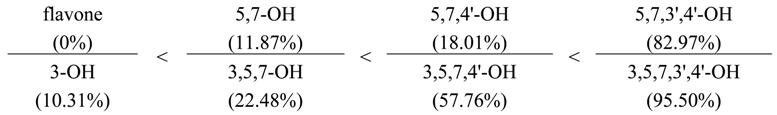2.1. Preliminary Clarification of Experimental Design
In order to reveal the effectiveness of the proposed technique investigating Fe2+-chelating activity (Fe-CA), the experimental part of the work was carried out on samples of standard compounds during the first stage of the study. Given the suggestion by various researchers of the leading role of phenolic compounds in the manifestation of Fe-CA, representatives of flavonoids (we used commercially available samples) were selected. The general scheme of analysis consists of preparing solutions of the compounds at a certain concentration, processing the sample with Fe2+ salt, incubation, filtration and then the HPLC study. A solution of a sample treated in a similar manner except that the solution of Fe2+ salt was substituted for distilled water was used as a reference.
Based on the data regarding the different abilities of the compounds to be solubilised in solvents that are used for working with phenolic compounds, we proposed DMSO as a universal solvent. In preliminary investigations, the presence of this solvent in the incubation medium did not affect the results of the analysis; moreover, the presence of DMSO allows analysis by HPLC.
FeSO
4∙7H
2O was used as the Fe
2+ salt, as it is soluble in water and does not precipitate when mixed with DMSO. The final concentration of the Fe
2+ ions in the 2% incubation medium creates an excess of ions, which in turn allows a more complete chelation. Because the optimal pH for complex formation is around 6.0, this level was used for process solutions [
21].
The incubation time selected was 30 min. During this time, changes in the residual concentration of the test compounds did not occur. The temperature of the incubation medium was 37 °C as being the most close to physiological body temperature.
It should be noted that excess FeSO4 in the sample does not affect the quality of chromatographic analysis by HPLC. Given the high hydrophilicity of this salt on reversed-phase sorbents, which are traditionally used for the analysis of phenols, residual amounts eluted with the dead volume of the column.
Efficacy of chelation was assessed by the value of ΔS (percentage), which is the reduction of the peak area of the compound after the reaction of Fe2+ ions with respect to the sample without the introduction of metal ions. The results are presented in the form of two superposed chromatograms, including chromatogram before treatment and inverted chromatogram after treatment to improve the visual perception of chelating effectiveness.
2.2. Characterization of Fe-CA of Flavonoid Standards by HPLC Using a Prechromatographic Reaction with Fe2+ Ions
Our study of Fe-CA of flavonoids was conducted on representatives of two structural types—flavones and flavonols (flavon-3-ols) whose derivatives are the most frequently identified in the plant world [
24]. During the first stage, we studied the possibility of using the previously described hypothesis on a sample of the compound having notoriously high chelating activity. Among the flavonoids, flavone baicalein is one of the well-known chelators with high binding capacity of Fe
2+ ions, and has been the subject of numerous studies.
For the implementation of the task, a solution of baicalein with a concentration of 1.48 mM (
ca. 400 μg/mL) was prepared, and solutions of FeSO
4 at different concentrations (0.154–19.656 mM) were introduced into it in ratio 1:1. In the presence of a chelating effect in the test samples of baicalein, a decrease of the peak area of the compound on the chromatograms should be observed in the course of the rise in the concentration of Fe
2+ ions. This relationship has been confirmed experimentally, namely, the peak area of baicalein in the sample without Fe
2+ ions was approximately 30,000 AU, and this value gradually decreased to a value that was less than the limit of detection (the compound was not detected at a concentration of Fe
2+ more than 2.5 mM) (
Figure 1a).
Figure 1.
(a) Reduction of peak areas (S) of baicalein after prechromatographic reaction with different concentration of Fe2+ ions. On cut—chromatograms of baicalein samples before (1) and after prechromatographic reaction with Fe2+ ions (2–6). (b) Residual concentration of Fe2+ ions after reaction of baicalein with Fe2+ ions versus initial concentration of Fe2+ ions (i). On ii—hypothetical curve in case of absence of chelation processes.
Figure 1.
