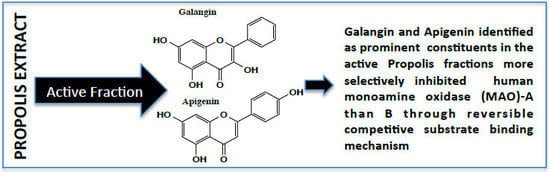
Monoamine Oxidase Inhibitory Constituents of Propolis: Kinetics and Mechanism of Inhibition of Recombinant Human MAO-A and MAO-B
Abstract
:1. Introduction
2. Results and Discussion
2.1. Determination of Inhibitory Effect of Galangin and Apigenin on MAO-A and -B
| Sample Name | Unit | Monoamine Oxidase-A (IC50) * | Monoamine Oxidase-B (IC50) * |
|---|---|---|---|
| Propolis Extract (prop-E) | μg/mL | 0.60 ± 0.12 | 6.99 ± 0.09 |
| Fraction 2 (9.0–17.5 min) # | μg/mL | 0.33 ± 0.03 | 0.31 ± 0.02 |
| Fraction 3 (17.5–20 min) # | μg/mL | 0.16 ± 0.11 | 0.23 ± 0.09 |
| Fraction 4 (20–30 min) # | μg/mL | 5.36 ± 0.22 | 0.98 ± 0.08 |
| Fraction 5 (30–35 min) # | μg/mL | 26.16 ± 0.29 | 22.19 ± 3.34 |
| Galangin | μM | 0.13 ± 0.01 | 3.65 ± 0.150 |
| Apigenin | μM | 0.64 ± 0.11 | 1.12 ± 0.27 |
| Quercetin | μM | 2.44 ± 0.12 | 38.66 ± 1.20 |
| Fisetin hydrate | μM | 2.10 ± 0.09 | >100 |
| Morin hydrate | μM | 5.88 ± 0.15 | 49.66 ± 0.56 |
| Toxifolin | μM | 65.06 ± 1.96 | 53.80 ± 3.91 |
| Clorgyline | μM | 0.0065 ± 0.0003 | - |
| Deprenyl | μM | - | 0.036 ± 0.0012 |
| Harmine | μM | 0.0039 ± 0.0003 | 27.50 ± 2.67 |
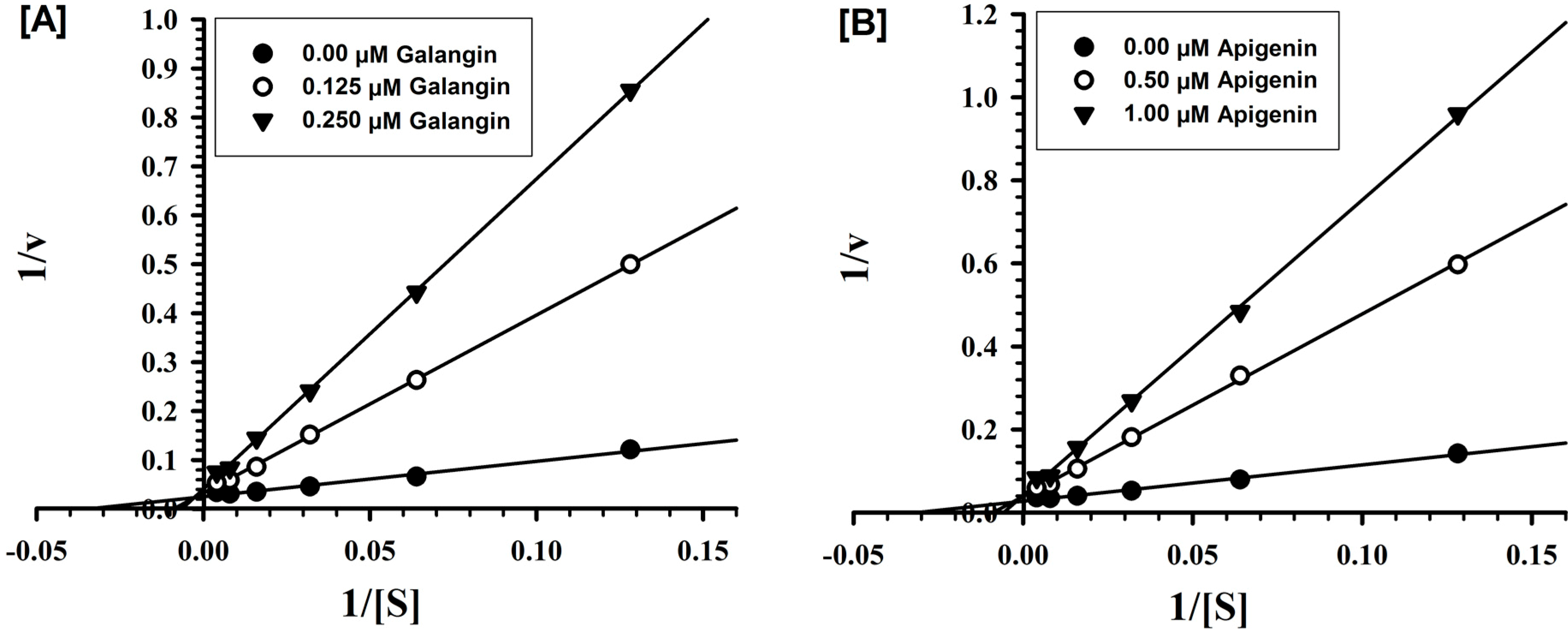
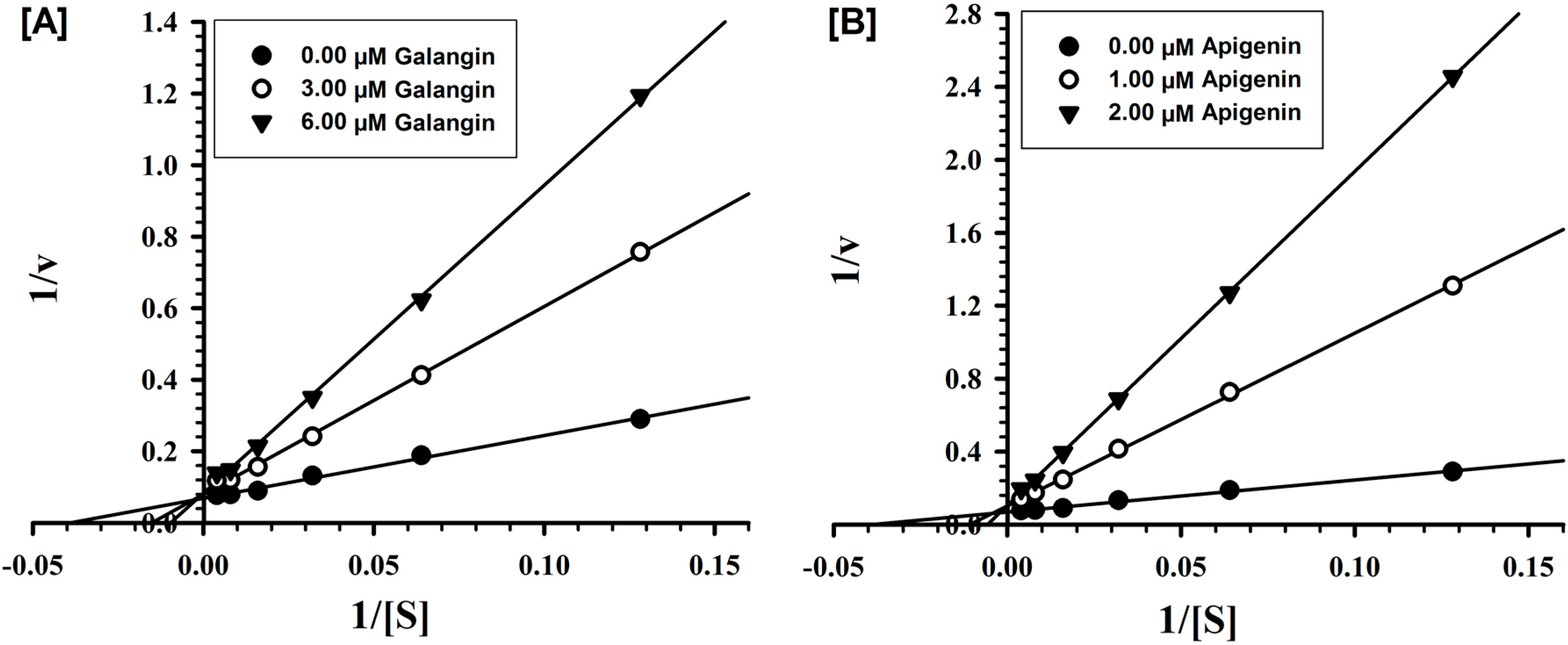
| Compound | Monoamine Oxidase-A | Monoamine Oxidase-B | ||
|---|---|---|---|---|
| Ki (μM) * | Type of Inhibition | Ki (μM) * | Type of Inhibition | |
| Galangin | 0.029 ± 0.004 | Competitive/Reversible | 1.998 ± 0.039 | Competitive/Reversible |
| Apigenin | 0.125 ± 0.014 | Competitive/Reversible | 0.238 ± 0.024 | Competitive/Reversible |
| Harmine | 0.0019 ± 0.0002 | Competitive/Reversible | - | - |
| Clorgyline | 0.0026 ± 0.006 | Mixed/Irreversible | - | - |
| Deprenyl | - | - | 0.0301 ± 0.003 | Mixed/Irreversible |
