, 78, 264–269. [Google Scholar] [CrossRef]
- Deepa, B.; Venkatraman Anuradha, C. Effects of linalool on inflammation, matrix accumulation and podocyte loss in kidney of streptozotocin-induced diabetic rats. Toxicol. Mech. Methods 2013, 23, 223–234. [Google Scholar] [CrossRef]
- Anjos, P.J.; Lima, A.O.; Cunha, P.S.; de Sousa, D.P.; Onofre, A.S.; Ribeiro, T.P.; Medeiros, I.A.; Antoniolli, A.R.; Quintans-Junior, L.J.; Santosa, M.R. Cardiovascular effects induced by linalool in normotensive and hypertensive rats. Z. Naturforsch. C 2013, 68, 181–190. [Google Scholar] [CrossRef]
- Peana, A.T.; de Montis, M.G.; Nieddu, E.; Spano, M.T.; D’Aquila, P.S.; Pippia, P. Profile of spinal and supra-spinal antinociception of (−)-linalool. Eur. J. Pharmacol. 2004, 485, 165–174. [Google Scholar] [CrossRef]
- Zhu, Z.; Sun, H.; Ma, G.; Wang, Z.; Li, E.; Liu, Y.; Liu, Y. Bufalin Induces Lung Cancer Cell Apoptosis via the Inhibition of PI3K/Akt Pathway. Int. J. Mol. Sci. 2012, 13, 2025–2035. [Google Scholar] [CrossRef]
- Park, H.S.; Hwang, H.J.; Kim, G.Y.; Cha, H.J.; Kim, W.J.; Kim, N.D.; Yoo, Y.H.; Choi, Y.H. Induction of apoptosis by fucoidan in human leukemia U937 cells through activation of p38 MAPK and modulation of Bcl-2 family. Mar. Drugs 2013, 11, 2347–2364. [Google Scholar] [CrossRef]
- Kong, C.S.; Jeong, C.H.; Choi, J.S.; Kim, K.J.; Jeong, J.W. Antiangiogenic effects of p-coumaric acid in human endothelial cells. Phytother. Res. 2013, 27, 317–323. [Google Scholar] [CrossRef]
- Yen, G.C.; Chen, Y.C.; Chang, W.T.; Hsu, C.L. Effects of polyphenolic compounds on tumor necrosis factor-alpha (TNF-alpha)-induced changes of adipokines and oxidative stress in 3T3-L1 adipocytes. J. Agric. Food Chem. 2011, 59, 546–551. [Google Scholar] [CrossRef]
- Janicke, B.; Hegardt, C.; Krogh, M.; Onning, G.; Akesson, B.; Cirenajwis, H.M.; Oredsson, S.M. The antiproliferative effect of dietary fiber phenolic compounds ferulic acid and p-coumaric acid on the cell cycle of Caco-2 cells. Nutr. Cancer 2011, 63, 611–622. [Google Scholar] [CrossRef]
- Scheepens, A.; Bisson, J.F.; Skinner, M. p-coumaric acid activates the GABA-A receptor in vitro and is orally anxiolytic in vivo. Phytother. Res. 2014, 28, 207–211. [Google Scholar] [CrossRef]
- Meltzer, M.S.; Benjamin, W.R.; Farrar, J.J. Macrophage activation for tumor cytotoxicity: Induction of macrophage tumoricidal activity by lymphokines from EL-4, a continuous T cell line. J. Immunol. 1982, 129, 2802–2807. [Google Scholar]
- Porakishvili, N.; Jackson, A.M.; de Souza, J.B.; Dalla Chiesa, M.; Roitt, I.M.; Delves, P.J.; Lund, T. Epitopes of human chorionic gonadotropin and their relationship to immunogenicity and cross-reactivity of beta-chain mutants. Am. J. Reprod. Immunol. 1998, 40, 210–214. [Google Scholar] [CrossRef]
- Zhu, R.; Zou, S.T.; Wan, J.M.; Li, W.; Li, X.L.; Zhu, W. BTG1 inhibits breast cancer cell growth through induction of cell cycle arrest and apoptosis. Oncol. Rep. 2013, 30, 2137–2144. [Google Scholar]
- CSElab. Available online: http://www.cse-lab.ethz.ch/software.html (aceessed on 22 May 2014).
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chang, M.-Y.; Shen, Y.-L. Linalool Exhibits Cytotoxic Effects by Activating Antitumor Immunity. Molecules 2014, 19, 6694-6706. https://doi.org/10.3390/molecules19056694
Chang M-Y, Shen Y-L. Linalool Exhibits Cytotoxic Effects by Activating Antitumor Immunity. Molecules. 2014; 19(5):6694-6706. https://doi.org/10.3390/molecules19056694
Chicago/Turabian StyleChang, Mei-Yin, and Yi-Ling Shen. 2014. "Linalool Exhibits Cytotoxic Effects by Activating Antitumor Immunity" Molecules 19, no. 5: 6694-6706. https://doi.org/10.3390/molecules19056694
APA StyleChang, M.-Y., & Shen, Y.-L. (2014). Linalool Exhibits Cytotoxic Effects by Activating Antitumor Immunity. Molecules, 19(5), 6694-6706. https://doi.org/10.3390/molecules19056694




