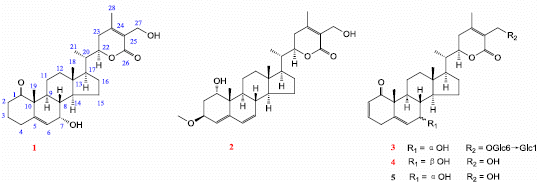
Five Withanolides from the Leaves of Datura metel L. and Their Inhibitory Effects on Nitric Oxide Production
Abstract
:1. Introduction
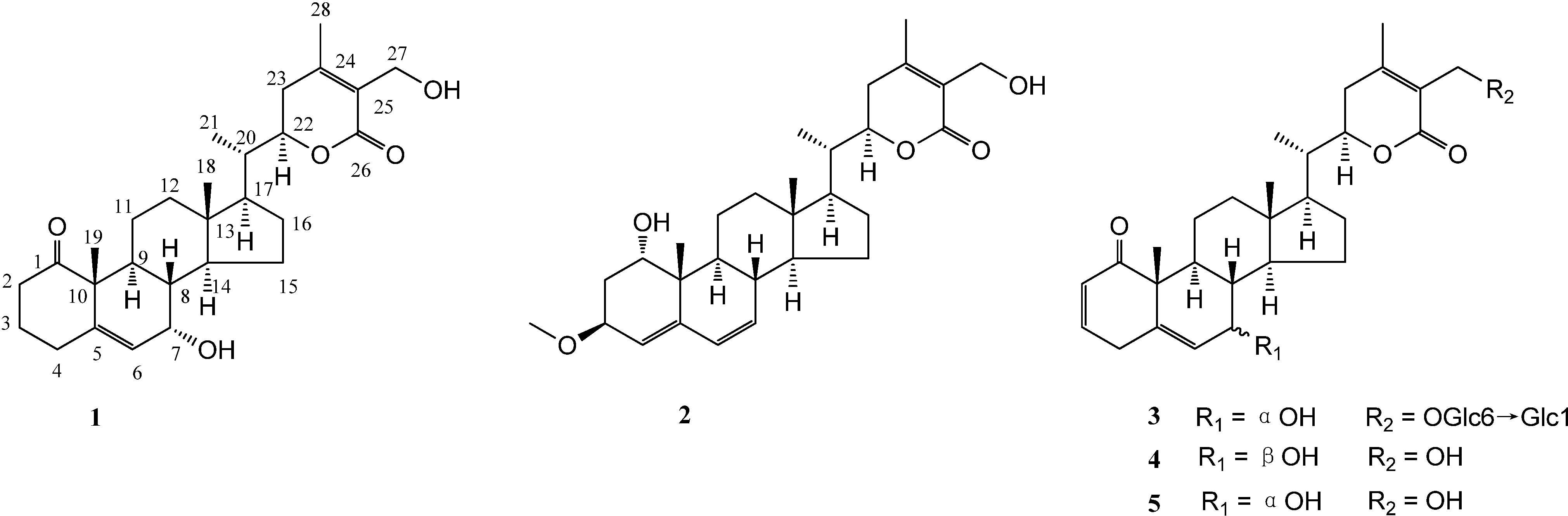
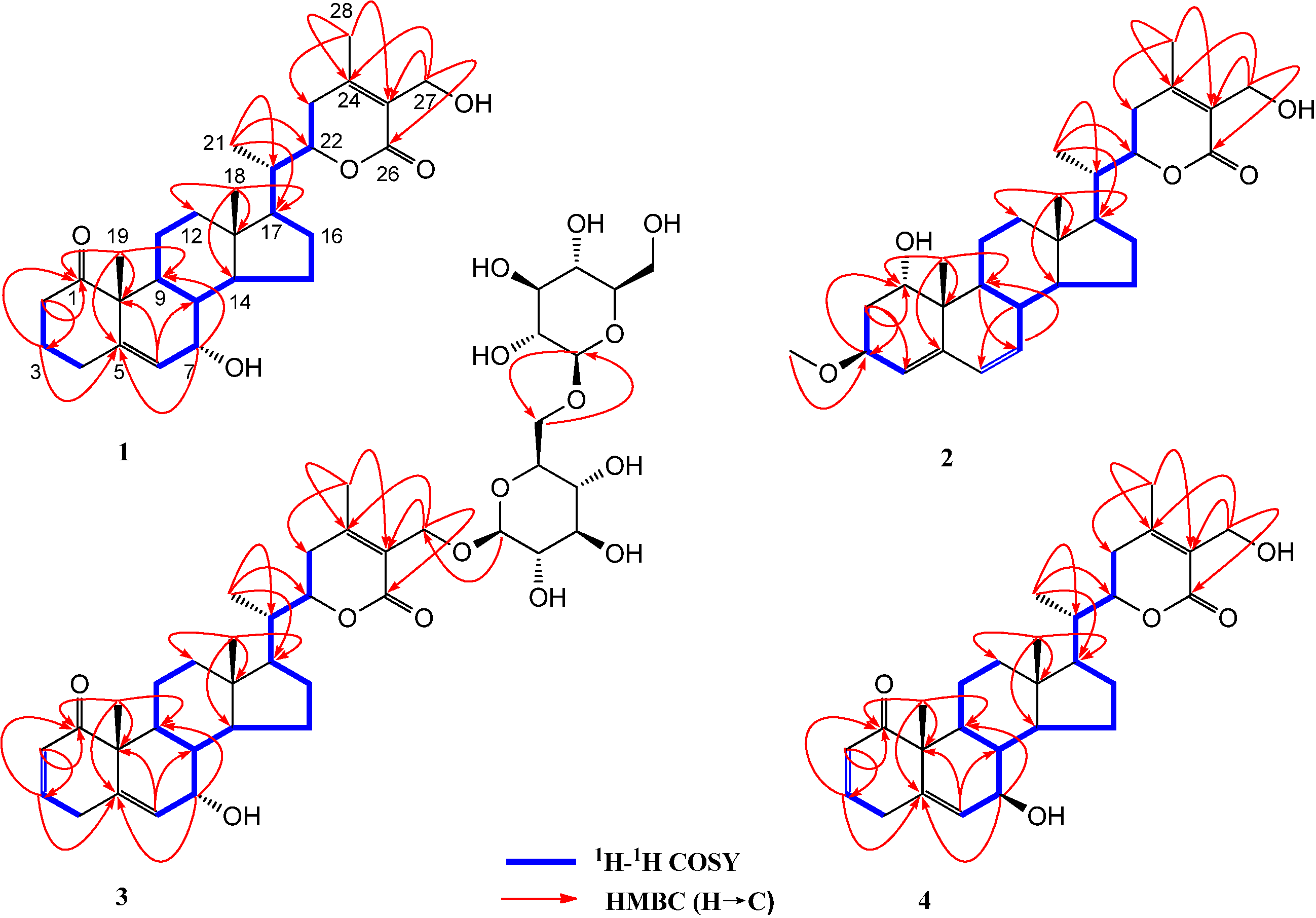
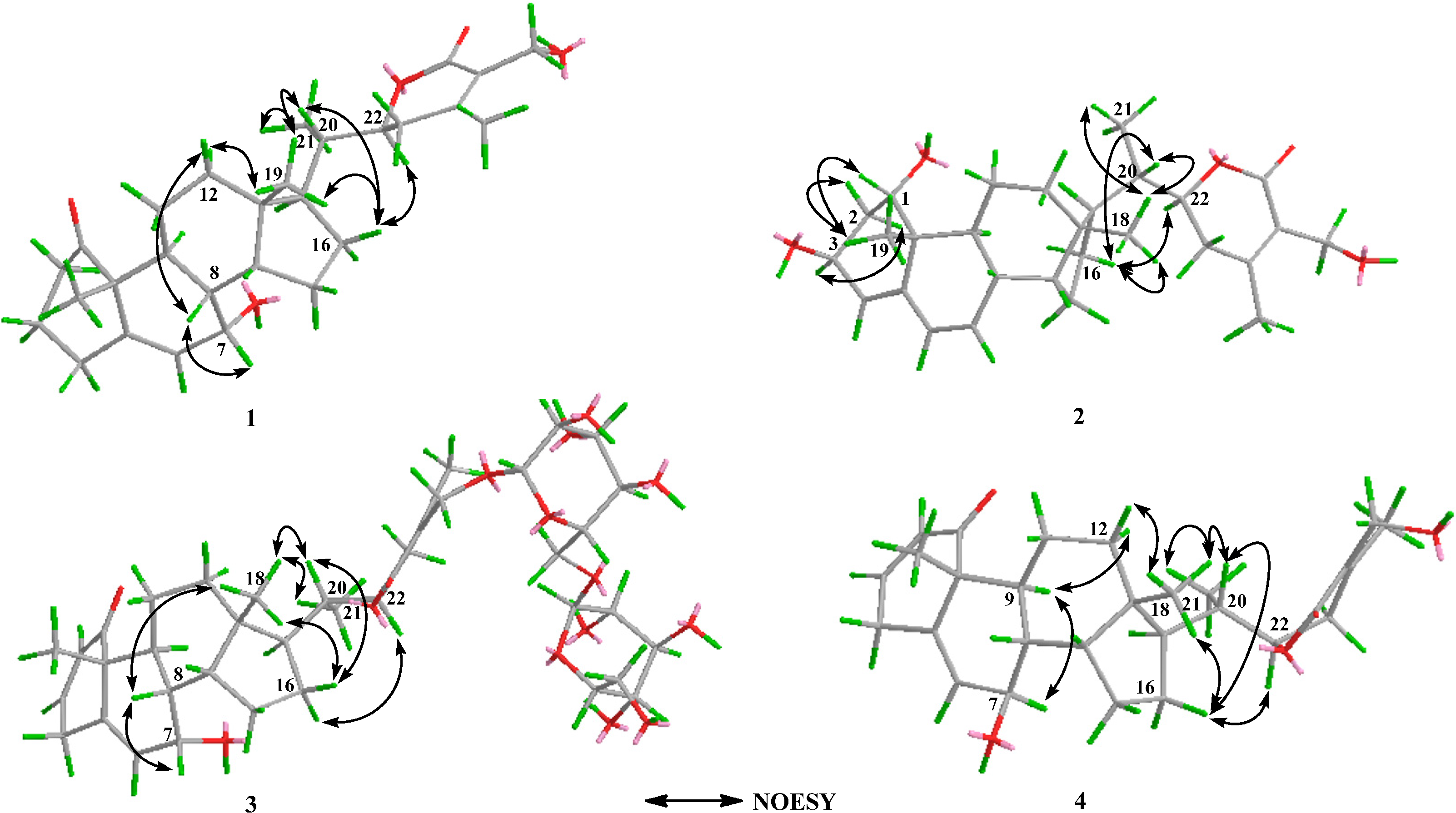
2. Results and Discussion
| No. | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1 | 3.88 br s | |||
| 2 | 2.32 m, 2.67 m | 1.85 m, 2.24 m | 5.86 dd (10.0, 2.4) | 5.84 dd (10.0, 2.5) |
| 3 | 2.00 m, 1.62 m | 4.11 t (7.4) | 6.93 ddd (10.0, 4.8, 2.4) | 6.91 ddd (10.0, 4.8, 2.5) |
| 4 | 2.22 m, 2.63 m | 5.48 br s | 3.44 m | 3.40 m |
| 2.96 dd (21.5, 4.8) | 2.93 dd (21.4, 4.8) | |||
| 6 | 5.70 dd (5.3, 1.2) | 5.98 dd (10.0, 2.3) | 5.80 dd (5.8, 1.5) | 5.48 br s |
| 7 | 3.77 t (3.8) | 5.63 br d (10.0) | 3.80 t (5.5) | 3.71 d (8.4) |
| 8 | 1.43 m | 2.07 m | 1.46 m | 1.42 m |