2.2. Evaluation of Inhibition Mechanism and Kinetics
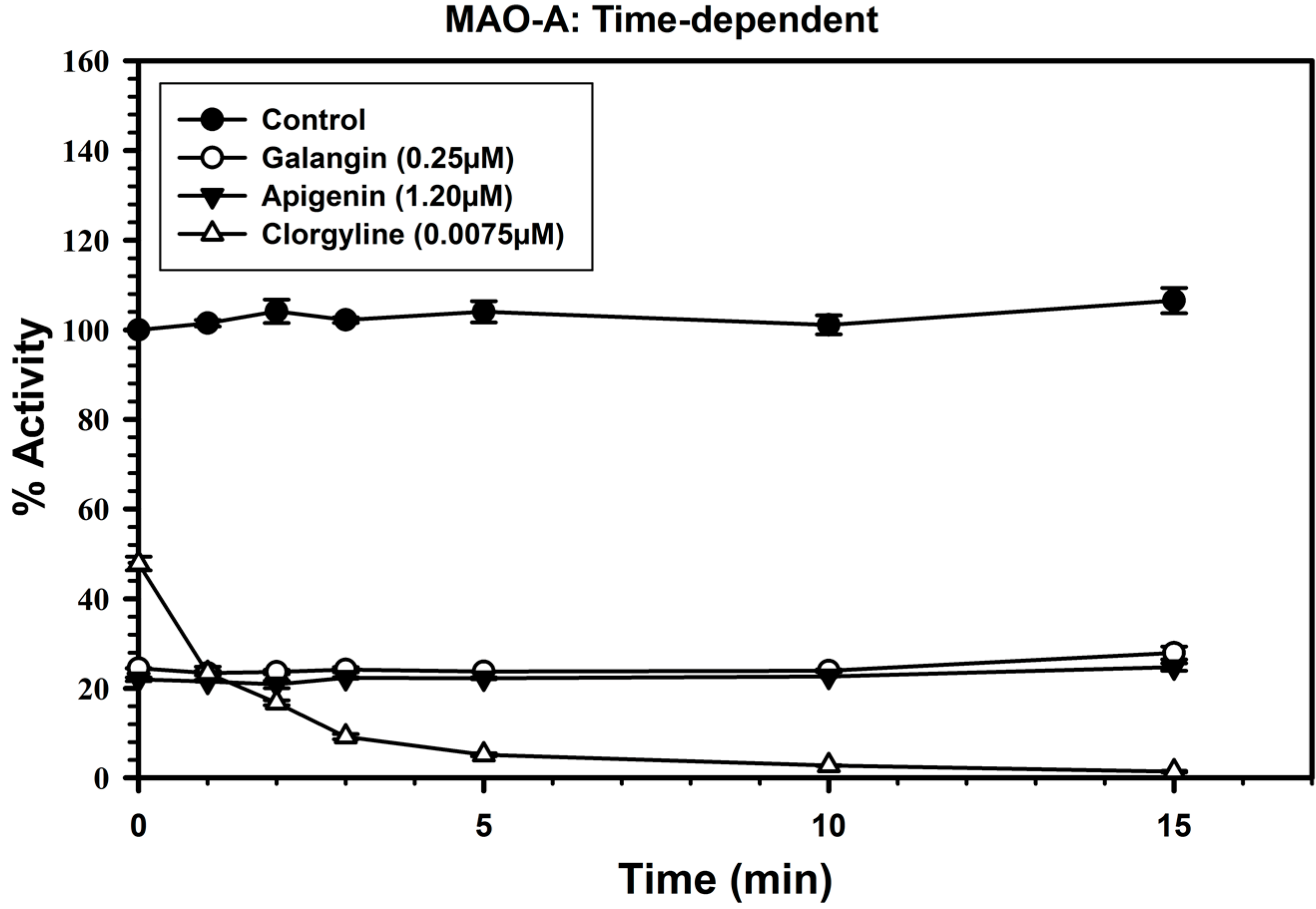
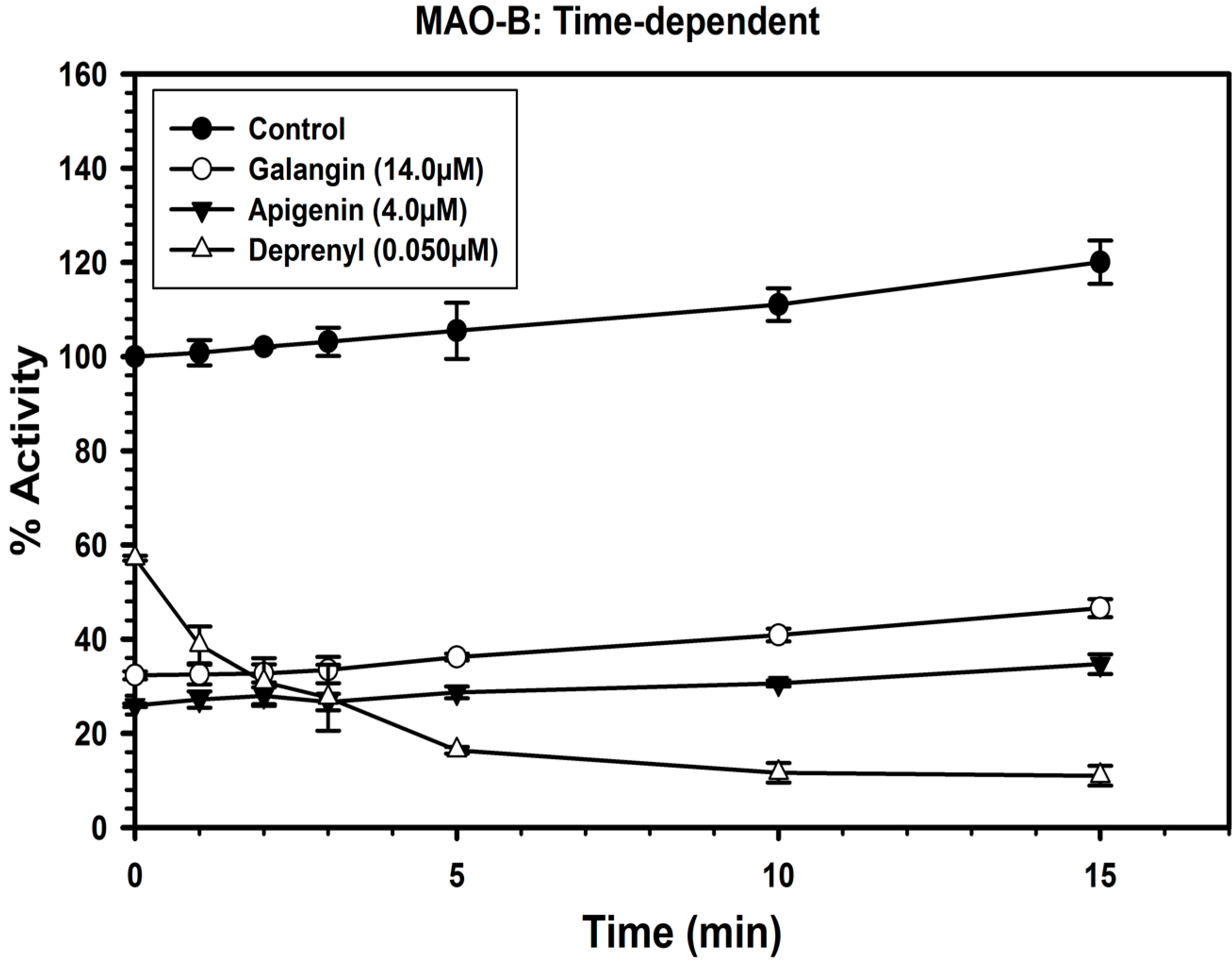
2.3. Analysis of Time-Dependent Enzyme Inhibition
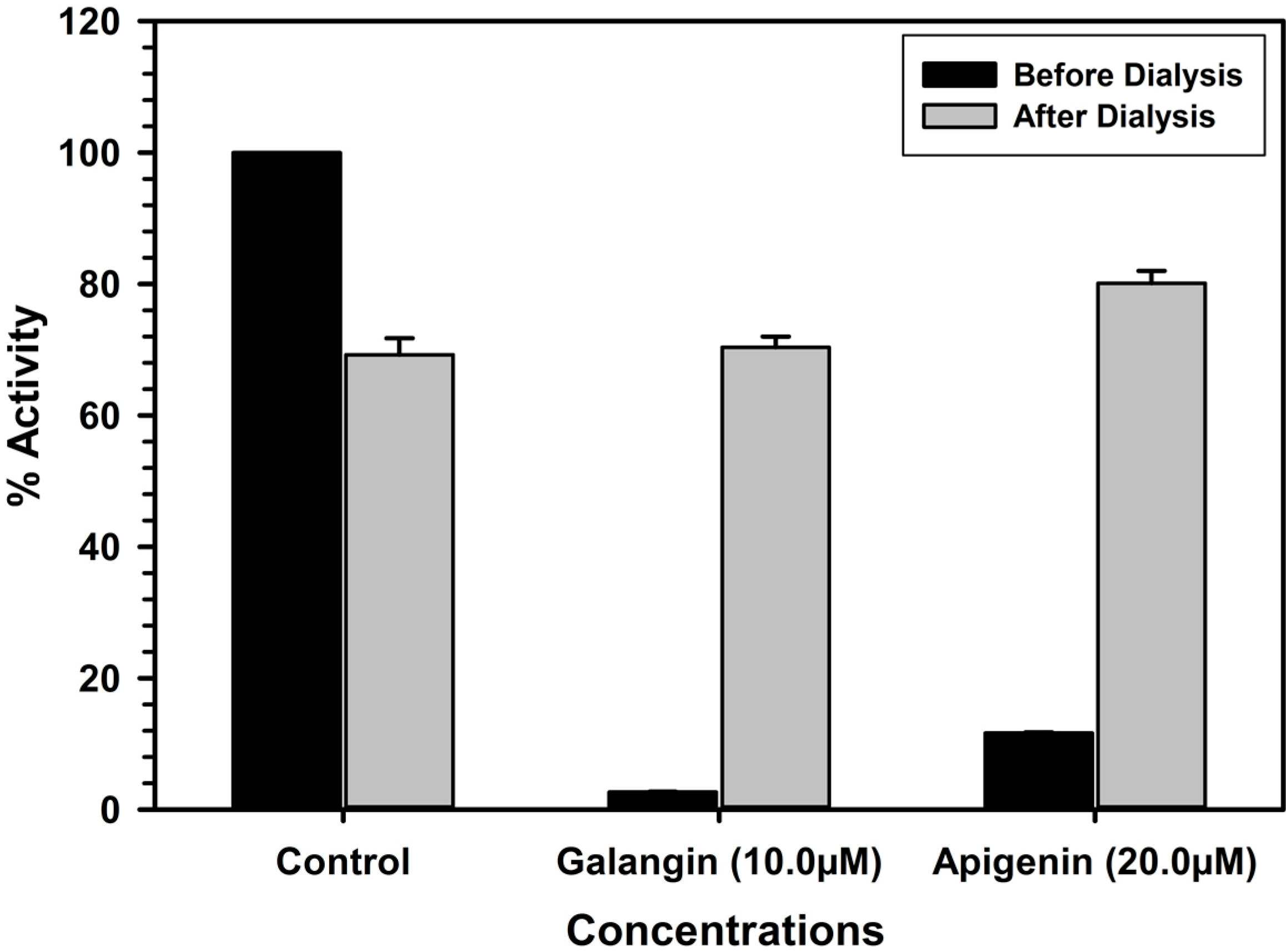
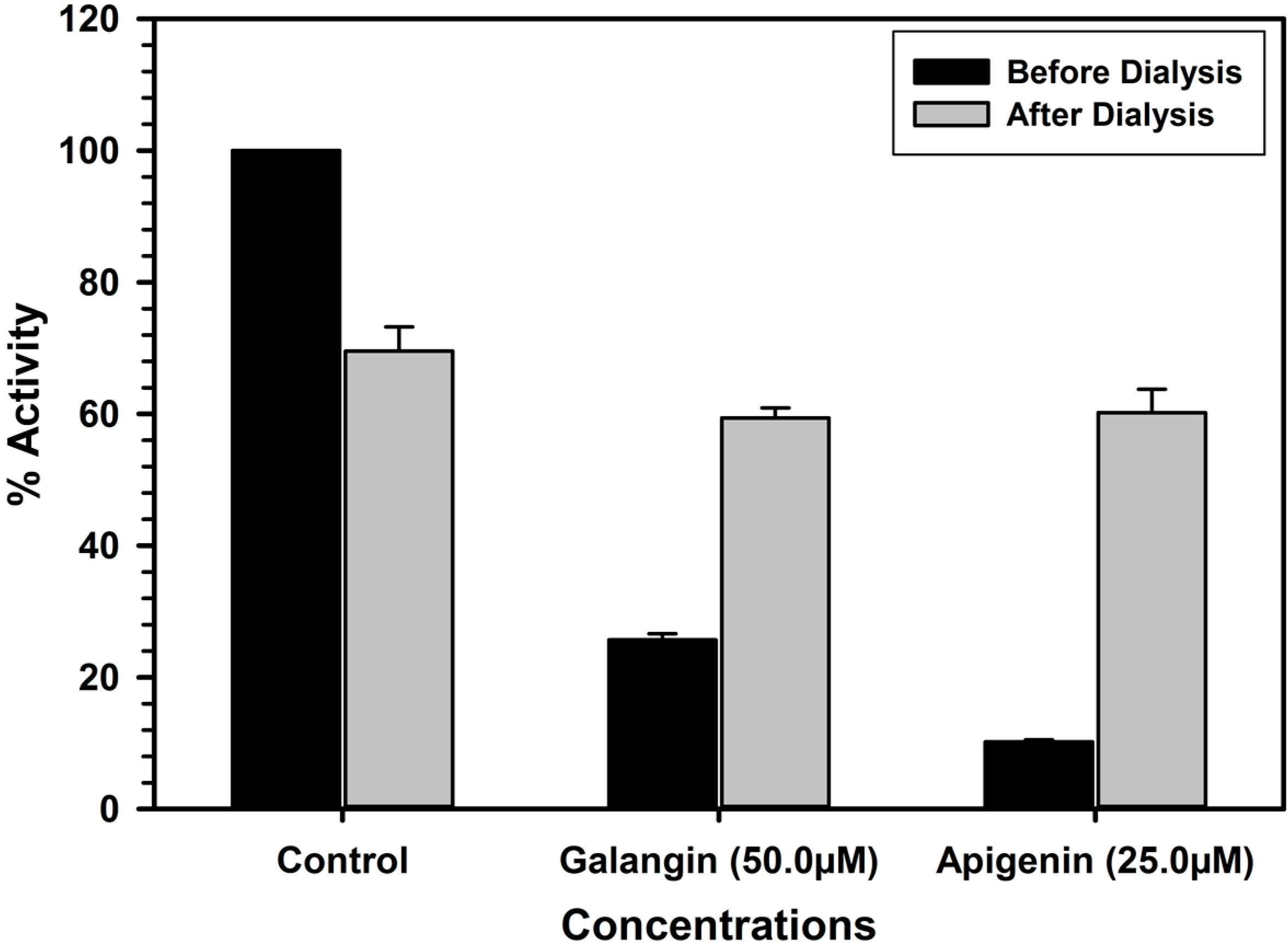
2.4. Analysis of Binding of Galangin and Apigenin with MAO-A and -B
2.5. Discussion
3. Experimental Section
3.1. Materials and Reagents
3.2. Preparation of Propolis Extracts
3.3. Fractionation of Propolis Extract and Analysis of MAO Inhibitory Constituents by HPLC
3.4. MAO Inhibition Assays
3.5. Determination of IC50 Values
3.6. Enzyme Kinetics Studies
3.7. Analysis of Time-Dependent Enzyme Inhibition
3.8. Analysis of Reversibility and Binding of the Inhibitor with MAO-A and B
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Groot, A.C. Propolis: A review of properties, applications, chemical composition, contact allergy, and other adverse effects. Dermatitis 2013, 24, 263–282. [Google Scholar]
- Toreti, V.C.; Sato, H.H.; Pastore, G.M.; Park, Y.K. Recent progress of propolis for its biological and chemical compositions and its botanical origin. Evid. Based Complement. Alternat. Med. 2013. [Google Scholar] [CrossRef]
- Chan, G.C.; Cheung, K.W.; Sze, D.M. The immunomodulatory and anticancer properties of propolis. Clin. Rev. Allergy Immunol. 2013, 44, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, T.; Farooqui, A.A. Beneficial effects of propolis on human health and neurological diseases. Front. Biosci. Elite Ed. 2012, 1, 779–793. [Google Scholar] [CrossRef]
- Watanabe, M.A.; Amarante, M.K.; Conti, B.J.; Sforcin, J.M. Cytotoxic constituents of propolis inducing anticancer effects: A review. J. Pharm. Pharmacol. 2011, 63, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Daleprane, J.B.; Abdalla, D.S. Emerging roles of propolis: Antioxidant, cardioprotective, and antiangiogenic actions. Evid. Based Complement. Alternat. Med. 2013, 2013, 175135. [Google Scholar] [CrossRef] [PubMed]
