Abstract
A new photoisomeric nucleoside dUAz bearing an azobenzene group at the C5-position of 2'-deoxyuridine was designed and synthesized. Photoisomerization of dUAz in oligodeoxynucleotides can be achieved rapidly and selectively with 365 nm (forward) and 450 nm (backward) irradiation. Thermal denaturation experiments revealed that dUAz stabilized the duplex in the cis-form and destabilized it in the trans-form with mismatch discrimination ability comparable to thymidine. These results indicate that dUAz could be a powerful material for reversibly manipulating nucleic acid hybridization with spatiotemporal control.
1. Introduction
Regulation of nucleic acid hybridization by some external stimuli is a rewarding challenge due to its potential to control gene expression flow from DNA to protein at a predetermined place and time. This technique could allow for spatiotemporal controllable pharmacotherapy based on nucleic acid agents. The regulation of nucleic acid hybridization is also important in the field of nanotechnology, such as in the construction of DNA-origami [1,2,3]. Modified oligonucleotides (ONs) that can reversibly alter the hybridization ability by noninvasive external stimuli are therefore necessary. The most promising external stimulus is light, due to the possibility of accurately controlling the location, dosage and time of the irradiation. For example, Asanuma et al. have reported reversible photoregulation of DNA duplex formation via installation of azobenzene moieties on ONs [4,5]. Azobenzene and its derivatives are commonly adopted due to their rapid photoisomerization and drastic changes in geometry and dipole moment [6,7].
In this study, we describe a new type of azobenzene-modified nucleoside that reversibly changes its properties upon photoisomerization by ultraviolet (365 nm) or visible light (450 nm). There are several positions to attach a photochromic moiety to a nucleoside, and we have selected the C5 position of 2'-deoxyuridine (dUAz, Figure 1) [8]. It is predicted that the azobenzene moiety of dUAz is projected into the major groove of the double helix via a rigid ethynyl linker. We assumed that the duplexes containing trans-dUAz would be destabilized because the hydrophobic azobenzene moiety extends to the outside of the groove [9] which surrounded by a highly polar aqueous phase, and interferes with hydration and the formation of interstrand cation bridges to stabilize the duplexes [10,11]. Meanwhile, cis-dUAz-modification would not affect the duplex stability due to compact conformation of the azobenzene moiety. In other words, the affinity of ONs containing dUAz for complementary single-stranded DNA or RNA may be reversibly changed, triggered by light.
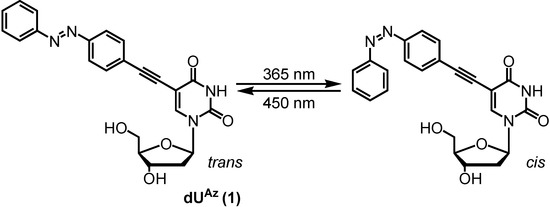
Figure 1.
Photoisomeric nucleoside used in this study.
2. Results and Discussion
2.1. Synthesis of dUAz Phosphoramidite and dUAz-Modified Oligodeoxynucleotides
The synthetic route of dUAz phosphoramidite is outlined in Scheme 1. dUAz nucleoside 1 was synthesized from the corresponding 2'-deoxy-5-iodouridine (2) through a palladium-catalyzed cross-coupling reaction [12] with 4-ethynylazobenzene 3[13]. Tritylation at the primary hydroxyl group of 1 with DMTrCl and phosphitylation at the secondary hydroxyl group yielded phosphoramidite 5. The amidite 5 was incorporated into the oligodeoxynucleotide using conventional solid-phase phosphoramidite synthesis and purified by reverse-phase HPLC (29% yield). The ON sequences used in this study are shown in Table 1.
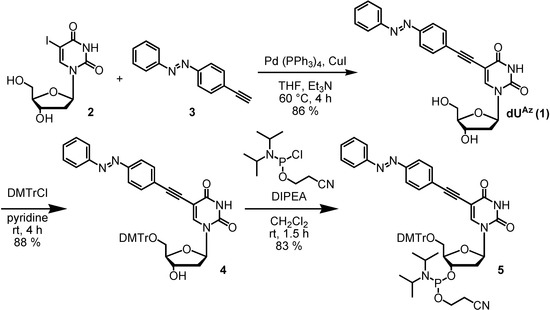
Scheme 1.
Route for the synthesis of dUAz phosphoramidite.

Table 1.
The oligonucleotides used in this study.
| ON | Sequence | |
|---|---|---|
| 6 | 5'-d(GCGTTTTTTGCT)-3' | control DNA |
| 7 | 5'-d(GCGTTUAzTTTGCT)-3' | dUAz-modified DNA |
| 8 | 5'-d(AGCAAAAAACGC)-3' | full match DNA |
| 9 | 5'-d(AGCAAATAACGC)-3' | mismatch DNA (T) |
| 10 | 5'-d(AGCAAACAACGC)-3' | mismatch DNA (C) |
| 11 | 5'-d(AGCAAAGAACGC)-3' | mismatch DNA (G) |
| 12 | 5'-r(AGCAAAAAACGC)-3' | full match RNA |
| 13 | 5'-r(AGCAAAUAACGC)-3' | mismatch RNA (U) |
| 14 | 5'-r(AGCAAACAACGC)-3' | mismatch RNA (C) |
| 15 | 5'-r(AGCAAAGAACGC)-3' | mismatch RNA (G) |
2.2. Photoisomerization Property of dUAz
We initially investigated the efficiency of the dUAz cis-trans photoisomerization property in ON by UV spectra and HPLC analysis. UV spectra of trans/cis ON 7, showed that photoisomerization of trans-dUAz to cis-dUAz decreased absorbance at 365 nm and increased absorbance at 310 nm and 450 nm (Figure 2a). The λmax of cis-form (340 nm) was blue-shifted compared to that of the trans-form (365 nm), as was the case with previous reports [6,7,14]. The trans-form dUAz was photoisomerized to the cis-form by a 10-second irradiation of 365 nm monochromic light with 60% conversion, as determined by the HPLC peak areas (Figure 2b). In addition, subsequent 10-second irradiation of 450 nm yielded the trans form isomer with 80%. The HPLC analysis showed no side products from the reactions.