| 9 | 1.99 m | 1.54 m | 2.00 m | 1.68 m |
| 11 | 1.50 m, 1.73 m | 1.44 m, 1.66 m | 1.59 m, 2.23 m | 1.53 m, 2.24 m |
| 12 | 1.29 m, 1.97 m | 1.32 m, 2.03 m | 1.34 m, 2.03 m | 1.32 m, 2.04 m |
| 14 | 1.54 m | 1.24 m | 1.32 m | 1.24 m |
| 15 | 1.78 m, 1.19 m | 1.85 m, 1.37 m | 1.84 m, 1.22 m | 1.92 m, 1.53 m |
| 16 | 1.79 m, 1.41 m | 1.83 m, 1.43 m | 1.83 m, 1.41 m | 1.78 m, 1.37 m |
| 17 | 1.24 m | 1.30 m | 1.28 m | 1.22 m |
| 18 | 0.78 s | 0.82 s | 0.79 s | 0.80 s |
| 19 | 1.28 s | 1.00 s | 1.24 s | 1.28 s |
| 20 | 1.95 m | 1.98 m | 1.98 m | 1.94 m |
| 21 | 1.04 d (6.6) | 1.03 d (6.7) | 1.05 d (6.6) | 1.04 d (6.6) |
| 22 | 4.47 dt (13.2, 3.4) | 4.47 dt (13.3, 3.4) | 4.50 dt (13.5, 3.6) | 4.48 dt (13.2, 3.4) |
| 23 | 2.56 dd (17.8, 13.7) | 2.54 dd (18.0, 13.4) | 2.58 dd (17.9, 13.6) | 2.54 dd (18.0, 13.5) |
| 2.18 dd (17.8, 3.4) | 2.21 dd (18.0, 3.3) | 2.22 dd (17.9, 3.0) | 2.20 dd (18.0, 3.1) | |