- Shih, J.C.; Chen, K.; Ridd, M.J. Monoamine oxidase: From genes to Behaviour. Annu. Rev. Neurosci. 1999, 22, 197–217. [Google Scholar] [CrossRef] [PubMed]
- Abell, C.W.; Kwan, S.W. Molecular characterization of monoamine oxidase A and B. Prog. Nucleic Acids Res. Mol. Biol. 2009, 65, 129–156. [Google Scholar]
- Cesura, A.M.; Pletscher, A. The new generation of monoamine oxidase inhibitors. Prog. Drug Res. 1992, 38, 171–297. [Google Scholar] [PubMed]
- Yamada, M.; Yasuhara, H. Clinical pharmacology of MAO inhibitors: Safety and future. Neurotoxico 2004, 25, 215–221. [Google Scholar] [CrossRef]
- Youdim, M.B.H.; Edmondson, D.; Tipton, K.F. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 2006, 7, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Shulman, K.I.; Herrmann, N.; Walker, S.E. Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs 2013, 27, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Finberg, J.P.; Gillman, K. Selective inhibitors of monoamine oxidase type B and the “cheese effect”. Int. Rev. Neurobiol. 2013, 100, 169–190. [Google Scholar]
- Mabberley, D.J. The Plant Book. A Portable Dictionary of the Higher Plants, 2nd ed.; Univ Press Cambridge: Cambridge, UK, 1977; p. 858. [Google Scholar]
- Kahn, D.; Silver, J.M.; Opler, L.A. The safety of switching rapidly from tricyclic antidepressants to monoamine oxidase inhibitors. J. Clin. Psychopharmacol. 1989, 9, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Youdim, M.B.; Bakhle, Y.S. Monoamine oxidase: Isoforms and inhibitors in Parkinson’s disease and depressive illness. Br. J. Pharmacol. 2006, 147, 287–296. [Google Scholar] [CrossRef]
