Vapour Phase Hydrogenation of Phenol over Rhodium on SBA-15 and SBA-16
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterisation of SBA-15 Support and Rh/SBA-15 Catalysts
2.1.1. Nitrogen Physisorption
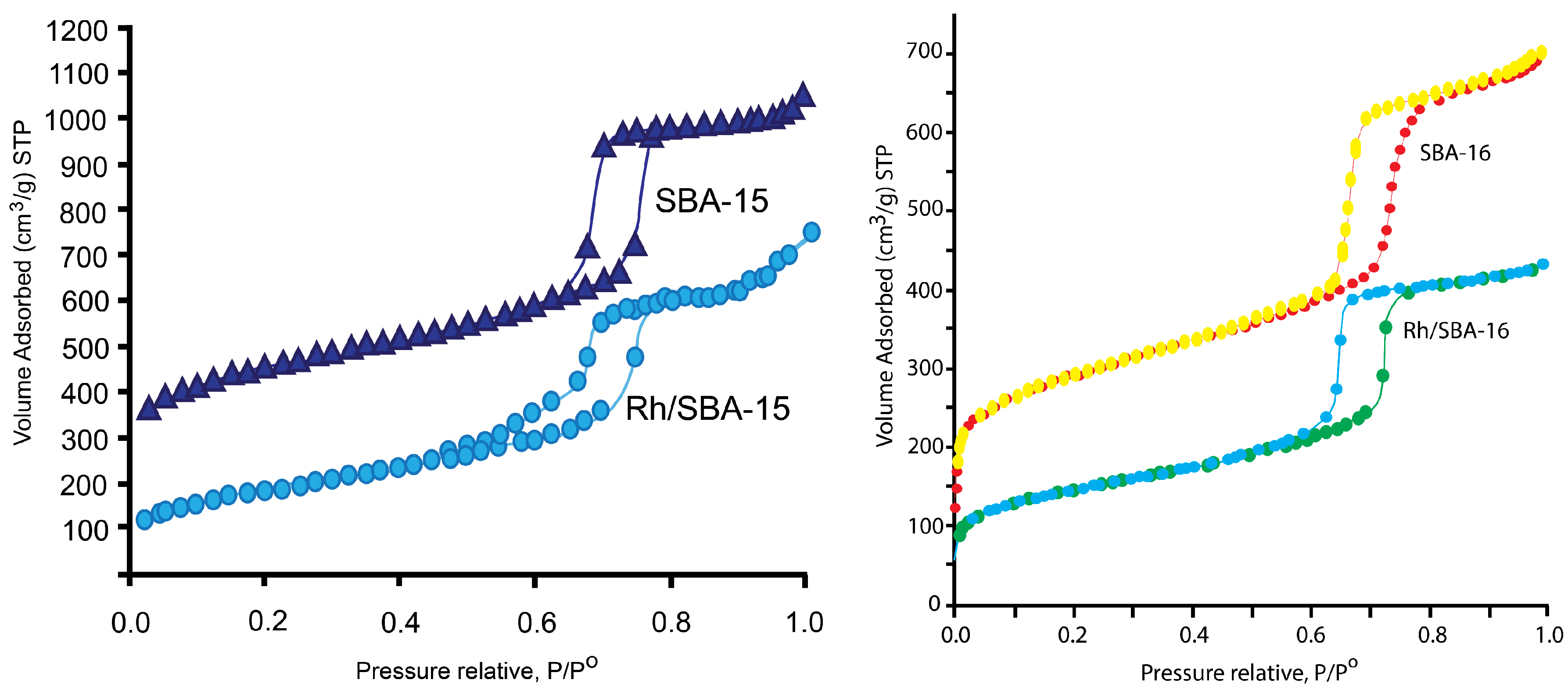
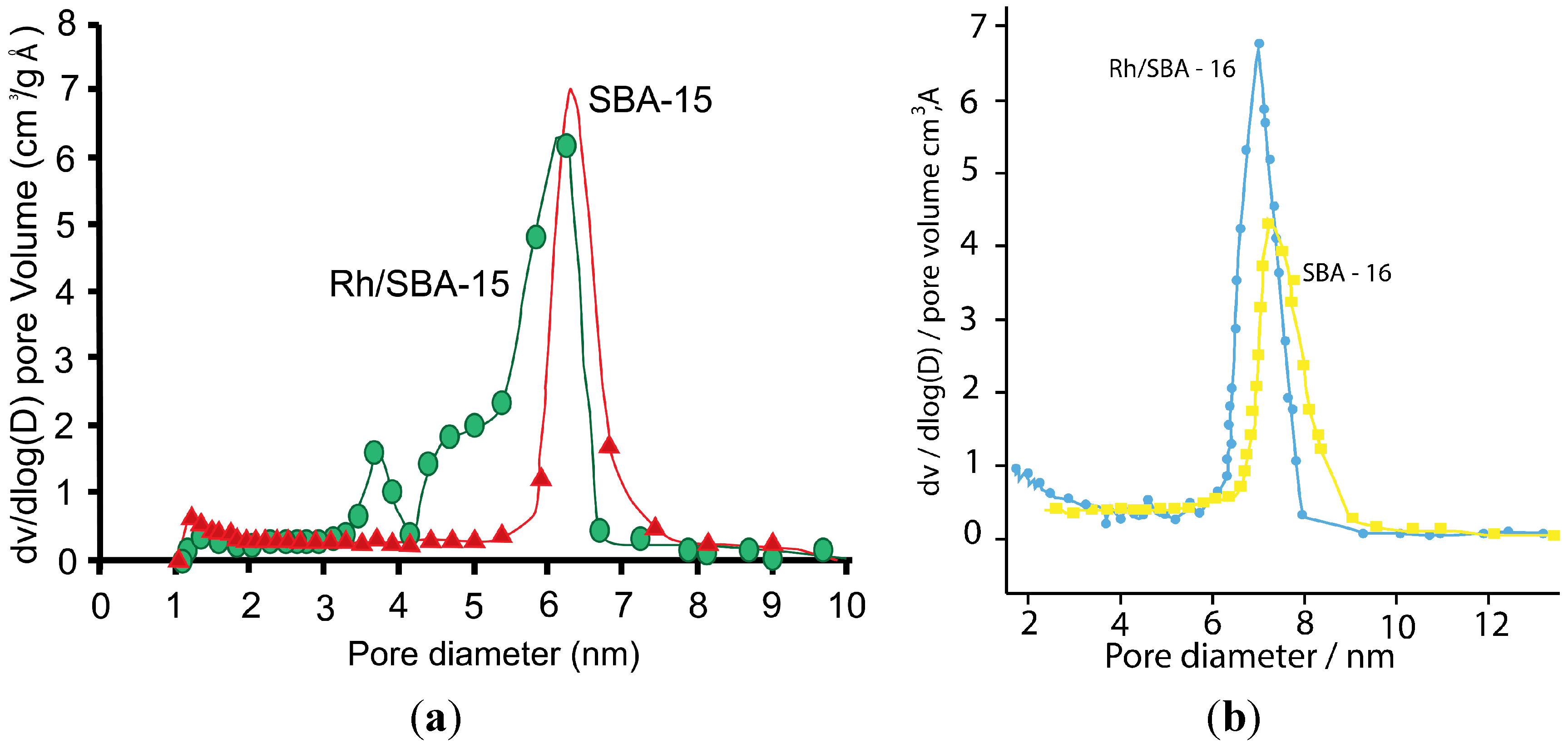
| Catalyst | SBET (m2 g−1) | DBJH (nm) | VBJH (cm3 g−1) |
|---|---|---|---|
| Rh/SBA-16 | 575 | 7.3 | 0.68 |
| SBA-16 | 655 | 7.6 | 1.01 |
| Rh/SBA-15 | 625 | 6.8 | 1.03 |
| SBA-15 | 910 | 7.2 | 1.39 |
2.1.2. Small and Wide Angle XRD
2.1.3. Transmission Electronic Microscope (TEM)
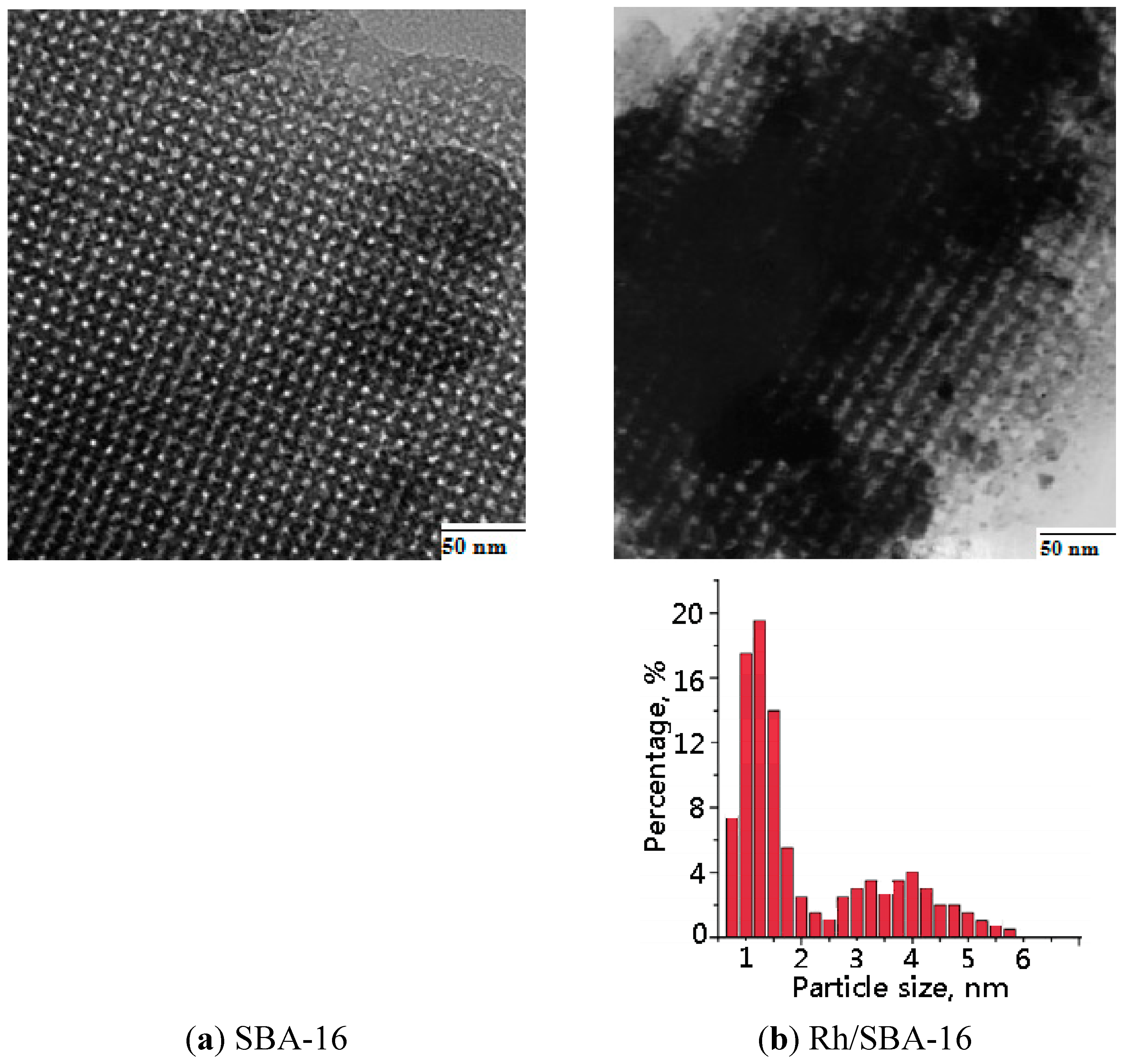
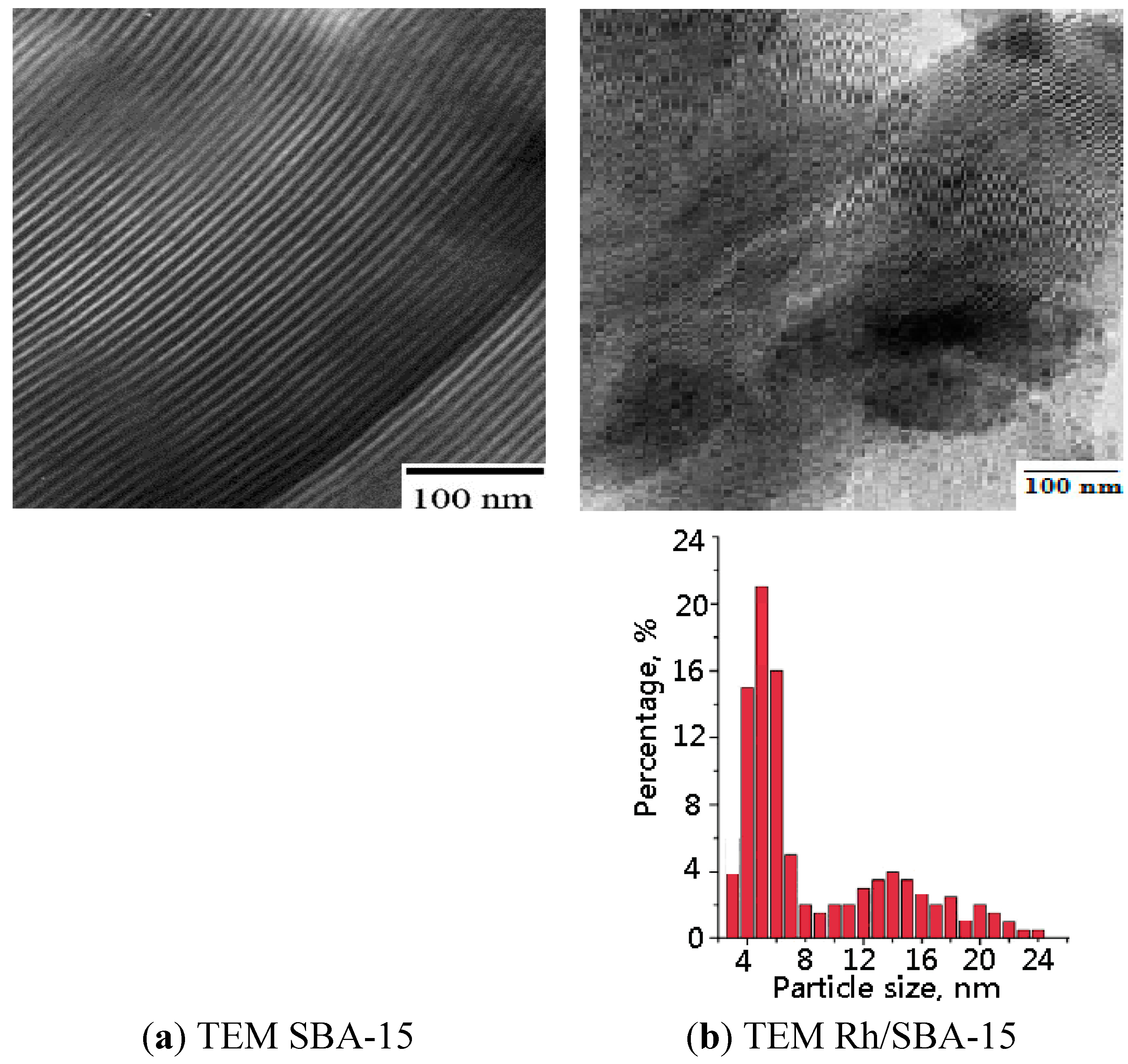
2.1.4. Fourier Transform Infrared Spectroscopy (FTIR)
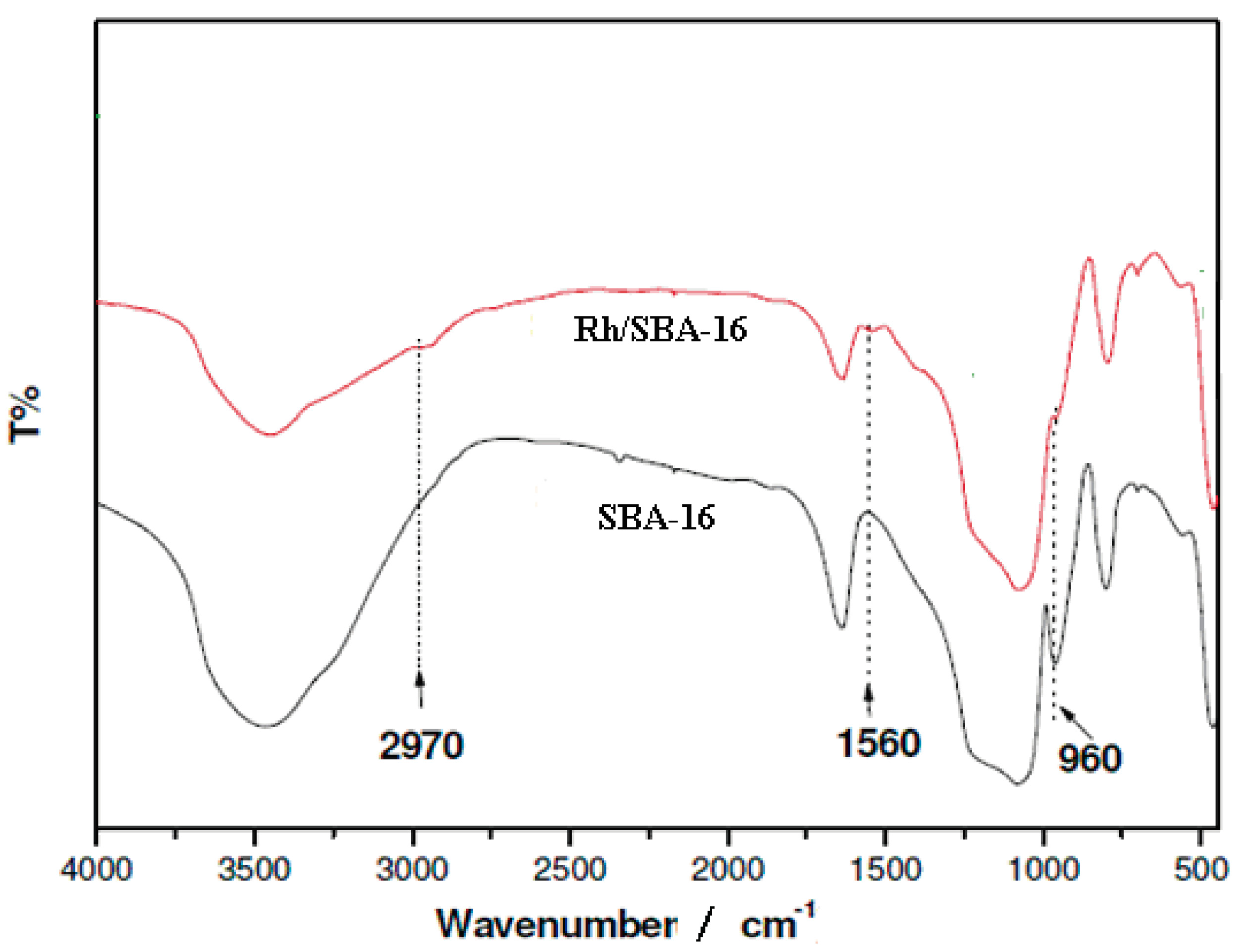
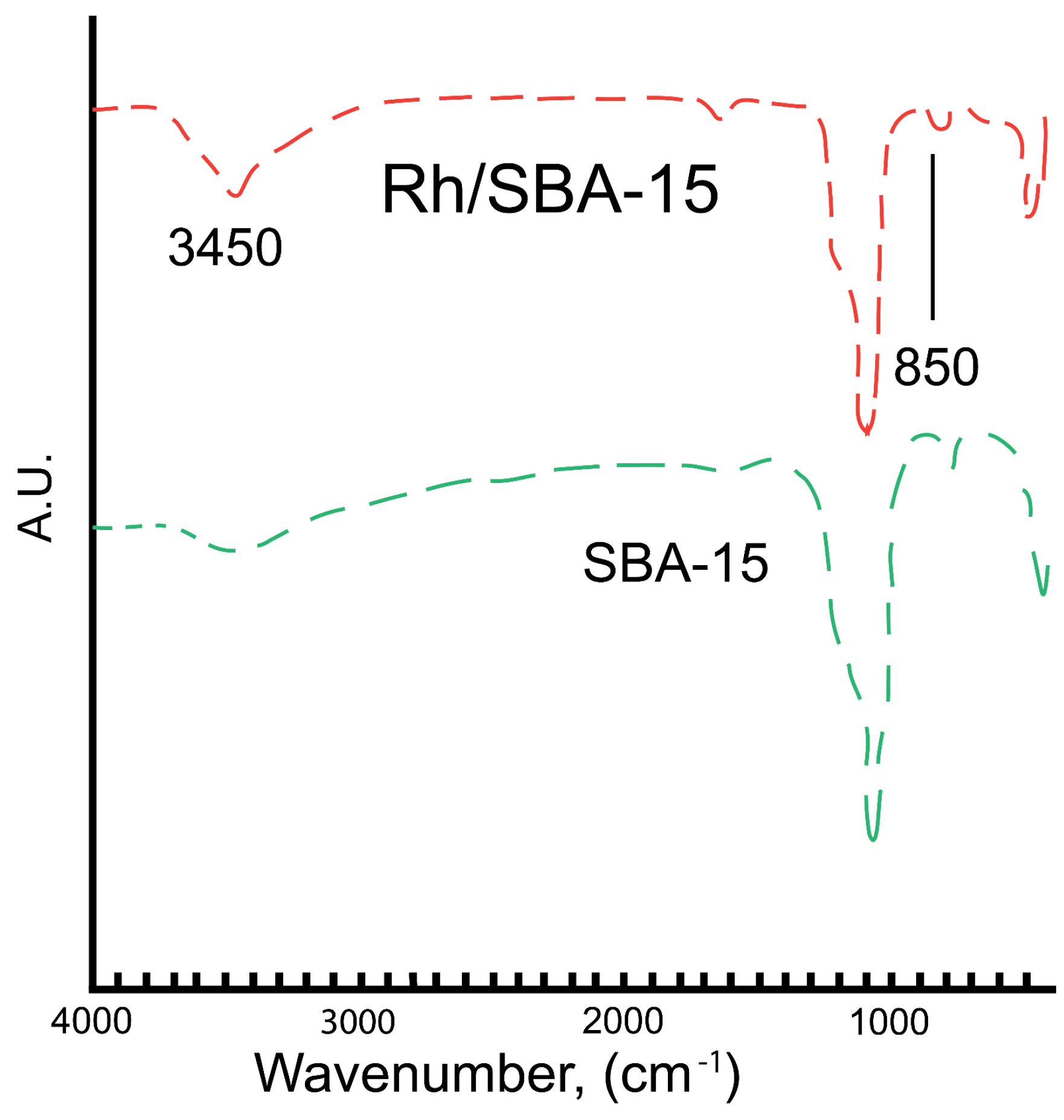
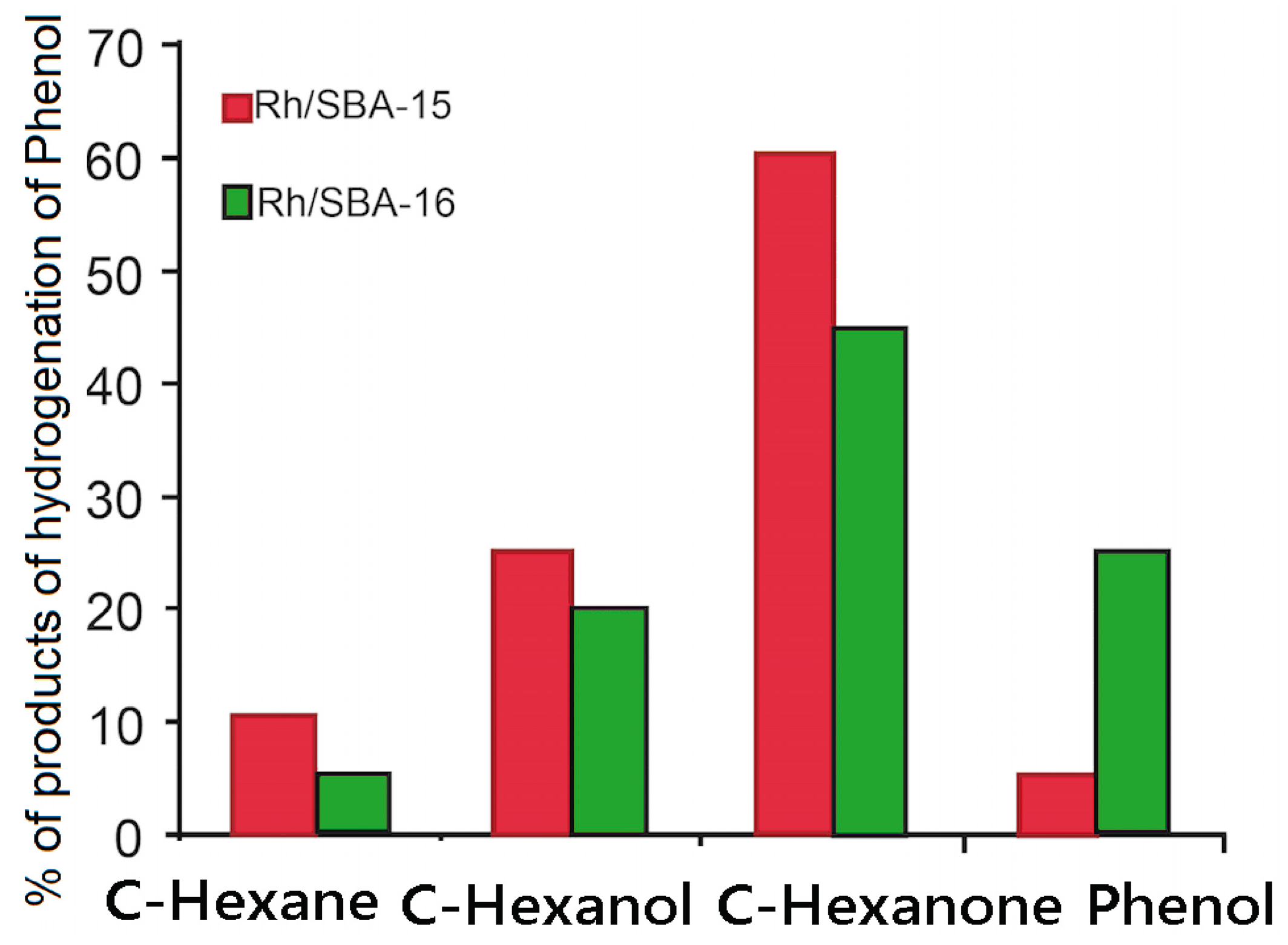
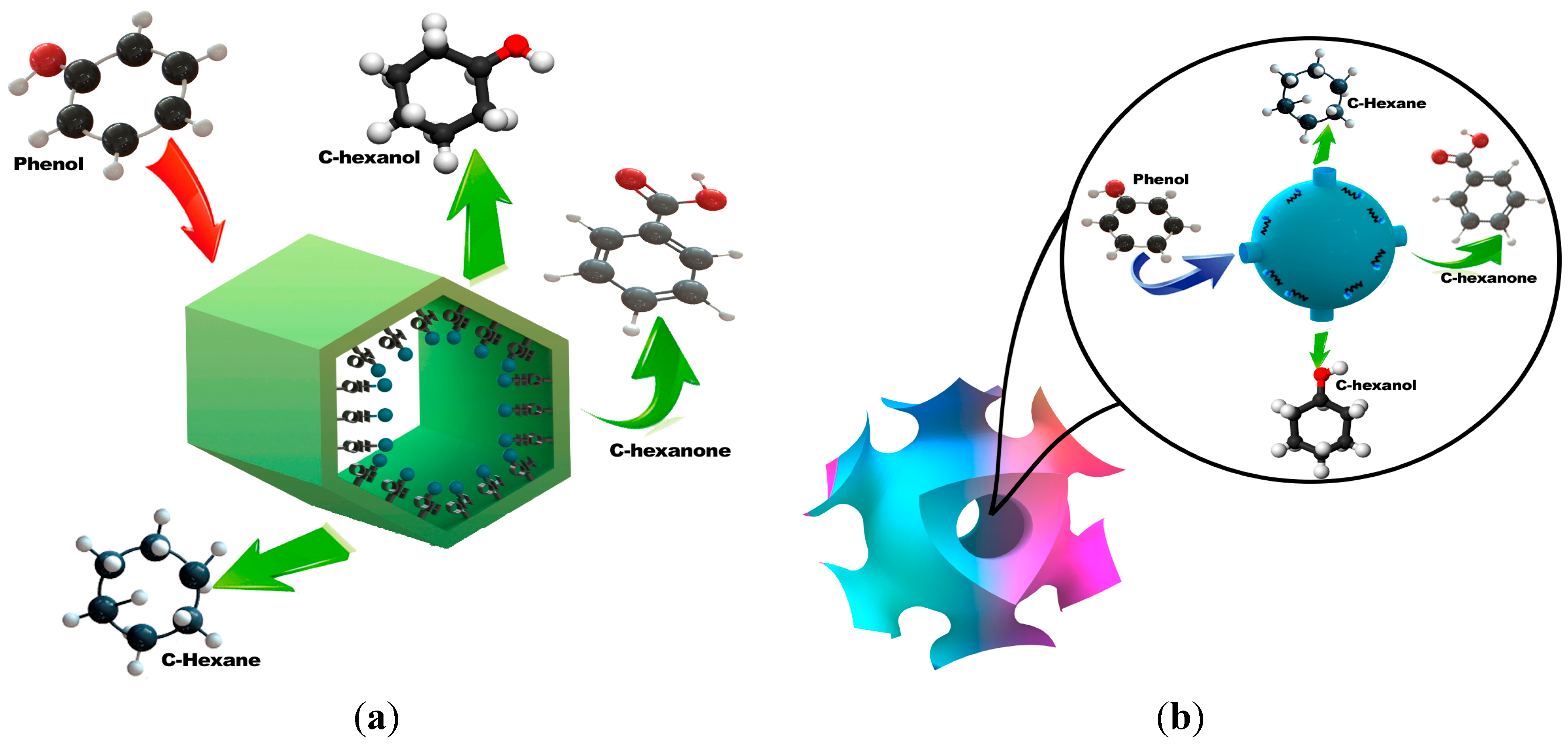
2.2. Hydrogenation of Phenol over Rh/SBA
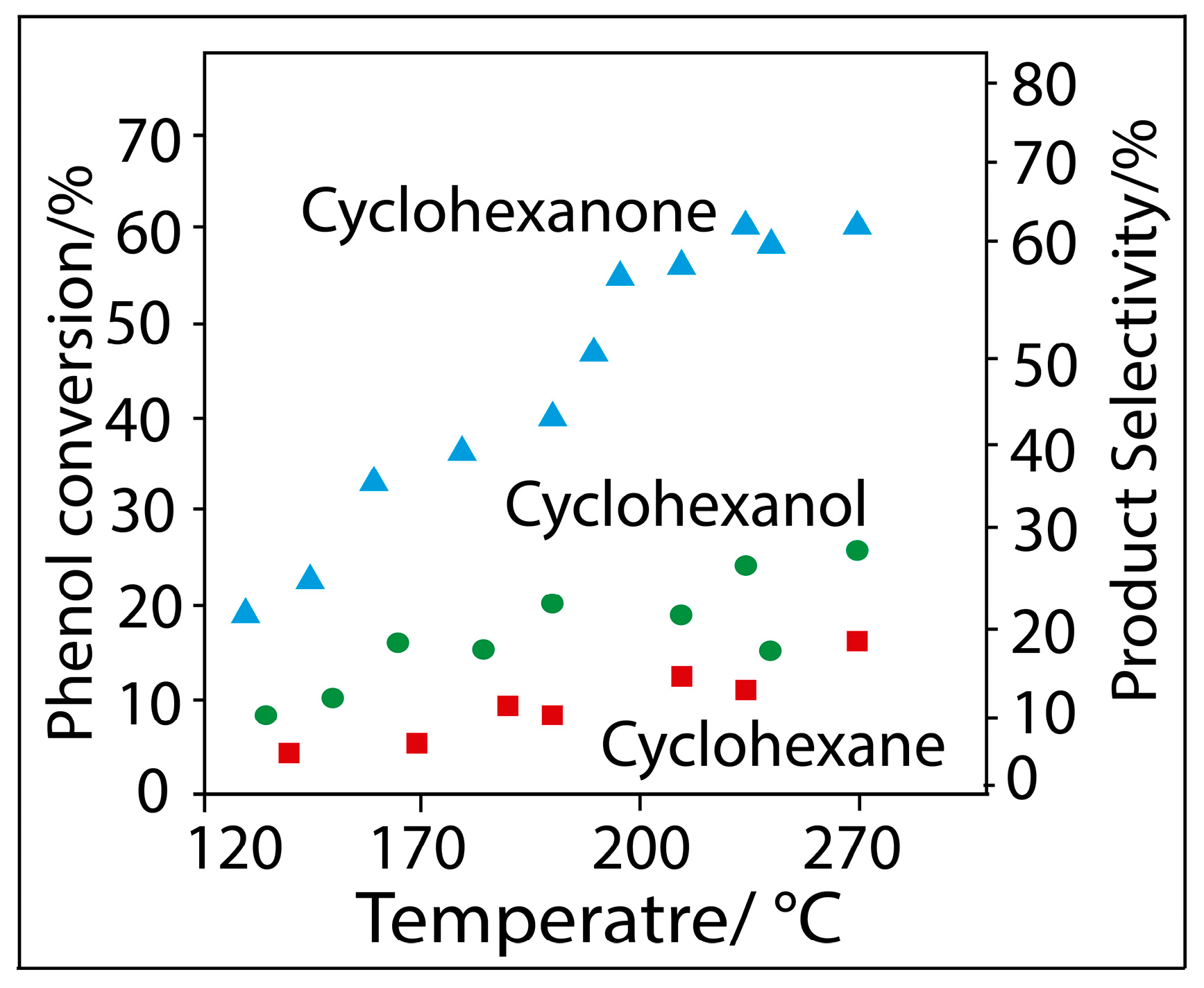

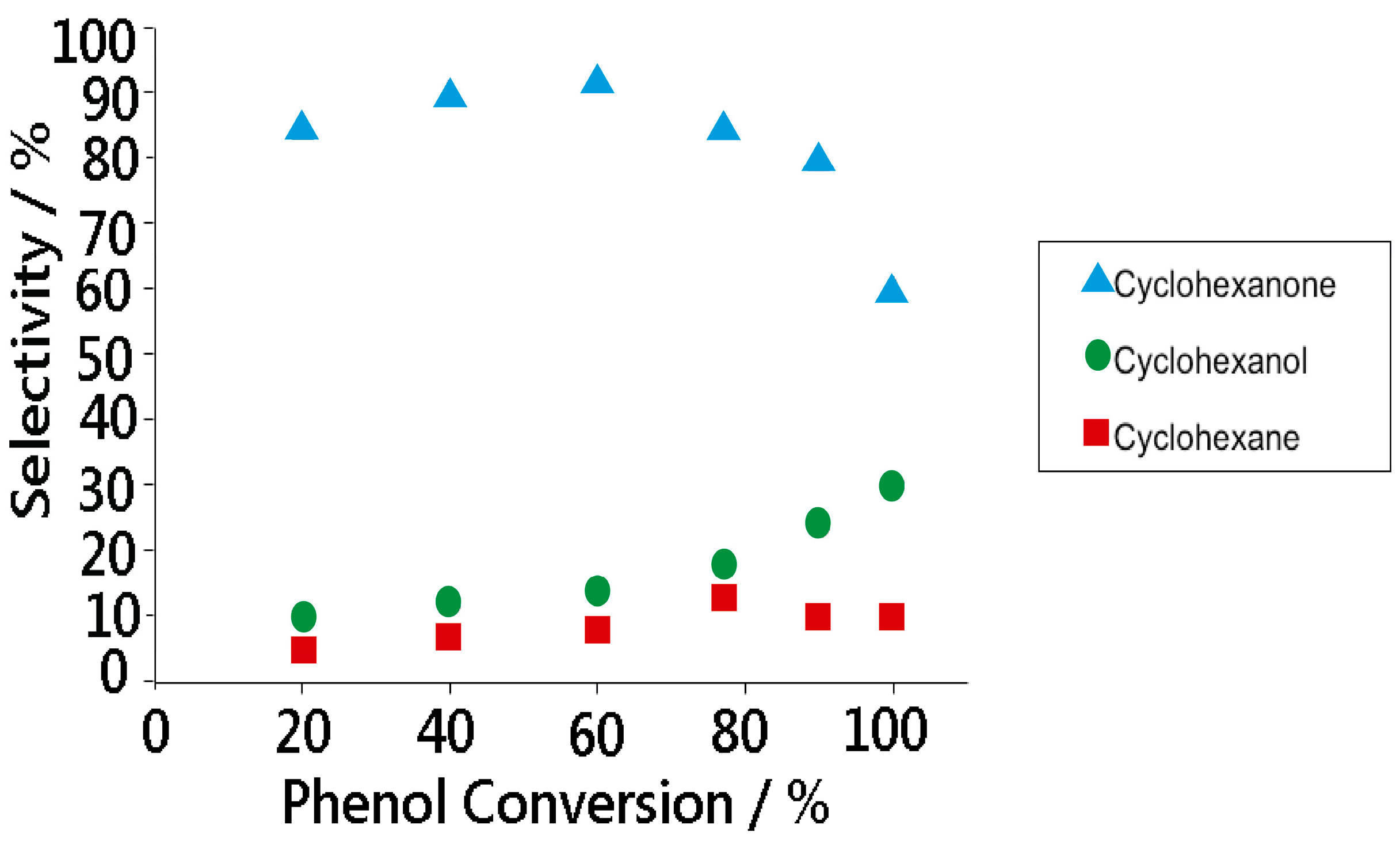
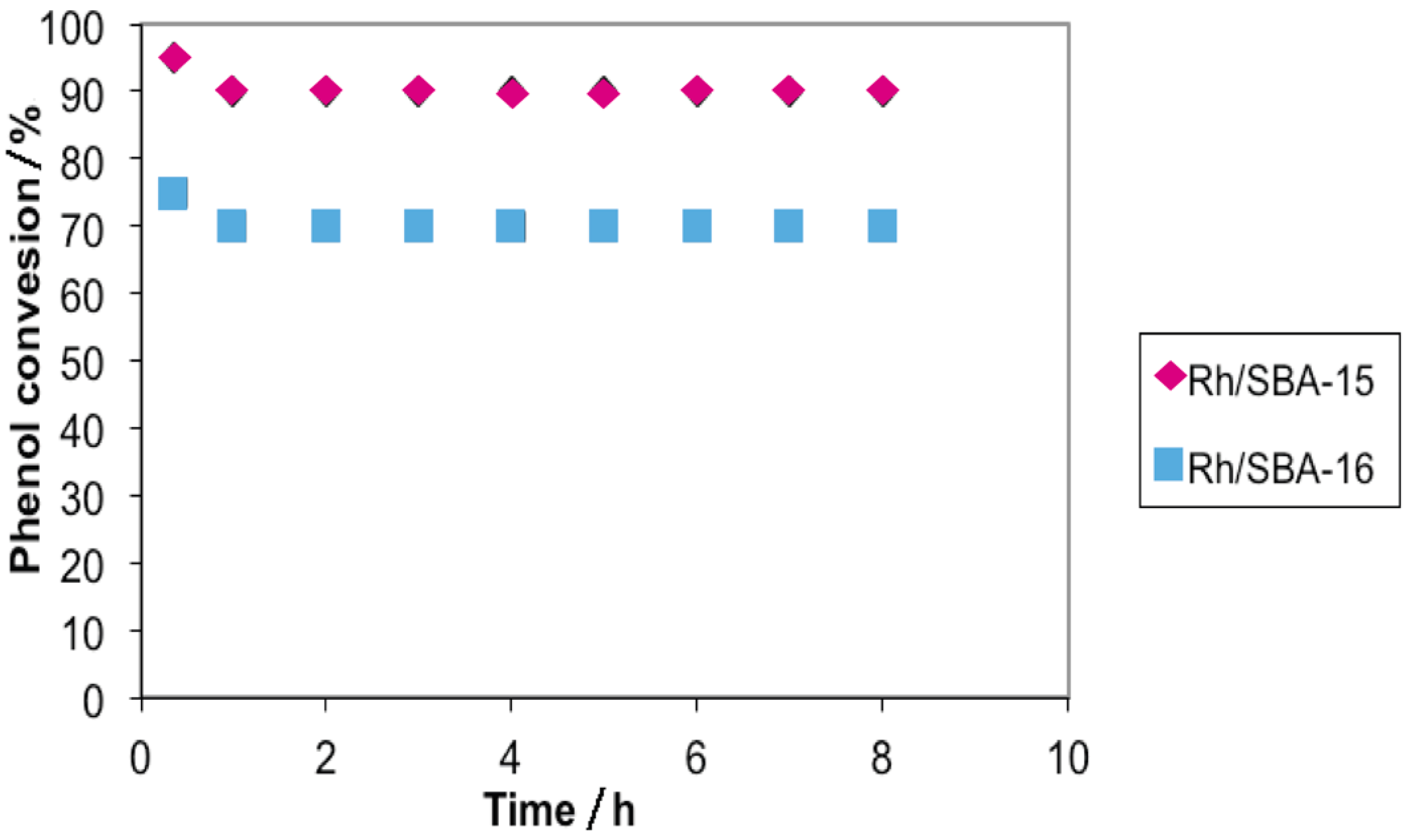
3. Experimental Section
3.1. Sample Preparation
3.1.1. Synthesis of SBA-15
3.1.2. Synthesis of SBA-16
3.1.3. Catalyst Synthesis
3.2. Sample Characterisation
3.3. Hydrogenation of Phenol
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dojido, J.R.; Best, G.A. Chemistry of Water and Water Pollution; Prentice Hall: New York, NY, USA, 1993; Chapter 4, Section 19. [Google Scholar]
- Park, C.; Keane, M.A. Catalyst support effects: Gas-phase hydrogenation of phenol over palladium. J. Colloid Interface Sci. 2003, 266, 183–194. [Google Scholar]