| 27 | 4.29 d (11.7) | 4.36 d (11.7) | 4.47 d (11.2) | 4.37 d (11.7) |
| 4.37 d (11.7) | 4.29 d (11.7) | 4.62 d (11.2) | 4.29 d (11.7) | |
| 28 | 2.10 s | 2.10 s | 2.14 s | 2.10 s |
| OCH3 | 3.38 s |
| No. | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1 | 215.9 | 72.5 | 205.7 | 205.9 |
| 2 | 39.0 | 33.0 | 128.4 | 128.4 |
| 3 | 26.2 | 75.4 | 147.7 | 147.6 |
| 4 | 31.9 | 123.4 | 34.4 | 33.8 |
| 5 | 146.6 | 143.3 | 141.7 | 138.2 |
| 6 | 125.9 | 130.1 | 127.5 | 130.8 |
| 7 | 65.1 | 132.1 | 64.8 | 72.1 |
| 8 | 38.6 | 38.5 | 39.5 | 42.2 |
| 9 | 36.2 | 45.3 | 36.4 | 42.6 |
| 10 | 55.5 | 40.4 | 52.3 | 51.4 |
| 11 | 23.3 | 21.4 | 24.6 | 24.9 |
| 12 | 40.5 | 40.9 | 40.8 | 41.0 |
| 13 | 43.7 | 45.1 | 43.6 | 44.4 |
| 14 | 50.7 | 55.2 | 51.0 | 57.4 |
| 15 | 25.1 | 25.0 | 25.0 | 27.7 |
| 16 | 28.3 | 28.3 | 28.2 | 28.5 |
| 17 | 53.2 | 53.2 | 53.2 | 52.7 |