- Huang, W.Y.; Davidge, S.T.; Wu, J. Bioactive natural constituents from food sources-potential use in hypertension prevention and treatment. Crit. Rev. Food Sci. Nutr. 2013, 53, 615–630. [Google Scholar] [CrossRef] [PubMed]
- Schultes, R.E.; Sirivon, R. Ethnobotany, Evolution of a Discipline; Dioscorides Press: Portland, OR, USA, 1995; p. 421. [Google Scholar]
- Viña, D.; Serra, S.; Lamela, M.; Delogu, G. Herbal natural products as source of monoamine oxidase inhibitors: A review. Curr. Top. Med. Chem. 2012, 12, 2131–2144. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, T.; González, D.; Ancín-Azpilicueta, C.; Arán, V.J.; Guillén, H. Beta-Carboline alkaloids in Peganum harmala and inhibition of human monoamine oxidase (MAO). Food Chem. Toxicol. 2010, 48, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, T. Identification and occurrence of beta-carboline alkaloids in raisins and inhibition of monoamine oxidase (MAO). J. Agric. Food Chem. 2007, 55, 8534–8540. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, T.; Chaparro, C. Human monoamine oxidase enzyme inhibition by coffee and beta-carbolines norharman and harman isolated from coffee. Life Sci. 2006, 78, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Samoylenko, V.; Rahman, M.M.; Tekwani, B.L.; Tripathi, L.M.; Wang, Y.H.; Khan, S.I.; Khan, I.A.; Miller, L.S.; Vaishali, C.J.; Muhammad, I. Banisteriopsis caapi, a unique combination of MAO inhibitory and antioxidative constituents for the activities relevant to neurodegenerative disorders and Parkinson’s disease. J. Ethnopharmacol. 2010, 127, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V.; Popova, M.; Trusheva, B. Propolis volatile compounds: Chemical diversity and biological activity: A review. Chem. Cent. J. 2014, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Basista-Sołtys, K. Allergy to Propolis in Beekeepers-A Literature Review. Occup. Med. Health Aff. 2013, 1. [Google Scholar] [CrossRef]