Even when the photoirradiation was repeated three times, the efficiency of the dUAz cis-trans photoisomerization was not attenuated (Figure 2c). It can therefore be concluded that dUAz has a rapid and highly efficient cis-trans photoisomerization property and the potential to work as a photo-switch for various biomolecules.
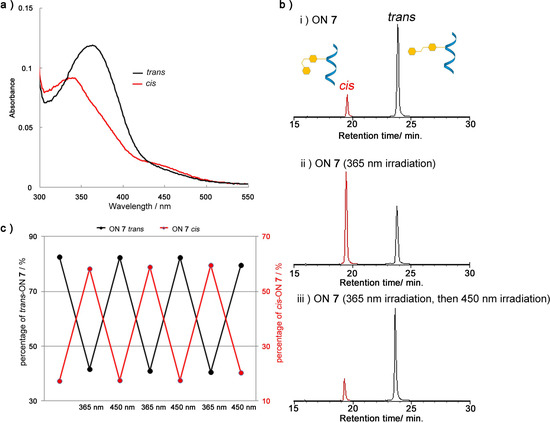
Figure 2.
Photoisomerization properties of dUAz in oligodeoxynucleotide. (a) Absorbance spectra of trans- (black line) and cis- (red line) ON 7. (b) HPLC analysis of the photoisomerization of ON 7; (i) Before irradiation; (ii) after 365 nm irradiation for 10 s; (iii) subsequent irradiation at 450 nm, 10 s. (c) Repetitive photoisomerization of ON 7 induced by alternative light irradiation at 365 nm and 450 nm. The percentages of trans- (black line) and cis- (red line) ON 7 obtained from the HPLC peak areas are shown. Conditions: ON 7 (4.0 µM), NaCl (100mM) in sodium phosphate buffer (10 mM, pH 7.0) was irradiated at room temperature.
We investigated the differences in the thermal stability of 12-bp duplexes containing dUAz in the trans- and cis-forms by monitoring the melting temperature (Tm) following the way of azobenzene- modified nucleoside containing ONs (Table 2) [15,16]. DNA duplex 7/8 showed a modest Tm difference (ΔTm) between the trans- and cis-forms, namely, the Tm value of the cis-form was 2 °C higher than that of the trans-form. On the other hand, the ON 7/RNA 12 duplex showed a larger Tm difference. The Tm value of the cis-form was 5 °C higher than that of the trans-form. It is noteworthy that the cis-ON 7/RNA 12 duplex showed a Tm value comparable to that of natural DNA 6/RNA 12 duplex. According to past studies, the cis-form photochromic moieties generically destabilize the duplex because of its interference with the vicinity bases stacking interaction [4,5,17,18,19].In this study, ON containing dUAz showed a higher hybridization ability when dUAz is cis-form rather than trans-form, unlike ONs containing the exiting photochromic nucleoside. Brown et al. have reported that hydrophobic buta-1,3-diynyl anthracene in ON leads to significant destabilization of the duplex, probably because the aromatic moiety is exposed to the aqueous environment [9]. The azobenzene moiety of trans-dUAz also would extend to the outside of the major groove, a highly polar aqueous phase. This may have an impact on the groove hydration and the formation of interstrand cation bridges, and lead to destabilization of the duplex containing trans-dUAz.

Table 2.
UV-melting points of 12-bp duplexes. a
| Duplex | Tm [°C] | ΔTm [°C] b (Tm cis - Tm trans) | ||
|---|---|---|---|---|
| trans c | cis d | |||
| 6/8 | 52 | - | ||
| 7/8 | 47 | 49 | 2 | |
| 6/12 | 47 | |||
| 7/12 | 42 | 47 | 5 | |
a All Tm values for the duplexes (4.0 µM) were determined in 10 mM sodium phosphate buffer (pH 7.0) containing 100 mM NaCl. The Tm values given are the average of at least three data points; b The change in the Tm value induced by the cis-trans photoisomerization; c The percentage of trans isomer was ca. 80%; d The percentage of cis isomer was ca. 60%.
Finally, we investigated the mismatch discrimination ability of ON containing dUAz. The Tm values of mismatched DNA duplexes containing dUAz were found to be 14 or 15 °C lower than that of ON7/DNA8 in both trans- and cis-form (Table 3). Toward complementary ssRNA, ON containing dUAz could also discriminate mismatched bases comparable to ON7 (Table S1 in Supplementary Material). These results indicate that the mismatch discrimination ability of ON containing trans-/cis-dUAz is not spoiled by the C5-substituted-azobenzene moiety of dUAz.

Table 3.