- Matatov-Meytal, Y.I.; Sheintuch, M. Catalytic abatement of water pollutants. Ind. Eng. Chem. Res. 1998, 37, 309–326. [Google Scholar] [CrossRef]
- Santos, A.; Yustos, P.; Quintanilla, A.; García-Ochoa, F.; Casas, J.A.; Rodríguez, J.J. Route of the catalytic oxidation of phenol in aqueous phase. Appl. Catal. B: Environ. 2002, 39, 97–113. [Google Scholar] [CrossRef]
- Pera-Titus, M.; Garcia-Molina, V.; Banos, M.A.; Gimenez, J.; Esplugas, S. Degradation of chlorophenols by means of advanced oxidation processes: A general review. Appl. Catal. B: Environ. 2004, 47, 219–256. [Google Scholar] [CrossRef]
- Zazo, J.A.; Casas, J.A.; Mohedano, A.F.; Gilarranz, M.A.; Rodríguez, J.J. Chemical pathway and kinetics of phenol oxidation by Fenton’s reagent. Environ. Sci. Technol. 2005, 39, 9295–9302. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, S.T.; Jhansi Lakshmi, L.; Kanta Rao, P. Selectivity dependence on the alloying element of carbon supported Pt-alloy catalysts in the hydrogenation of phenol. Appl. Catal. A 1994, 110, 167–172. [Google Scholar] [CrossRef]
- Shin, E.-J.; Keane, M.A. Gas-phase hydrogenation/hydrogenolysis of phenol over supported nickel catalysts. Ind. Eng. Chem. Res. 2000, 39, 883–892. [Google Scholar] [CrossRef]
- Talukdar, A.K.; Bhattacharyya, K.G. Hydrogenation of phenol over supported platinum and palladium catalysts. Appl. Catal. A 1993, 96, 229–239. [Google Scholar] [CrossRef]