- Yildiz, O.; Karahalil, F.; Can, Z.; Sahin, H.; Kolayli, S. Total monoamine oxidase (MAO) inhibition by chestnut honey, pollen and propolis. J. Enzyme Inhib. Med. Chem. 2013, 29, 690–694. [Google Scholar] [CrossRef] [PubMed]
- McCabe, B.J. Dietary tyramine and other pressure amines in MAOI regimens: A review. J. Am. Diet. Assoc. 1986, 86, 1059–1064. [Google Scholar] [PubMed]
- Kiray, M.; Bagriyanik, H.A.; Pekcetin, C.; Ergur, B.U.; Uysal, N. Protective effects of deprenyl in transient cerebral ischemia in rats. Chin. J. Physiol. 2008, 51, 275–281. [Google Scholar] [PubMed]
- Gregoris, E.; Stevanato, R. Correlations between polyphenolic composition and antioxidant activity of Venetian propolis. Food Chem. Toxicol. 2010, 48, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.M.; Wu, H.L.; Wang, J.Y.; Liu, Z.; Zhai, M.; Yu, R.Q. Simultaneous determination of eight flavonoids in propolis using chemometrics-assisted high performance liquid chromatography-diode array detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2014, 962, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Barbarić, M.; Mišković, K.; Bojić, M.; Lončar, M.B.; Smolčić-Bubalo, A.; Debeljak, Z.; Medić-Šarić, M. Chemical composition of the ethanolic propolis extracts and its effect on HeLa cells. J. Ethnopharmacol. 2011, 135, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.E.D.; Ramsay, R.R. Dietary inhibitors of monoamine oxidase A. J. Neural Transm. 