(a) Reduction of peak areas (S) of baicalein after prechromatographic reaction with different concentration of Fe2+ ions. On cut—chromatograms of baicalein samples before (1) and after prechromatographic reaction with Fe2+ ions (2–6). (b) Residual concentration of Fe2+ ions after reaction of baicalein with Fe2+ ions versus initial concentration of Fe2+ ions (i). On ii—hypothetical curve in case of absence of chelation processes.
c(Fe2+), mM: 1. 0; 2. 0.077; 3. 0.154; 4. 0.307; 5. 0.614; 6. 1.228; 7. 2.457; 8. 4.914; 9. 9.828.
It is known that flavonoids in presence of metal ions undergo an autoxidation process with formation of
p-quinonemethide intermediates in neutral solution with production of hydrogen peroxide [
25]. The influence of Fe
2+ ions on this process is less extensive
versus Cu
+ or Cu
2+ ions that resulted to low rate of intermediates formation.
To prove the existence of the chelation process in the reaction mixture, we determined the residual concentration of Fe
2+ ions after reaction of baicalein solutions with Fe
2+ ions. The results have shown that working solutions with a concentration of Fe
2+ ions up to 1.23 mM are characterized by undetectable levels of residual Fe
2+ ions (
Figure 1b, curve i). The sharp increase of the residual concentration of Fe
2+ ions is observed after application of the solutions with a concentration more 1.23 mM. With the absence of chelation process the curve would have been a line (
Figure 1b, curve ii).
Thus, by the example of a compound having chelating ability, the efficacy of the hypotheses of using HPLC as a tool for the detection of compounds that have the ability to bind metal ions was demonstrated.
Given the huge variety of the structural types of flavonoids, we selected a few “basic” compounds with different types of replacement in the main flavonoid framework. The representatives of the studied groups of flavonoids showed different efficacy of chelation of Fe
2+ ions (
Figure 2).