| 18 | 12.1 | 12.2 | 12.2 | 12.4 |
| 19 | 18.7 | 19.9 | 18.8 | 19.2 |
| 20 | 40.5 | 40.4 | 40.5 | 40.4 |
| 21 | 13.8 | 13.7 | 13.8 | 13.8 |
| 22 | 80.2 | 80.1 | 80.2 | 80.2 |
| 23 | 30.7 | 30.7 | 30.8 | 30.7 |
| 24 | 157.9 | 157.9 | 160.4 | 157.9 |
| 25 | 126.4 | 126.4 | 123.6 | 126.4 |
| 26 | 168.6 | 168.5 | 168.6 | 168.6 |
| 27 | 56.4 | 56.4 | 63.6 | 56.4 |
| 28 | 20.2 | 20.2 | 20.9 | 20.2 |
| OCH3 | 55.8 |
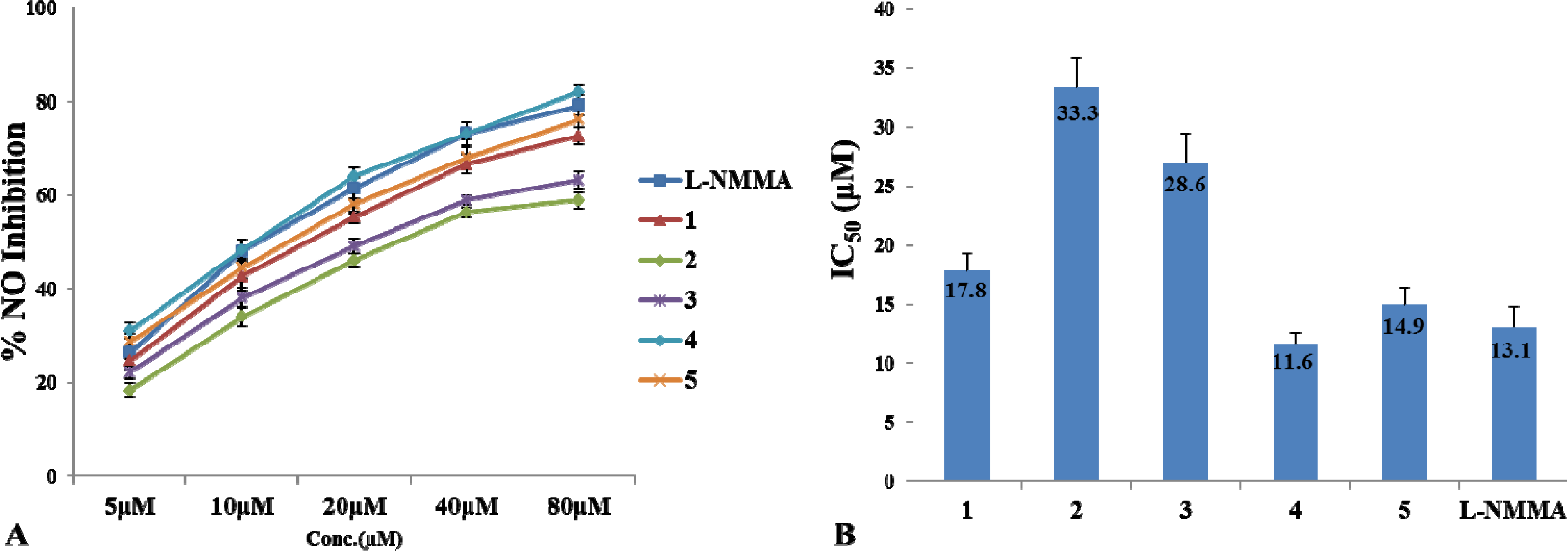
3. Experimental Section
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
 + 28.0 (c = 0.10, MeOH); UV (MeOH) λmax (logε): 224 (4.37) nm; IR (KBr) vmax: 3401, 3169, 2933, 2688, 1701, 1689, 1262, 1068, 1023, 821 cm−1; 1H and 13C-NMR data, see Table 1 and Table 2; HRESIMS m/z: 479.2779 [M+Na]+ (calcd. for C28H40O5Na, 479.2773).