2011, 118, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, N.; Wu, J.; Su, L.; Chen, X.; Lin, B.; Luo, H. Galangin inhibits proliferation of HepG2 cells by activating AMPK via increasing the AMP/TAN ratio in a LKB1-independent manner. Eur. J. Pharmacol. 2013, 718, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yang, H.; Chen, J.; Fang, J.; Chen, C.; Liang, R.; Yang, G.; Wu, H.; Wu, C.; Li, S. Analysis of serum metabolites for the discovery of amino acid biomarkers and the effect of galangin on cerebral ischemia. Mol. Biosyst. 2013, 9, 2311–2321. [Google Scholar] [CrossRef] [PubMed]
- Zha, W.J.; Qian, Y.; Shen, Y.; Du, Q.; Chen, F.F.; Wu, Z.Z.; Li, X.; Huang, M. Galangin abrogates ovalbumin-induced airway inflammation via negative regulation of NF-κB. Evid. Based Complement. Alternat. Med. 2013. [Google Scholar] [CrossRef]
- Wang, X.; Gong, G.; Yang, W.; Li, Y.; Jiang, M.; Li, L. Antifibrotic activity of galangin, a novel function evaluated in animal liver fibrosis model. Environ. Toxicol. Pharmacol. 2013, 36, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Bae, Y.; Kim, S.H. Galangin attenuates mast cell-mediated allergic inflammation. Food Chem. Toxicol. 2013, 57, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Alagawadi, K.R. Anti-obesity effects of galangin, a pancreatic lipase inhibitor in cafeteria diet fed female rats. Pharm. Biol. 2013, 51, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.E.; Jung, I.T.; Choi, J.; Baek, Y.H.; Lee, J.D.; Park, D.S.; Choi, D.Y. The natural flavonoid galangin inhibits osteoclastic bone destruction and osteoclastogenesis by suppressing NF-κB in collagen-induced arthritis and bone marrow-derived macrophages. Eur. J. Pharmacol. 2013, 698, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Gupta, S. Apigenin: A promising molecule for cancer prevention. Pharm. Res. 2010, 27, 962–278. [Google Scholar] [CrossRef] [PubMed]