- Mahata, N.; Vishwanathan, V. Influence of palladium precursors on structural properties and phenol hydrogenation characteristics of supported palladium catalysts. J. Catal. 2000, 196, 262–270. [Google Scholar] [CrossRef]
- Calvo, L.; Mohedano, A.F.; Casas, J.A.; Gilarranz, M.A.; Rodríguez, J.J. Treatment of chlorophenols-bearing wastewaters through hydrodechlorination using Pd/activated carbon catalysts. Carbon 2004, 42, 1377–1381. [Google Scholar] [CrossRef]
- Schuth, C.; Reinhard, M. Hydrodechlorination and Hydrogenation of Aromatic Compounds over Palladium on Alumina in Hydrogen-Saturated Water. Appl. Catal. B-Environ. 1998, 18, 215–221. [Google Scholar] [CrossRef]
- Calvo, L.; Mohedano, A.F.; Casas, J.A.; Gilarranz, M.A.; Rodríguez, J.J. Effects of support surface composition on the activity and selectivity of Pd/C catalysts in aqueous-phase hydrodechlorination reactions. Ind. Eng. Chem. Res. 2005, 44, 6661–6667. [Google Scholar] [CrossRef]
- Díaz, E.; Mohedano, A.F.; Calvo, L.; Gilarranz, M.A.; Casas, J.A.; Rodríguez, J.J. Hydrogenation of phenol in aqueous phase with palladium on activated carbon catalysts. Chem. Eng. J. 2007, 131, 65–71. [Google Scholar] [CrossRef]
- Dodgson, I.; Griffin, K.; Barberis, G.; Piganttaro, F.; Tauszik, G. A low cost phenol to cyclohexanone process. Chem. Ind. 1989, 830–841. [Google Scholar]
- Neri, G.; Visco, A.M.; Donato, A.; Milone, C.; Malentacchi, M.; Guibitosa, G. Hydrogenation of phenol to cyclohexanone over palladium and alkali-doped palladium catalysts. Appl. Catal. A 1994, 110, 49–55. [Google Scholar] [CrossRef]