+ 28.0 (c = 0.10, MeOH); UV (MeOH) λmax (logε): 224 (4.37) nm; IR (KBr) vmax: 3401, 3169, 2933, 2688, 1701, 1689, 1262, 1068, 1023, 821 cm−1; 1H and 13C-NMR data, see Table 1 and Table 2; HRESIMS m/z: 479.2779 [M+Na]+ (calcd. for C28H40O5Na, 479.2773). + 16.0 (c = 0.10, MeOH); UV (MeOH) λmax (logε): 225 (5.60) nm; IR (KBr) vmax: 3367, 2963, 2869, 1685, 1388, 1075 cm−1; 1H and 13C-NMR data, see Table 1 and Table 2; HRESIMS m/z 493.2921 [M+Na]+ (calcd. for C29H42O5Na, 493.2930).
+ 16.0 (c = 0.10, MeOH); UV (MeOH) λmax (logε): 225 (5.60) nm; IR (KBr) vmax: 3367, 2963, 2869, 1685, 1388, 1075 cm−1; 1H and 13C-NMR data, see Table 1 and Table 2; HRESIMS m/z 493.2921 [M+Na]+ (calcd. for C29H42O5Na, 493.2930). + 44.0 (c = 0.10, MeOH); UV (MeOH) λmax (logε): 222 (5.28) nm; IR (KBr) vmax: 3400, 2937, 2360, 2349, 1716, 1695, 1681, 1219, 1126, 1028 cm−1; 1H and 13C-NMR data, see Table 1, Table 2, and Table 3; HRESIMS m/z 801.3694 [M+Na]+ (calcd. for C40H58O15Na, 801.3673).