- Lum, C.T.; Stahl, S.M. Opportunities for reversible inhibitors of monoamine oxidase-A (RIMAs) in the treatment of depression. CNS Spectr. 2012, 17, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Finberg, J.P. Update on the pharmacology of selective inhibitors of MAO-A and MAO-B: Focus on modulation of CNS monoamine neurotransmitter release. Pharmacol. Ther. 2014, 143, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, F.; Fioravanti, R.; Bolasco, A.; Chimenti, P.; Secci, D.; Rossi, F.; Yáñez, M.; Orallo., F.; Ortuso, F.; Alcaro, S.; et al. A new series of flavones, thioflavones, and flavanones as selective monoamine oxidase-B inhibitors. Bioorg. Med. Chem. 2010, 18, 1273–1289. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, F.; Cottiglia, F.; Bonsignore, L.; Casu, L.; Casu, M.; Floris, C.; Secci, D.; Bolasco, A.; Chimenti, P.; Granese, A.; et al. Quercetin as the active principle of Hypericum hircinum exerts a selective inhibitory activity against MAO-A: Extraction, biological analysis, and computational study. J. Nat. Prod. 2006, 69, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Bandaruk, Y.; Mukai, R.; Kawamura, T.; Nemoto, H.; Terao, J. Evaluation of the inhibitory effects of quercetin-related flavonoids and tea catechins on the monoamine oxidase-A reaction in mouse brain mitochondria. J. Agric. Food Chem. 2012, 60, 10270–10277. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Qin, G.W.; Wang, J.; Chu, W.J.; Guo, L.H. Functional activation of monoamine transporters by luteolin and apigenin isolated from the fruit of Perilla frutescens (L.) Britt. Neurochem. Int. 2010, 56, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Taupin, P. Apigenin and related