UV-melting points of DNA duplexes with a mismatched base pair. a
| Duplex | Base pair | Tm [°C] | ΔTm [°C] b | ||
|---|---|---|---|---|---|
| trans c | cis d | trans c | cis d | ||
| 6/9 | T:T | 40 | −12 | ||
| 6/10 | T:C | 37 | −15 | ||
| 6/11 | T:G | 41 | −11 | ||
| 7/9 | UAz:T | 33 | 35 | −14 | −14 |
| 7/10 | UAz:C | 33 | 34 | −14 | −15 |
| 7/11 | UAz:G | 33 | 35 | −14 | −14 |
a All Tm values for the duplexes (4.0 µM) were determined in 10 mM sodium phosphate buffer (pH 7.0) containing 100 mM NaCl. The Tm values given are the average of at least three data points; b ΔTm values are calculated relative to the Tm values of matched DNA 6/DNA 8 (52 °C) or ON 7/DNA 8 (47 °C for trans and 49 °C for cis) duplexes.; c The percentage of trans isomer was ca. 80%; d The percentage of cis isomer was ca. 60%.
We achieved synthesis of the photoisomeric nucleoside, dUAz, for which the hybridization can be controlled by using different wavelengths of light. The ΔTm value between the trans- and cis-form is more remarkable in the DNA/RNA duplex than the DNA duplex. Although dUAz photoisomerization induced modest Tm differences, the modification of ONs with multiple dUAz units or the introduction of substituents to the azobenzene moiety [20] could enhance the ΔTm value between the trans- and cis-forms. Our strategy indicated the possibility of photo-switches based on dUAz-modified ONs for the development of unique molecular machines and the control of various biological phenomena.
3. Experimental
3.1. General
Reagents and solvents were purchased from commercial suppliers and were used without purification unless otherwise specified. All experiments involving air and/or moisture-sensitive compounds were carried out under N2 or Ar atmosphere. All reactions were monitored with analytical TLC (Merck Kieselgel 60 F254). Column chromatography was carried out with a Fuji Silysia FL-100D. Physical data were measured as follows: NMR spectra were recorded on a JEOL JNM-ECS-500 spectrometer in CDCl3 or DMSO-d6 as the solvent with tetramethylsilane as an internal standard. IR spectra were recorded on a JASCO FT/IR-4200 spectrometer. Optical rotations were recorded on a JASCO P-2200 instrument. FAB mass spectra were measured on a JEOL JMS-700 mass spectrometer.
3.2. Preparation of 5-(4-Phenyldiazenylphenyl)ethynyl-2'-deoxyuridine (1)
Under an argon atmosphere, 4-ethynylazobenzene (3 [13], 1.06 g, 5.12 mmol), Pd(PPh3)4 (592 mg, 0.512 mmol), and CuI (113 mg,0.512 mmol) was dissolved in dry DMF (50 mL). Then, Et3N (3.6 mL) and 2'-deoxy-5-iodouridine (2, 1.81 g, 5.12 mmol) were added. The reaction mixture was stirred at 60 °C for 4 h. The resultant mixture was filtered over Celite. The filtrate was concentrated in vacuo. The residue was purified by silica gel column chromatography and eluted with CHCl3/MeOH (20:1), to give compound 1 (1.80 g, 81%) as a light-orange powder: M.p. 208–210 °C; IR (KBr): ν 3439 (NH, OH), 1617 (C=O), 1289 (N=N) cm−1; 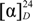 −3.7 (c 1.00, DMSO); 1H-NMR (500 MHz, DMSO-d6): δ 11.7 (1H, brs, NH), 8.47 (1H, s, H-6), 7.94–7.90 (4H , m), 7.69–7.57 (5H, m), 6.14 (1H, t, J = 6.5 Hz, H-1'), 5.27 (1H, d, J = 4.0 Hz, H-3'), 5.20 (1H, t, J = 5.0 Hz, C-H4'), 4.30–4.26 (1H, m, OH), 3.82 (1H, m, OH), 3.71–3.58 (2H, m, H-5'), 2.21–2.17 (2H, m, H-2'); 13C-NMR (125 MHz, DMSO-d6): δ 161.3, 151.9, 151.0, 149.4, 132.2, 131.8, 129.5, 125.4, 122.9, 122.6, 97.8, 91.5, 87.6, 85.6, 84.9, 69.8, 60.8, 40.2; FAB-LRMS m/z = 433 (MH+); FAB-HRMS calcd for C23H21N4O5 433.1506, found 433.1524.
−3.7 (c 1.00, DMSO); 1H-NMR (500 MHz, DMSO-d6): δ 11.7 (1H, brs, NH), 8.47 (1H, s, H-6), 7.94–7.90 (4H , m), 7.69–7.57 (5H, m), 6.14 (1H, t, J = 6.5 Hz, H-1'), 5.27 (1H, d, J = 4.0 Hz, H-3'), 5.20 (1H, t, J = 5.0 Hz, C-H4'), 4.30–4.26 (1H, m, OH), 3.82 (1H, m, OH), 3.71–3.58 (2H, m, H-5'), 2.21–2.17 (2H, m, H-2'); 13C-NMR (125 MHz, DMSO-d6): δ 161.3, 151.9, 151.0, 149.4, 132.2, 131.8, 129.5, 125.4, 122.9, 122.6, 97.8, 91.5, 87.6, 85.6, 84.9, 69.8, 60.8, 40.2; FAB-LRMS m/z = 433 (MH+); FAB-HRMS calcd for C23H21N4O5 433.1506, found 433.1524.