+ 44.0 (c = 0.10, MeOH); UV (MeOH) λmax (logε): 222 (5.28) nm; IR (KBr) vmax: 3400, 2937, 2360, 2349, 1716, 1695, 1681, 1219, 1126, 1028 cm−1; 1H and 13C-NMR data, see Table 1, Table 2, and Table 3; HRESIMS m/z 801.3694 [M+Na]+ (calcd. for C40H58O15Na, 801.3673). + 20.0 (c = 0.10, MeOH); UV (MeOH) λmax (logε): 221 (6.52) nm; IR (KBr) vmax: 3402, 3367, 3129, 2963, 2915, 2869, 1685, 1075, 799 cm−1; 1H and 13C-NMR data, see Table 1 and Table 2; HRESIMS m/z 455.2715 [M+H]+ (calcd. for C28H39O5, 455.2797).
+ 20.0 (c = 0.10, MeOH); UV (MeOH) λmax (logε): 221 (6.52) nm; IR (KBr) vmax: 3402, 3367, 3129, 2963, 2915, 2869, 1685, 1075, 799 cm−1; 1H and 13C-NMR data, see Table 1 and Table 2; HRESIMS m/z 455.2715 [M+H]+ (calcd. for C28H39O5, 455.2797).| NO. | δH | δC | NO. | δH | δC | |
|---|---|---|---|---|---|---|
| 1′ | 4.34 d (7.8) | 103.9 | 1″ | 4.41 d (7.8) | 104.9 | |
| 2′ | 3.18 t (8.9) | 75.2 | 2″ | 3.36 t (9.2) | 75.0 | |
| 3′ | 3.41 m | 77.9 | 3″ | 3.30 m | 78.0 | |
| 4′ | 3.28 m | 71.7 | 4″ | 3.36 m | 71.5 | |
| 5′ | 3.43 m | 77.2 | 5″ | 3.30 m | 78.0 | |
| 6′ | 3.78 dd (11.6, 5.8) | 69.9 | 6″ | 3.68 dd (11.8, 5.0) | 62.8 | |
| 4.16 dd (11.6, 1.8) | 3.86 dd (11.8, 2.0) |
3.4. Acid Hydrolysis of Compound 3 and GC Analysis
3.5. Cell Culture
3.6. Cell Viability Assay
3.7. The Determination of NO Production from RAW 264.7
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, H.P.; Samadi, A.K.; Gallagher, R.J.; Araya, J.J.; Tong, X.Q.; Day, V.W.; Cohen, M.S.; Kindscher, K.; Gollapudi, R.; Timmermann, B.N. Cytotoxic withanolide constituents of Physalis longifolia. J. Nat. Prod. 2011, 74, 2532–2544. [Google Scholar] [CrossRef]
- Jayaprakasam, B.; Zhang, Y.J.; Seeram, N.P.; Nair, M.G. Growth inhibition of human tumor cell lines by withanolides from Withania somnifera leaves. Life Sci. 2003, 74, 125–132. [Google Scholar] [CrossRef]
- Minguzzi, S.; Barata, L.S.; Shin, Y.G.; Jonas, P.F.; Chai, H.B.; Park, E.J.; Pezzuto, J.M.; Cordell, G.A. Cytotoxic withanolides from Acnistus arborescens. Phytochemistry 2002, 59, 635–641. [Google Scholar] [CrossRef]
- Habtemariam, S. Cytotoxicity and immunosuppressive activity of withanolides from Discopodium penninervium. Planta Med. 1997, 63, 15–17. [Google Scholar] [CrossRef]
- Jayaprakasam, B.; Nair, M.G. Cyclooxygenase-2 enzyme inhibitory withanolides from Withania somnifera leaves. Tetrahedron 2003, 59, 841–849. [Google Scholar] [CrossRef]
- Budhiraja, R.D.; Sudhir, S.; Garg, K.N. Antiinflammatory Activity of 3 β-Hydroxy-2,3-dihydro-withanolide F. Planta Med. 1984, 50, 134–136. [Google Scholar] [CrossRef]
- Su, B.N.; Park, E.J.; Nikolic, D.; Santarsiero, B.D.; Mesecar, A.D.; Vigo, J.S.; Graham, J.G.; Cabieses, F.; Breemen, R.B.; Fong, H.H.S.; et al. Activity-guided isolation of novel norwithanolides from Deprea subtriflora with potential cancer chemopreventive activity. J. Org. Chem. 2003, 68, 2350–2361. [Google Scholar]