- Mahata, N.; Raghavan, K.V.; Vishwanathan, V.; Park, C.; Keane, M.A. Phenol hydrogenation over palladium supported on magnesia: Relationship between catalyst structure and performance. Phys. Chem. Chem. Phys. 2001, 3, 2712–2722. [Google Scholar] [CrossRef]
- Cosimo, J.I.D.; Díez, V.K.; Xu, M.; Iglesia, E.; Apesteguía, C.R. Structure and Surface and Catalytic Properties of Mg-Al Basic Oxides. J. Catal. 1998, 178, 499–510. [Google Scholar] [CrossRef]
- Narayanan, S.; Krishna, K. Hydrotalcite-supported palladium catalysts: Part I: Preparation, characterization of hydrotalcites and palladium on uncalcined hydrotalcites for CO chemisorption and phenol hydrogenation. Catal. Today 1999, 49, 57–64. [Google Scholar] [CrossRef]
- Hu, S.; Xue, M.; Chen, H.; Shen, J. The effect of surface acidic and basic properties on the hydrogenation of aromatic rings over the supported nickel catalysts. Chem. Eng. J. 2010, 162, 371–379. [Google Scholar] [CrossRef]
- Graham, G.W.; Jen, H.-W.; McCabe, R.W.; Straccia, A.M.; Haack, L.P. Characterization of model automotive exhaust catalysts: Pd on Zr-rich ceria–zirconia supports. Catal. Lett. 2000, 67, 99–105. [Google Scholar] [CrossRef]
- Cheng, L.; Dai, Q.; Li, H.; Wang, X. Highly selective hydrogenation of phenol and derivatives over Pd catalysts supported on SiO2 and γ-Al2O3 in aqueous media. Catal. Commun. 2014, 57, 23–28. [Google Scholar] [CrossRef]
- González-Velasco, J.R.; González-Marcos, M.P.; Arnaiz, S.; Gutiérrez-Ortiz, J.I.; Gutiérrez-Ortiz, M.A. Activity and selectivity of palladium catalysts during the liquid-phase hydrogenation of phenol. Influence of temperature and pressure. Ind. Eng. Chem. Res. 1995, 34, 1031–1036. [Google Scholar]