compounds stimulate adult neurogenesis. Mars, Inc., the Salk Institute for Biological Studies: WO2008147483. Expert Opin. Ther. Pat. 2009, 19, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.; Hanscom, S.; Gagne, P.; Crespi, C.; Patten, C. A Fluorescent-Based, High-Throughput Assay for Detecting Inhibitors of Human Monoamine Oxidase A and B; S02T081R2; BD Biosciences Discovery Labware: Woburn, MA, USA, 2002. [Google Scholar]
- Sample Availability: Samples of the compounds 1–2 and the própolis extracts used in these studies are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaurasiya, N.D.; Ibrahim, M.A.; Muhammad, I.; Walker, L.A.; Tekwani, B.L. Monoamine Oxidase Inhibitory Constituents of Propolis: Kinetics and Mechanism of Inhibition of Recombinant Human MAO-A and MAO-B. Molecules 2014, 19, 18936-18952. https://doi.org/10.3390/molecules191118936
Chaurasiya ND, Ibrahim MA, Muhammad I, Walker LA, Tekwani BL. Monoamine Oxidase Inhibitory Constituents of Propolis: Kinetics and Mechanism of Inhibition of Recombinant Human MAO-A and MAO-B. Molecules. 2014; 19(11):18936-18952. https://doi.org/10.3390/molecules191118936
Chicago/Turabian StyleChaurasiya, Narayan D., Mohamed A. Ibrahim, Ilias Muhammad, Larry A. Walker, and Babu L. Tekwani. 2014. "Monoamine Oxidase Inhibitory Constituents of Propolis: Kinetics and Mechanism of Inhibition of Recombinant Human MAO-A and MAO-B" Molecules 19, no. 11: 18936-18952. https://doi.org/10.3390/molecules191118936
APA StyleChaurasiya, N. D., Ibrahim, M. A., Muhammad, I., Walker, L. A., & Tekwani, B. L. (2014). Monoamine Oxidase Inhibitory Constituents of Propolis: Kinetics and Mechanism of Inhibition of Recombinant Human MAO-A and MAO-B. Molecules, 19(11), 18936-18952. https://doi.org/10.3390/molecules191118936