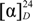 −3.7 (c 1.00, DMSO); 1H-NMR (500 MHz, DMSO-d6): δ 11.7 (1H, brs, NH), 8.47 (1H, s, H-6), 7.94–7.90 (4H , m), 7.69–7.57 (5H, m), 6.14 (1H, t, J = 6.5 Hz, H-1'), 5.27 (1H, d, J = 4.0 Hz, H-3'), 5.20 (1H, t, J = 5.0 Hz, C-H4'), 4.30–4.26 (1H, m, OH), 3.82 (1H, m, OH), 3.71–3.58 (2H, m, H-5'), 2.21–2.17 (2H, m, H-2'); 13C-NMR (125 MHz, DMSO-d6): δ 161.3, 151.9, 151.0, 149.4, 132.2, 131.8, 129.5, 125.4, 122.9, 122.6, 97.8, 91.5, 87.6, 85.6, 84.9, 69.8, 60.8, 40.2; FAB-LRMS m/z = 433 (MH+); FAB-HRMS calcd for C23H21N4O5 433.1506, found 433.1524.
−3.7 (c 1.00, DMSO); 1H-NMR (500 MHz, DMSO-d6): δ 11.7 (1H, brs, NH), 8.47 (1H, s, H-6), 7.94–7.90 (4H , m), 7.69–7.57 (5H, m), 6.14 (1H, t, J = 6.5 Hz, H-1'), 5.27 (1H, d, J = 4.0 Hz, H-3'), 5.20 (1H, t, J = 5.0 Hz, C-H4'), 4.30–4.26 (1H, m, OH), 3.82 (1H, m, OH), 3.71–3.58 (2H, m, H-5'), 2.21–2.17 (2H, m, H-2'); 13C-NMR (125 MHz, DMSO-d6): δ 161.3, 151.9, 151.0, 149.4, 132.2, 131.8, 129.5, 125.4, 122.9, 122.6, 97.8, 91.5, 87.6, 85.6, 84.9, 69.8, 60.8, 40.2; FAB-LRMS m/z = 433 (MH+); FAB-HRMS calcd for C23H21N4O5 433.1506, found 433.1524.3.3. Preparation of 5'-O-(4,4'-Dimethoxytrityl)-5-(4-phenyldiazenylphenyl)ethynyl-2'-deoxyuridine (4)
To a solution of compound 1 (141 mg, 0.324 mmol) in dry pyridine (3 mL) was added DMTrCl (131 mg, 0.389 mmol) at room temperature, and the reaction mixture was stirred for 4 h. The reaction was quenched by the addition of MeOH with 10 min stirring. The solvent was removed in vacuo, and the residue was partitioned between CHCl3 and H2O. The separated organic layer was washed with H2O, followed by brine. The organic layer was dried (Na2SO4) and concentrated in vacuo. The residue was purified by silica gel column chromatography and eluted with CHCl3/MeOH (20:1 with 0.5% Et3N) to give Compound 4 (239 mg, 88%) as an orange foam: IR (KBr): ν 3437, 3410(NH, OH), 1701 (C=O), 1272 (N=N) cm−1; 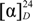 36.2 (c 1.00, CHCl3); 1H-NMR (500 MHz, CDCl3): δ 8.51 (1H, brs, NH), 8.29 (1H, s, H-6), 7.90 (2H, d, J = 7.5 Hz), 7.70 (2H, d, J = 8.5 Hz), 7.52–7.45 (5H, m), 7.37–7.28 (6H, m), 7.16 (1H, dd, J = 6.5 and 1.0 Hz), 7.10 (2H, d, J = 8.0 Hz), 6.82–6.79 (4H, m) 6.38 (1H, dd, J = 7.5, 6.5 Hz, H-1'), 4.60–4.59 (1H, m, H-3'), 4.14–4.13 (1H, m, H-4'), 3.70 (3H, s, OMe), 3.69 (3H, s, OMe), 3.50 (1H, dd, J = 8.0 and 3.0 Hz, H-5'), 3.34 (1H, dd, J = 8.0 and 3.0 Hz, H-5'), 2.57–2.53 (1H, m, H-2'), 2.40–2.34 (1H, m, H-2'), 2.09 (1H, brs, OH); 13C-NMR (125 MHz, CDCl3): δ 158.6, 152.6, 151.7, 148.8, 144.3, 135.4, 132.4, 131.3, 129.9, 129.1, 128.1, 127.9, 127.1, 125.1, 122.9, 122.5, 113.4, 100.4, 93.6, 87.2, 86.7, 85.9, 82.2, 72.4, 63.3, 55.2, 41.7; FAB-LRMS m/z = 757 (MNa+); FAB-HRMS calcd for C44H38N4O7Na 757.2633, found 757.2633.
36.2 (c 1.00, CHCl3); 1H-NMR (500 MHz, CDCl3): δ 8.51 (1H, brs, NH), 8.29 (1H, s, H-6), 7.90 (2H, d, J = 7.5 Hz), 7.70 (2H, d, J = 8.5 Hz), 7.52–7.45 (5H, m), 7.37–7.28 (6H, m), 7.16 (1H, dd, J = 6.5 and 1.0 Hz), 7.10 (2H, d, J = 8.0 Hz), 6.82–6.79 (4H, m) 6.38 (1H, dd, J = 7.5, 6.5 Hz, H-1'), 4.60–4.59 (1H, m, H-3'), 4.14–4.13 (1H, m, H-4'), 3.70 (3H, s, OMe), 3.69 (3H, s, OMe), 3.50 (1H, dd, J = 8.0 and 3.0 Hz, H-5'), 3.34 (1H, dd, J = 8.0 and 3.0 Hz, H-5'), 2.57–2.53 (1H, m, H-2'), 2.40–2.34 (1H, m, H-2'), 2.09 (1H, brs, OH); 13C-NMR (125 MHz, CDCl3): δ 158.6, 152.6, 151.7, 148.8, 144.3, 135.4, 132.4, 131.3, 129.9, 129.1, 128.1, 127.9, 127.1, 125.1, 122.9, 122.5, 113.4, 100.4, 93.6, 87.2, 86.7, 85.9, 82.2, 72.4, 63.3, 55.2, 41.7; FAB-LRMS m/z = 757 (MNa+); FAB-HRMS calcd for C44H38N4O7Na 757.2633, found 757.2633.