- Wang, H.; Zhao, F.; Fujita, S.I.; Arai, M. Hydrogenation of phenol in scCO2 over carbon nanofiber supported Rh catalyst. Catal. Commun. 2008, 9, 362–368. [Google Scholar] [CrossRef]
- Shore, S.G.; Ding, E.; Park, C.; Keane, M.A. Vapor phase hydrogenation of phenol over silica supported Pd and PdAYb catalysts. Catal. Commun. 2002, 3, 77–84. [Google Scholar] [CrossRef]
- Pérez, Y.; Fajardo, M.; Corma, A. Highly selective palladium supported catalyst for hydrogenation of phenol in aqueous phase. Catal. Commun. 2011, 12, 1071–1074. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Liaw, C.W.; Lee, L.I. Selective hydrogenation of phenol to cyclohexanone over palladium supported on calcined Mg/Al hydrotalcite. App. Catal. A: Gen. 1999, 177, 1–8. [Google Scholar] [CrossRef]
- Lee, B.I.; Bae, D.; Kang, J.-K.; Kim, H.; Byeon, S.-H. Synthesis of SBA-15 Supported Rh Nanoparticles with High Loading Density and its Catalytic Hydrogenation of Phenol in Supercritical Carbon Dioxide. Bull. Korean Chem. Soc. 2009, 30, 1701–1702. [Google Scholar] [CrossRef]
- Sharma, R.V.; Baroi, C.; Dalai, A.K. Production of biodiesel from unrefined canola oil using mesoporous sulfated Ti-SBA-15 catalyst. Catal. Today 2014, 237, 3–12. [Google Scholar] [CrossRef]
- Jin, M.; Kim, J.H.; Kim, J.M.; Jeon, J.-K.; Jurng, J.; Bae, G.-N.; Park, Y.-K. Benzene oxidation with ozone over MnOx/SBA-15 catalysts. Catal. Today 2013, 204, 108–113. [Google Scholar] [CrossRef]