- Kuang, H.X.; Yang, B.Y.; Xia, Y.G.; Wang, Q.H. Two new withanolide lactones from Flos Daturae. Molecules 2011, 16, 5833–5839. [Google Scholar] [CrossRef]
- Tang, L.; Wang, Q.H.; Yang, B.Y.; Xiao, H.B.; Sun, Y.P.; Kuang, H.X. Protective effects of active fraction and constituents from Flos Daturae on Chinese hamster ovary cells injuried by dimethyl sulfoxide. In Chin. Trad. Herbal Drug.; 2006; Volume 37, pp. 1826–1831. (In Chinese) [Google Scholar]
- Wang, Y.X. The report on Traditional Chinese medicine yangjinhua (Datura metel) is given priority to treat 242 patients with psoriasis. J. Trad. Chin. Med. 1985, 35, 32–33. (In Chinese) [Google Scholar]
- Wang, Q.H.; Xiao, H.B.; Yang, B.Y.; Yao, F.Y.; Kuang, H.X. Studies on pharmacological actions of the effective parts for psoriasis in Flos Daturae (I). Chin. J. Exp. Trad. Med. Formulae 2008, 14, 49–51. (In Chinese) [Google Scholar]
- Wang, Q.H.; Xiao, H.B.; Yang, B.Y.; Yao, F.Y.; Kuang, H.X. Studies on pharmacological actions of the active parts in Flos Daturae for psoriasis (II). Chin. J. Exp. Trad. Med. Formulae 2008, 14, 32–34. (In Chinese) [Google Scholar]
- Yang, B.Y.; Wang, Q.H.; Xia, Y.G.; Feng, W.S.; Kuang, H.X. Withanolide compounds from the flower of Datura metel L. Helv. Chim. Acta 2007, 90, 1522–1528. [Google Scholar] [CrossRef]
- Yang, B.Y.; Wang, Q.H.; Xia, Y.G.; Feng, W.S.; Kuang, H.X. Baiman-tuoluolines D–F, three new withanolides from the lower of Datura metel L. Helv. Chim. Acta 2008, 91, 964–971. [Google Scholar] [CrossRef]
- Yang, B.Y.; Xia, Y.G.; Wang, Y.Y.; Wang, Q.H.; Kuang, H.X. Two novel norwithasteroids with unusual Six- and seven-membered ether rings in side chain from flos Daturae. Evid. Based Complement. Alternat. Med. 2013. [Google Scholar] [CrossRef]
- Kuang, H.X.; Yang, B.Y.; Tang, L.; Xia, Y.G.; Dou, D.Q. Baimantuoluosides A-C, Three new withanolide glucosides from the flower of Datura metel L. Helv. Chim. Acta 2009, 92, 1315–1323. [Google Scholar] [CrossRef]
- Yang, B.Y.; Xia, Y.G.; Wang, Q.H.; Dou, D.Q.; Kuang, H.X. Baimantuoluosides D–G, four new withanolide glucosides from the lower of Datura metel L. Arch. Pharm. Res. 2010, 33, 1143–1148. [Google Scholar] [CrossRef]
- Yang, B.Y.; Xia, Y.G.; Liu, Y.; Li, L.; Jiang, H.; Yang, L.; Wang, Q.H.; Kuang, H.X. New antiproliferaive and immunosuppressive withanolides from the seeds of Datura metel. Phytochem. Lett. 2014, 8, 92–96. [Google Scholar] [CrossRef]
- Ma, L.; Xie, C.M.; Li, J.; Lou, F.C.; Hu, L.H. Daturametelins H, I, and J: Three new withanolide glycosides from Datura metel L. Chem. Biodivers. 2006, 3, 180–186. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S. Introduction to Spectroscopy, 1st ed.; Saunders College Publishing Co.: Philadelphia, PA, USA, 1979; pp. 41–60. [Google Scholar]
- Rahman, A.U.; Shahwar, D.E.; Naz, A.; Choudhary, M.I. Withanolides from Withania coagulans. Phytochemistry 2003, 63, 387–390. [Google Scholar] [CrossRef]