Baicalein (97.35%), scutellarein (100%) and 6-hydroxyluteolin (100%) were the most effective chelators of Fe
2+ ions from the flavone series (
Table 1). Unsubstituted flavone lacks the ability to bind metal ions. The insertion of two hydroxyl groups at positions C-5 and C-7 of the A-ring (chrysin) increases activity by 11.87%. Hydroxyl substitution at position C-7 of the methoxyl group (tectochrysin) slightly reduces the efficiency index, while the substitution of both groups (dimethoxychrysin) leads to a completely inactive compound.
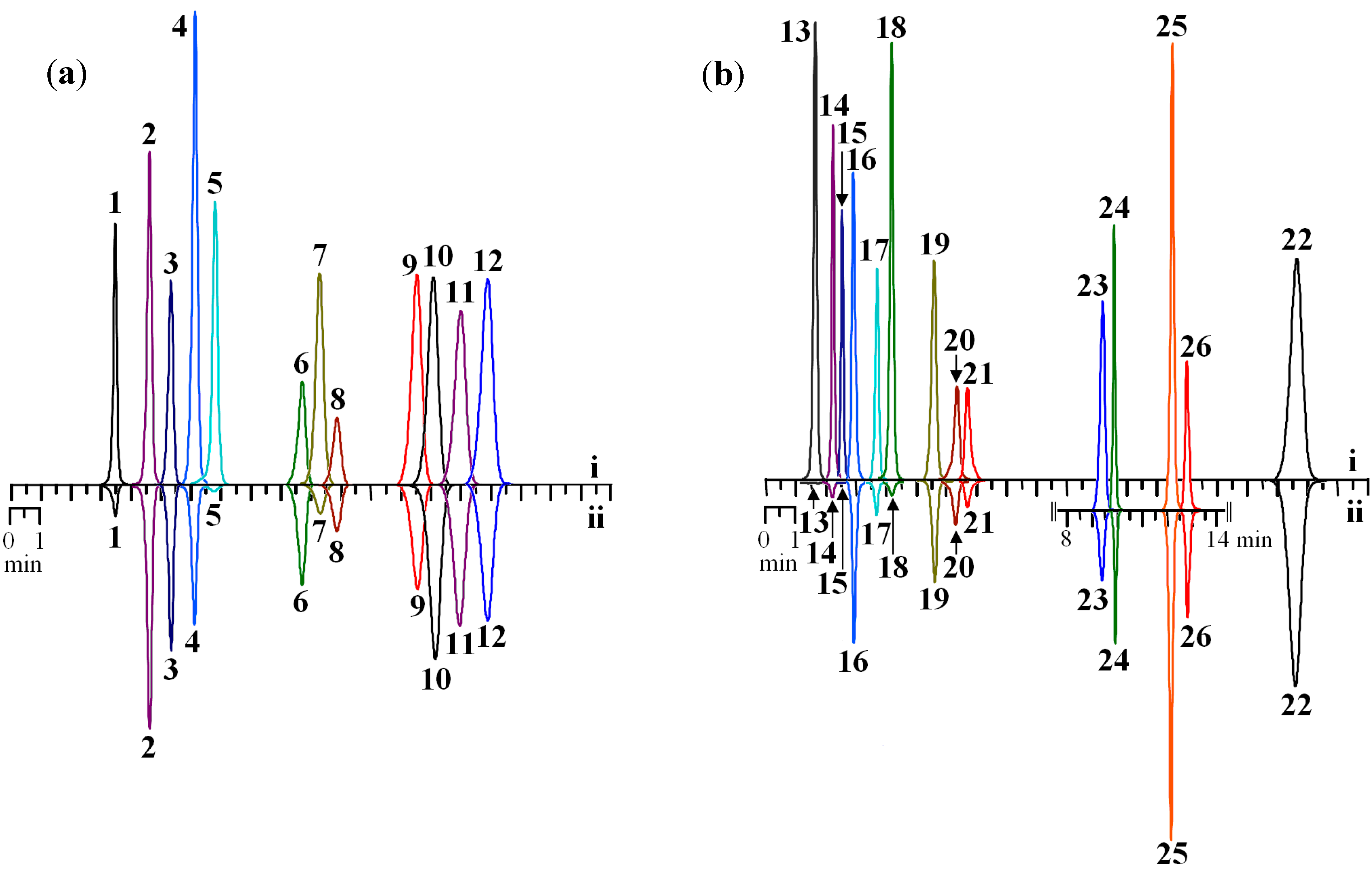
Figure 2.
HPLC chromatograms of selected flavonoids standards (125 μg/mL) before (i) and after prechromatographic reaction with Fe2+ ions (ii). Samples: (a) flavones; (b) flavonols (on cut—chromatogram of flavonol glycosides). Detector λ—270 nm.
Figure 2.
HPLC chromatograms of selected flavonoids standards (125 μg/mL) before (i) and after prechromatographic reaction with Fe2+ ions (ii). Samples: (a) flavones; (b) flavonols (on cut—chromatogram of flavonol glycosides). Detector λ—270 nm.
1. luteolin; 2. apigenin; 3. chrysoeriol; 4. norwogonin; 5. baicalein; 6. sinensetin; 7. oroxylin A; 8. eupatorin; 9. wogonin; 10. chrysin; 11. acacetin; 12. genkwanin; 13. quercetagetin; 14. patuletin; 15. myricetin; 16. morin; 17. fisetin; 18. quercetin; 19. kaempferol; 20. isorhamnetin; 21. rhamnetin; 22. galangin; 23. isomyricitrin; 24. isoquercitrin; 25. spiraeoside; 26. astragalin.
The presence in the A-ring of flavone of three ordinary (C-5, C-6, C-7) hydroxyl groups (baicalein) sharply increases the activity up to 97.35%, the substitution one of them at C-6 (oroxylin A) leads to a reduction in activity (82.86%). The presence of 5,7,8-trihydroxy-type substitution (norwogonin) also increases the activity of the compound to 68.25% compared with the 5,7-dihydroxy-analogue (chrysin); the insertion of methoxyl at C-8 (wogonin) affects the reduction of activity (47.46%).
Table 1.
Reduction of peak areas (ΔS) of flavones and flavonols after prechromatographic reaction with Fe2+ ions.
Table 1.