- Cao, Y.-L.; Wang, J.-W.; Li, Q.-F.; Yin, N.; Liu, Z.-M.; Kang, M.-Q.; Zhu, Y.L. Hydrolytic hydrogenation of cellulose over Ni-WO3/SBA-15. J. Fuel Chem. Technol. 2013, 41, 943–949. [Google Scholar] [CrossRef]
- Zhu, Q.; Maeno, S.; Nishimoto, R.; Miyamoto, T.; Fukushima, M. Oxidative degradation of pentabromophenol in the presence of humic substances catalyzed by a SBA-15 supported iron-porphyrin catalyst. J. Mol. Catal. A. Chem. 2014, 385, 31–37. [Google Scholar] [CrossRef]
- Ojeda, M.L.; Esparza, J.M.; Campero, A.; Cordero, S.; Kornhauser, I.; Rojas, F. On comparing BJH and NLDFT pore-size distributions determined from N2 sorption on SBA-15 substrata. Phys. Chem. Chem. Phys. 2003, 5, 1859–1866. [Google Scholar] [CrossRef]
- Villarroel-Rocha, J.; Barrera, D.; Sapag, K. Introducing a self-consistent test and the corresponding modification in the Barrett, Joyner and Halenda method for pore-size determination. Microporous Mesoporous Mater. 2014, 200, 68–78. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Broekhoff, J.C.P. Mesopore determination from nitrogen sorption isotherms: Fundamentals, scope, limitations. Stud. Surf. Sci. Catal. 1979, 3, 663–684. [Google Scholar]
- Moreno-Piraján, J.C.; Giraldo, L. Lipase supported on mesoporous materials as a catalyst in the synthesis of biodiesel from Persea americana mill oil. J. Mol. Catal. B: Enzym. 2012, 156, 45–50. [Google Scholar]
- Yang, Y.; Karmakar, S.; Zhang, J.; Yu, M.; Mitter, N.; Yu, C. Synthesis of SBA-15 rods with small sizes for enhanced cellular uptake. J. Mater. Chem. B 2014, 2, 4929–4934. [Google Scholar] [CrossRef]