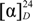 36.2 (c 1.00, CHCl3); 1H-NMR (500 MHz, CDCl3): δ 8.51 (1H, brs, NH), 8.29 (1H, s, H-6), 7.90 (2H, d, J = 7.5 Hz), 7.70 (2H, d, J = 8.5 Hz), 7.52–7.45 (5H, m), 7.37–7.28 (6H, m), 7.16 (1H, dd, J = 6.5 and 1.0 Hz), 7.10 (2H, d, J = 8.0 Hz), 6.82–6.79 (4H, m) 6.38 (1H, dd, J = 7.5, 6.5 Hz, H-1'), 4.60–4.59 (1H, m, H-3'), 4.14–4.13 (1H, m, H-4'), 3.70 (3H, s, OMe), 3.69 (3H, s, OMe), 3.50 (1H, dd, J = 8.0 and 3.0 Hz, H-5'), 3.34 (1H, dd, J = 8.0 and 3.0 Hz, H-5'), 2.57–2.53 (1H, m, H-2'), 2.40–2.34 (1H, m, H-2'), 2.09 (1H, brs, OH); 13C-NMR (125 MHz, CDCl3): δ 158.6, 152.6, 151.7, 148.8, 144.3, 135.4, 132.4, 131.3, 129.9, 129.1, 128.1, 127.9, 127.1, 125.1, 122.9, 122.5, 113.4, 100.4, 93.6, 87.2, 86.7, 85.9, 82.2, 72.4, 63.3, 55.2, 41.7; FAB-LRMS m/z = 757 (MNa+); FAB-HRMS calcd for C44H38N4O7Na 757.2633, found 757.2633.
36.2 (c 1.00, CHCl3); 1H-NMR (500 MHz, CDCl3): δ 8.51 (1H, brs, NH), 8.29 (1H, s, H-6), 7.90 (2H, d, J = 7.5 Hz), 7.70 (2H, d, J = 8.5 Hz), 7.52–7.45 (5H, m), 7.37–7.28 (6H, m), 7.16 (1H, dd, J = 6.5 and 1.0 Hz), 7.10 (2H, d, J = 8.0 Hz), 6.82–6.79 (4H, m) 6.38 (1H, dd, J = 7.5, 6.5 Hz, H-1'), 4.60–4.59 (1H, m, H-3'), 4.14–4.13 (1H, m, H-4'), 3.70 (3H, s, OMe), 3.69 (3H, s, OMe), 3.50 (1H, dd, J = 8.0 and 3.0 Hz, H-5'), 3.34 (1H, dd, J = 8.0 and 3.0 Hz, H-5'), 2.57–2.53 (1H, m, H-2'), 2.40–2.34 (1H, m, H-2'), 2.09 (1H, brs, OH); 13C-NMR (125 MHz, CDCl3): δ 158.6, 152.6, 151.7, 148.8, 144.3, 135.4, 132.4, 131.3, 129.9, 129.1, 128.1, 127.9, 127.1, 125.1, 122.9, 122.5, 113.4, 100.4, 93.6, 87.2, 86.7, 85.9, 82.2, 72.4, 63.3, 55.2, 41.7; FAB-LRMS m/z = 757 (MNa+); FAB-HRMS calcd for C44H38N4O7Na 757.2633, found 757.2633.3.4. Preparation of 3-O-{2-Cyanoethyl(diisopropylamino)phosphino}-5'-O-(4,4'-Dimethoxytrityl)-5-(4-phenyldiazenylphenyl)ethynyl-2'-deoxyuridine (5)
To a solution of compound 4 (188 mg, 0.26 mmol) in dry MeCN (5 mL) was added N, N-diisopropylamine (0.13 mL,0.76 mmol) and 2-cyanoethyl-N, N'-diisopropylchlorophosphoramidite (0.09 mL, 0.40mmol) at room temperature, and the reaction mixture was stirred for 1.5 h. The resultant mixture was partitioned between AcOEt and H2O. The separated organic layer was washed with saturated aqueous NaHCO3, followed by brine. The organic layer was dried (Na2SO4) and concentrated in vacuo. The residue was purified by silica gel column chromatography and eluted with CHCl3/MeOH (20:1 with 0.5% Et3N), to give a 17:3 diastereomeric mixture of 5 (324 mg, 82%) as an orange foam: IR (KBr): ν 3610 (NH), 1699 (C=O), 1272 (N=N) cm−1; 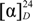 32.5 (c 1.00, CHCl3); 1H-NMR (500 MHz, CDCl3): δ 9.08 (1H, brs, NH), 8.35 (0.85H, s, H-6), 8.30 (0.15H, s, H-6), 7.89 (2H, d,J = 7.5 Hz), 7.67 (2H, d, J = 8.5 Hz), 7.55–7.04 (14H, m), , 6.67–6.75 (4H, m), , 6.35 (1H, dd, J = 7.5, 6.0 Hz, H-1'), 4.68–4.61 (1H, m, H-3'), 4.26 (1H, m, H-4'), 3.70 (3H, s, OMe), 3.69 (3H, s, OMe), 3.67–3.53 (5H, m, CH2CH2CN, H-5'), 3.31 (1H, dd, J = 8.5, 2.5 Hz, H-5'), 2.65–2.56 (1H, m, H-2'), 2.47–2.36 (3H, m, H-2', ((CH3)2CH)2N), 1.18 (12H, d, J = 6.5 Hz, ((CH3)2CH)2N); 13C-NMR (125 MHz, CDCl3): δ 161.2, 158.5(9), 158.5(6), 152.6, 151.5, 149.1, 144.35, 142.5, 135.4, 132.3, 132.0, 131.1, 130.0 (d, J (C, P) = 6.0 Hz), 129.1, 128.7, 128.0, 127.9 ,127.0, 125.1, 122.8, 122.4, 120.5, 117.3, 113.3, 100.3, 93.4, 86.3 (d, J (C, P) = 3.5 Hz), 85.9, 82.4, 77.3, 77.0, 76.8, 73.4, 73.2, 63.0, 58.2, 58.1, 55.1, 43.2 (d, J (C, P) = 13.0 Hz), 40.8 (d, J (C, P) = 5.0 Hz), 25.6, 24.5(9), 24.5(3), 24.4(8), 20.2 (d, J (C, P ) = 7.0 Hz); 31P-NMR (200 MHz, CDCl3): δ 149.09, 148.66; FAB-LRMS m/z = 957 (MNa+); FAB-HRMS calcd for C53H55N6O8PNa 957.3711, found 957.3711.