- Chao, C.H.; Chou, K.J.; Wen, Z.H.; Wang, G.H.; Wu, Y.C.; Dai, C.F.; Sheu, J.H. Paraminabeolides A–F, cytotoxic and anti-inflammatory marine withanolides from the Soft Coral Paraminabea acronocephala. J. Nat. Prod. 2011, 74, 1132–1141. [Google Scholar] [CrossRef]
- Haq, I.U.; Youn, U.J.; Chai, X.; Park, E.J.; Kondratyuk, T.P.; Simmons, C.J.; Borris, R.P.; Mirza, B.; Pezzuto, J.M.; Chang, L.C. Biologically active withanolides from Withania coagulans. J. Nat. Prod. 2013, 76, 22–28. [Google Scholar] [CrossRef]
- Maldonado, E.; Amador, S.; Martínez, M.; Pérez-Castorena, A.L. Virginols A-C, three new withanolides from Physalis virginiana. Steroids 2010, 75, 346–349. [Google Scholar] [CrossRef]
- Lai, P.K.K.; Chan, J.Y.W; Wu, S.B.; Cheng, G.K.; Ho, G.K.W.; Lau, C.P.; Kennelly, E.J.; Leung, K.P. Anti-inflammatory activities of an active fraction isolated from the root of Astragalus memberanaceus in Raw 264.7 Macrophages. Phytother. Res. 2013. [Google Scholar] [CrossRef]
- Wu, S.B.; Su, J.J.; Sun, L.H.; Wang, W.X.; Zhao, Y.; Li, H.; Zhang, S.P.; Dai, G.H.; Wang, C.G.; Hu, J.F. Triterpenoids and steroids from the fruits of Melia toosendan and their cytotoxic effects on two human cancer cell lines. J. Nat. Prod. 2010, 73, 1898–1906. [Google Scholar] [CrossRef]
- Wu, S.B.; Fan, H.; Wen, Y.; Zhang, H.F.; Zhao, Y.; Hu, J.F. Antiproliferative and apoptotic activities of linear furocoumarins from Notopterygium incisum on cancer cell lines. Planta Med. 2010, 76, 82–85. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–5 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yang, B.-Y.; Guo, R.; Li, T.; Liu, Y.; Wang, C.-F.; Shu, Z.-P.; Wang, Z.-B.; Zhang, J.; Xia, Y.-G.; Jiang, H.; et al. Five Withanolides from the Leaves of Datura metel L. and Their Inhibitory Effects on Nitric Oxide Production. Molecules 2014, 19, 4548-4559. https://doi.org/10.3390/molecules19044548
Yang B-Y, Guo R, Li T, Liu Y, Wang C-F, Shu Z-P, Wang Z-B, Zhang J, Xia Y-G, Jiang H, et al. Five Withanolides from the Leaves of Datura metel L. and Their Inhibitory Effects on Nitric Oxide Production. Molecules. 2014; 19(4):4548-4559. https://doi.org/10.3390/molecules19044548
Chicago/Turabian StyleYang, Bing-You, Rui Guo, Ting Li, Yan Liu, Chang-Fu Wang, Zun-Peng Shu, Zhi-Bin Wang, Jing Zhang, Yong-Gang Xia, Hai Jiang, and et al. 2014. "Five Withanolides from the Leaves of Datura metel L. and Their Inhibitory Effects on Nitric Oxide Production" Molecules 19, no. 4: 4548-4559. https://doi.org/10.3390/molecules19044548
APA StyleYang, B.-Y., Guo, R., Li, T., Liu, Y., Wang, C.-F., Shu, Z.-P., Wang, Z.-B., Zhang, J., Xia, Y.-G., Jiang, H., Wang, Q.-H., & Kuang, H.-X. (2014). Five Withanolides from the Leaves of Datura metel L. and Their Inhibitory Effects on Nitric Oxide Production. Molecules, 19(4), 4548-4559. https://doi.org/10.3390/molecules19044548