- Titirici, M.M.; Thomas, A.; Antonietti, M. Aminated hydrophilic ordered mesoporous carbons. J. Mater. Chem. 2007, 17, 3412–3418. [Google Scholar] [CrossRef]
- Margolese, D.; Melero, J.A.; Christiansen, S.C.; Chmelka, B.F.; Stucky, G.D. Direct syntheses of ordered SBA-15 mesoporous silica containing sulfonic acid groups. Chem. Mater. 2000, 12, 2448–2459. [Google Scholar] [CrossRef]
- Chen, G.; Guo, C.-Y.; Zhang, X.; Huang, Z.; Yuan, G. Direct conversion of syngas to ethanol over Rh/Mn-supported on modified SBA-15 molecular sieves: Effect of supports. Fuel Process. Technol. 2011, 92, 456–461. [Google Scholar] [CrossRef]
- Wang, H.-B.; Zhang, Y.-H.; Yang, H.-L.; Ma, Z.-Y.; Zhang, F.-W.; Sun, J.; Ma, J.-T. Palladium immobilized in the nanocages of SBA-16: An efficient and recyclable catalyst for Suzuki coupling reaction. Microporous Mesoporous Mater. 2013, 168, 65–72. [Google Scholar] [CrossRef]
- Rivera-Muñoz, E.M.; Huirache-Acuña, R. Sol gel-derived SBA-16 mesoporous material. Int. J. Mol. Sci. 2010, 11, 3069–3086. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Tang, Q.; Du, Y.; Liu, X.; Chen, Y.; Yang, Y. Mesostructured SBA-16 with excellent hydrothermal, thermal and mechanical stabilities: Modified synthesis and its catalytic application. J. Colloid Interface Sci. 2009, 333, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Jeon, G.S.; Chung, J.S. Preparation and characterization of silica-supported copper catalysts for the dehydrogenation of cyclohexanol to cyclohexanone. Appl. Catal. A 1994, 115, 29–44. [Google Scholar] [CrossRef]