32.5 (c 1.00, CHCl3); 1H-NMR (500 MHz, CDCl3): δ 9.08 (1H, brs, NH), 8.35 (0.85H, s, H-6), 8.30 (0.15H, s, H-6), 7.89 (2H, d,J = 7.5 Hz), 7.67 (2H, d, J = 8.5 Hz), 7.55–7.04 (14H, m), , 6.67–6.75 (4H, m), , 6.35 (1H, dd, J = 7.5, 6.0 Hz, H-1'), 4.68–4.61 (1H, m, H-3'), 4.26 (1H, m, H-4'), 3.70 (3H, s, OMe), 3.69 (3H, s, OMe), 3.67–3.53 (5H, m, CH2CH2CN, H-5'), 3.31 (1H, dd, J = 8.5, 2.5 Hz, H-5'), 2.65–2.56 (1H, m, H-2'), 2.47–2.36 (3H, m, H-2', ((CH3)2CH)2N), 1.18 (12H, d, J = 6.5 Hz, ((CH3)2CH)2N); 13C-NMR (125 MHz, CDCl3): δ 161.2, 158.5(9), 158.5(6), 152.6, 151.5, 149.1, 144.35, 142.5, 135.4, 132.3, 132.0, 131.1, 130.0 (d, J (C, P) = 6.0 Hz), 129.1, 128.7, 128.0, 127.9 ,127.0, 125.1, 122.8, 122.4, 120.5, 117.3, 113.3, 100.3, 93.4, 86.3 (d, J (C, P) = 3.5 Hz), 85.9, 82.4, 77.3, 77.0, 76.8, 73.4, 73.2, 63.0, 58.2, 58.1, 55.1, 43.2 (d, J (C, P) = 13.0 Hz), 40.8 (d, J (C, P) = 5.0 Hz), 25.6, 24.5(9), 24.5(3), 24.4(8), 20.2 (d, J (C, P ) = 7.0 Hz); 31P-NMR (200 MHz, CDCl3): δ 149.09, 148.66; FAB-LRMS m/z = 957 (MNa+); FAB-HRMS calcd for C53H55N6O8PNa 957.3711, found 957.3711.
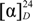 32.5 (c 1.00, CHCl3); 1H-NMR (500 MHz, CDCl3): δ 9.08 (1H, brs, NH), 8.35 (0.85H, s, H-6), 8.30 (0.15H, s, H-6), 7.89 (2H, d,J = 7.5 Hz), 7.67 (2H, d, J = 8.5 Hz), 7.55–7.04 (14H, m), , 6.67–6.75 (4H, m), , 6.35 (1H, dd, J = 7.5, 6.0 Hz, H-1'), 4.68–4.61 (1H, m, H-3'), 4.26 (1H, m, H-4'), 3.70 (3H, s, OMe), 3.69 (3H, s, OMe), 3.67–3.53 (5H, m, CH2CH2CN, H-5'), 3.31 (1H, dd, J = 8.5, 2.5 Hz, H-5'), 2.65–2.56 (1H, m, H-2'), 2.47–2.36 (3H, m, H-2', ((CH3)2CH)2N), 1.18 (12H, d, J = 6.5 Hz, ((CH3)2CH)2N); 13C-NMR (125 MHz, CDCl3): δ 161.2, 158.5(9), 158.5(6), 152.6, 151.5, 149.1, 144.35, 142.5, 135.4, 132.3, 132.0, 131.1, 130.0 (d, J (C, P) = 6.0 Hz), 129.1, 128.7, 128.0, 127.9 ,127.0, 125.1, 122.8, 122.4, 120.5, 117.3, 113.3, 100.3, 93.4, 86.3 (d, J (C, P) = 3.5 Hz), 85.9, 82.4, 77.3, 77.0, 76.8, 73.4, 73.2, 63.0, 58.2, 58.1, 55.1, 43.2 (d, J (C, P) = 13.0 Hz), 40.8 (d, J (C, P) = 5.0 Hz), 25.6, 24.5(9), 24.5(3), 24.4(8), 20.2 (d, J (C, P ) = 7.0 Hz); 31P-NMR (200 MHz, CDCl3): δ 149.09, 148.66; FAB-LRMS m/z = 957 (MNa+); FAB-HRMS calcd for C53H55N6O8PNa 957.3711, found 957.3711.