- Scirè, S.; Minicò, S.; Crisafulli, C. Selective hydrogenation of phenol to cyclohexanone over supported Pd and Pd-Ca catalysts: An investigation on the influence of different supports and Pd precursors. Appl. Catal. A 2002, 235, 21–31. [Google Scholar] [CrossRef]
- Velu, S.; Kapoor, M.P.; Inagaki, S.; Suzuki, K. Vapor phase hydrogenation of phenol over palladium supported on mesoporous CeO2 and ZrO2. Appl. Catal. A 2003, 245, 317–331. [Google Scholar] [CrossRef]
- Sample Availability: Samples are not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giraldo, L.; Bastidas-Barranco, M.; Moreno-Piraján, J.C. Vapour Phase Hydrogenation of Phenol over Rhodium on SBA-15 and SBA-16. Molecules 2014, 19, 20594-20612. https://doi.org/10.3390/molecules191220594
Giraldo L, Bastidas-Barranco M, Moreno-Piraján JC. Vapour Phase Hydrogenation of Phenol over Rhodium on SBA-15 and SBA-16. Molecules. 2014; 19(12):20594-20612. https://doi.org/10.3390/molecules191220594
Chicago/Turabian StyleGiraldo, Liliana, Marlon Bastidas-Barranco, and Juan Carlos Moreno-Piraján. 2014. "Vapour Phase Hydrogenation of Phenol over Rhodium on SBA-15 and SBA-16" Molecules 19, no. 12: 20594-20612. https://doi.org/10.3390/molecules191220594
APA StyleGiraldo, L., Bastidas-Barranco, M., & Moreno-Piraján, J. C. (2014). Vapour Phase Hydrogenation of Phenol over Rhodium on SBA-15 and SBA-16. Molecules, 19(12), 20594-20612. https://doi.org/10.3390/molecules191220594