32.5 (c 1.00, CHCl3); 1H-NMR (500 MHz, CDCl3): δ 9.08 (1H, brs, NH), 8.35 (0.85H, s, H-6), 8.30 (0.15H, s, H-6), 7.89 (2H, d,J = 7.5 Hz), 7.67 (2H, d, J = 8.5 Hz), 7.55–7.04 (14H, m), , 6.67–6.75 (4H, m), , 6.35 (1H, dd, J = 7.5, 6.0 Hz, H-1'), 4.68–4.61 (1H, m, H-3'), 4.26 (1H, m, H-4'), 3.70 (3H, s, OMe), 3.69 (3H, s, OMe), 3.67–3.53 (5H, m, CH2CH2CN, H-5'), 3.31 (1H, dd, J = 8.5, 2.5 Hz, H-5'), 2.65–2.56 (1H, m, H-2'), 2.47–2.36 (3H, m, H-2', ((CH3)2CH)2N), 1.18 (12H, d, J = 6.5 Hz, ((CH3)2CH)2N); 13C-NMR (125 MHz, CDCl3): δ 161.2, 158.5(9), 158.5(6), 152.6, 151.5, 149.1, 144.35, 142.5, 135.4, 132.3, 132.0, 131.1, 130.0 (d, J (C, P) = 6.0 Hz), 129.1, 128.7, 128.0, 127.9 ,127.0, 125.1, 122.8, 122.4, 120.5, 117.3, 113.3, 100.3, 93.4, 86.3 (d, J (C, P) = 3.5 Hz), 85.9, 82.4, 77.3, 77.0, 76.8, 73.4, 73.2, 63.0, 58.2, 58.1, 55.1, 43.2 (d, J (C, P) = 13.0 Hz), 40.8 (d, J (C, P) = 5.0 Hz), 25.6, 24.5(9), 24.5(3), 24.4(8), 20.2 (d, J (C, P ) = 7.0 Hz); 31P-NMR (200 MHz, CDCl3): δ 149.09, 148.66; FAB-LRMS m/z = 957 (MNa+); FAB-HRMS calcd for C53H55N6O8PNa 957.3711, found 957.3711.3.5. Synthesis of dUAz-Modified Oligodeoxynucleotides
Solid-phase oligonucleotide synthesis was performed on an nS-8 Oligonucleotides Synthesizer (GeneDesign, Inc., Osaka, Japan) using commercially available reagents and phosphoramidites with 5-(bis-3, 5-trifluoromethylphenyl)-1H-tetrazole (0.25 M concentration in acetonitrile) as the activator. dUAz phosphoramidite was chemically synthesized as described above. All of the reagents were assembled, and the oligonucleotides were synthesized according to the standard synthesis cycle (trityl on mode). Cleavage from the solid support and deprotection were accomplished with concentrated ammonium hydroxide solution at 55 °C for 12 h. The crude oligonucleotides were purified with Sep-Pak Plus C18 cartridges (Waters) followed by RP-HPLC on a XBridgeTM OST C18 Column, 2.5 μm, 10 × 50 mm (Waters) using MeCN in 0.1 M triethylammonium acetate buffer (pH 7.0). The purified oligonucleotides were quantified by UV absorbance at 260 nm and confirmed by MALDI-TOF mass spectrometry (Table 4).

Table 4.
Yields and MALDI-TOF MS data of dUAz-modified oligonucleotide.
| Oligodeoxynucleotide | Yield | MALDI-TOF MS | ||
|---|---|---|---|---|
| Calcd. [M-H]− | found [M-H]− | |||
| 5'-d(GCGTTUAzTTTGCT)-3' | 7 | 29% | 3822.6 | 3822.4 |
3.6. UV Melting Experiments
Melting temperatures (Tm) were determined by measuring the change in absorbance at 260 nm as a function of temperature using a Shimadzu UV-Vis Spectrophotometer UV-1650PC equipped with a Tm analysis accessory TMSPC-8. Equimolecular amounts of the target DNA/RNA and oligonucleotides were dissolved in 10 mM sodium phosphate buffer (pH 7.0) containing 100 mM NaCl to give a final strand concentration of 4.0 µM. The melting samples were denatured at 100 °C and annealed slowly to room temperature. Absorbance was recorded in the forward and reverse directions at temperatures of 5 to 90 °C at a rate of 0.5 °C/min.
3.7. Photoisomerization of dUAz
The trans-to-cis isomerization was performed with a UV-LED lamp (ZUV-C30H; OMRON) and a ZUV-L10H lens unit (760 mW/cm2). The cis-to-trans isomerization was performed with a Xenon lamp (MAX-303; Asahi Spectra Co., Ltd., Tokyo, Japan) and XHQA420 optical filter. Absorbance spectra of trans-cis ON 7 were measured by a Shimadzu UV-Vis Spectrophotometer UV-1650PC. Conditions: ON 7 (4.0 µM), NaCl (100mM) in sodium phosphate buffer (10 mM, pH 7.0).
4. Conclusions
We have synthesized a new photoisomeric nucleoside, C5-azobenzene-modified 2'-deoxyuridine dUAz using Sonogashira-type cross-coupling as a key step. dUAz showed very rapid reversible cis-trans photoisomerization with monochromic light at the appropriate wavelength in oligodeoxynucleotide. dUAz-modified oligodeoxynucleotide showed an interesting duplex-forming property, namely, the Tm values of both the dUAz-modified ON/DNA and dUAz-modified ON/RNA were higher for the cis-form than for the trans-form, unlike conventional azobenzene-modified ONs. Additionally, it was revealed that installation of dUAz into oligodeoxynucleotide had little influence on the mismatch recognition ability.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/19/4/5109/s1.
Acknowledgments
This work was supported by the Japan Society for the Promotion of Science (JSPS), the Ministry of Education, Culture, Sports, Science and Technology (MEXT), and theAdvanced Research for Medical Products Mining Programme of the National Institute of Biomedical Innovation (NIBIO).
Author Contributions
K.M. and S.O. designed the research. S.M. and K.M. performed the experiments and analyzed the data. S.M. was mainly responsible for writing the manuscript, with contributions from K.M. and S.O.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kuzuya, A.; Komiyama, M. DNA origami: Fold, stick, and beyond. Nanoscale 2010, 2, 309–321. [Google Scholar]
- Torring, T.; Voigt, N.V.; Nangreave, J.; Yan, H.; Gothelf, K.V. DNA origami: A quantum leap for self-assembly of comples structures. Chem. Soc. Rev. 2011, 40, 5636–5646. [Google Scholar] [CrossRef]
- Pinheiro, A.V.; Han, D.; Shin, W.M.; Yan, H. Challenges and opportunities for structural DNA nanotechnology. Nat. Nanotechnol. 2011, 6, 763–772. [Google Scholar] [CrossRef]
- Asanuma, H.; Ito, T.; Yoshida, T.; Liang, X.; Komiyama, M. Photoregulation of the formation and dissociation of a DNA duplex by using the cis-trans isomerization of azobenzene. Angew. Chem. Int. Ed. 1999, 38, 2393–2395. [Google Scholar] [CrossRef]
- Asanuma, H.; Liang, X.; Yoshida, T.; Komiyama, M. Photocontrol of DNA duplex formation by using azobenzene-bearing oligonucleotides. ChemBioChem 2001, 2, 39–44. [Google Scholar]
- Beharry, A.A.; Woolley, G.A. Azobenzene photoswitches for biomolecules. Chem. Soc. Rev. 2011, 40, 4422–4437. [Google Scholar] [CrossRef]
- Dhammika, H.M.; Burdette, S.C. Photoisomerization in different classes of azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825. [Google Scholar]
- Barrois, S.; Wagenknecht, H.A. Diarylehtene-modified nucleotides for switching optical properties inDNA. Beilstein. J. Org. Chem. 2012, 8, 905–914. [Google Scholar] [CrossRef]
- Xiao, Q.; Ranasinghe, R.T.; Tang, A.M.P.; Brown, T. Naphthalenyl- and anthracenyl-ethynyl dT analogues as base discriminating fluorescent nucleosides and intramolecular energy transfer donors in oligonucleotide probes. Tetrahedron 2007, 63, 3483–3490. [Google Scholar] [CrossRef]
- Franklin, R.E.; Gosling, R.G. Molecular configuration in sodium thymonucleate. Nature 1953, 171, 740–741. [Google Scholar] [CrossRef]
- Anderson, C.F.; Record, M.T., Jr. Salt-nucleic acid interactions. Annu. Rev. Phys. Chem. 1995, 46, 657–700. [Google Scholar] [CrossRef]
- Sonogashira, K.; Tohda, Y.; Hagihara, N. A convenient synthesis of acetylenes: Catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines. Tetrahedron Lett. 1975, 16, 4467–4470. [Google Scholar] [CrossRef]
- Shirai, Y.; Sasaki, T; Guerrero, J.M.; Yu, B.; Hodge, P.; Tour, J.M. Synthesis and photoisomerization of Fullerene- and oligo(phenylene ethynylene)-azobenzene derivatives. ACS Nano 2008, 2, 97–106. [Google Scholar] [CrossRef]
- Matharu, A.S.; Jeeva, S.; Ramanujam, P.S. Liquid crystals for holographic optical data storage. Chem. Soc. Rev. 2007, 36, 1868–1880. [Google Scholar] [CrossRef]
- Liang, X.; Asanuma, H.; Komiyama, M. Photoregulation of DNA triplex formation by azobenzene. J. Am. Chem. Soc. 2002, 124, 1877–1883. [Google Scholar] [CrossRef]
- Nishioka, H.; Liang, X.; Asanuma, H. Effect of the ortho modification of azobenzene on the photoregulatory efficiency of DNA hybridization and the thermal stability of its cis form. Chem. Eur. J. 2010, 16, 2054–2062. [Google Scholar] [CrossRef]
- Asanuma, H.; Yoshida, T.; Ito, T.; Komiyama, M. Photo-responsive oligonucleotides carrying azobenzene at the 2'-position of uridine. Tetrahedron Lett. 1999, 40, 7995–7998. [Google Scholar] [CrossRef]
- Patnaik, S.; Kumar, P.; Garg, B.S.; Gandni, R.P.; Gupta, K.C. Photomodulation of PS-modified oligonucleotides containing azobenzene substituent at pre-selected positions in phosphate backbone. Bioorg. Med. Chem. 2007, 15, 7840–7849. [Google Scholar] [CrossRef]
- Ogasawara, S.; Maeda, M. Straightforward and reversible photoregulation of hybridization by using a photochromic nucleoside. Angew. Chem. Int. Ed. 2008, 47, 8839–8842. [Google Scholar] [CrossRef]
- Nishioka, H.; Liang, X.; Kashida, H.; Asanuma, H. 2',6'-Dimethylazobenzene as an efficient and thermo-stable photoregulator for the photoregulation of DNA hybridization. Chem. Commun. 2007, 4354–4356. [Google Scholar]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).