Reduction of peak areas (ΔS) of flavones and flavonols after prechromatographic reaction with Fe2+ ions.
| Compound | Substituents Positions | ΔS, % |
|---|
| OH | MeO | Glucosyl |
|---|
| Flavones |
| Flavone | - | - | - | 0 |
| Dimethoxychrysin | - | 5,7 | - | 0 |
| Sinensetin | - | 5,6,7,3',4' | - | 0 |
| Tectochrysin | 5 | 7 | - | 9.30 ± 0.21 a |
| Acacetin | 5,7 | 4' | - | 10.23 ± 0.23 b |
| Chrysin | 5,7 | - | - | 11.87 ± 0.25 c |
| Chrysoeriol | 5,7,4' | 3' | - | 17.83 ± 0.37 d |
| Apigenin | 5,7,4' | - | - | 18.01 ± 0.39 ce |
| Genkwanin | 5,4' | 7 | - | 19.02 ± 0.41 de |
| Eupatorin | 5,3' | 6,7,4' | - | 24.75 ± 0.47 f |
| Wogonin | 5,7 | 8 | - | 47.46 ± 0.85 i |
| Diosmetin | 5,7,3' | 4' | - | 48.63 ± 0.97 hj |
| Norwogonin | 5,7,8 | - | - | 68.25 ± 1.50 k |
| Oroxylin A | 5,7 | 6 | - | 82.86 ± 1.74 mo |
| Luteolin | 5,7,3',4' | - | - | 82.97 ± 1.91 mo |
| Baicalein | 5,6,7 | - | - | 100 |
| Scutellarein | 5,6,7,4' | - | - | 100 |
| 6-Hydroxyluteolin | 5,6,7,3',4' | - | - | 100 |
| Flavon-3-ols |
| 3-Hydroxyflavone | 3 | - | - | 10.31 ± 0.24 ac |
| Galangin | 3,5,7 | - | - | 22.48 ± 0.54 e |
| Spiraeoside | 3,5,7,3',4' | - | 4' | 30.49 ± 0.73 g |
| Astragalin | 3,5,7,3' | - | 3 | 32.76 ± 0.78 h |
| Isoquercitrin | 3,5,7,3',4' | - | 3 | 57.73 ± 1.33 j |
| Kaempferol | 3,5,7,4' | - | - | 57.76 ± 1.38 i |
| Isorhamnetin | 3,5,7,4' | 3' | - | 59.67 ± 1.47 jk |
| Datiscetin | 3,5,7,2' | - | - | 61.38 ± 1.65 jk |
| Morin | 3,5,7,2',4' | - | - | 69.65 ± 1.67 jl |
| Tamarixetin | 3,5,7,3' | 4' | - | 71.38 ± 1.71 km |
| Rhamnetin | 3,5,3',4' | 7 | - | 73.41 ± 1.83 l |
| Isomyricitrin | 3,5,7,3',4',5' | - | 3 | 76.63 ± 1.84 m |
| Fisetin | 3,7,3',4' | - | - | 82.34 ± 1.97 n |
| Azaleatin | 3,7,3',4' | 5 | - | 83.10 ± 1.98 mo |
| Patuletin | 3,5,7,3',4' | 6 | - | 88.50 ± 2.30 o |
| Quercetin | 3,5,7,3',4' | - | - | 95.50 ± 2.48 p |
| Myricetin | 3,5,7,3',4',5' | - | - | 100 |
| Quercetagetin | 3,5,6,7,3',4' | - | - | 100 |
During the transition from chrysin (5,7-OH) to apigenin (5,7,4'-OH), a slight rise in activity is observed (from 11.87% to 18.01%). The significant influence of the hydroxyl at the C-4' position proves the fact that its substitution (as in acacetin) decreases the activity of the compound (10.23%), while a genkwanin having a methoxy group at C-7 is similar to apigenin (19.02%).
A further increase in the amount of hydroxyl groups in the B-ring leads to a sharp increase in activity, for example, the activity of luteolin (5,7,3',4'-OH) amounts to 82.97%. Interestingly, the effect of the hydroxyl groups in positions C-3' and C-4' on chelating activity of the compounds manifests in different ways: the replacement of the hydroxyl at C-4' (diosmetin) decreases the efficiency to 48.63%, but in the case of 3'-methoxy-luteolin (chrysoeriol), the activity falls by more than fourfold.
Flavone derivatives are characterized by the presence of a hydroxyl group at the C-3 position. The maximum intensity of the activity was found for the compounds containing three ordinary hydroxyls in the A-ring, i.e., quercetagetin (3,5,6,7,3',4'-OH), and in the B-ring, i.e., myricetin (3,5,7,3',4',5'-OH). Basic patterns identified for flavone derivatives are typical for derivatives of flavon-3-ol.
The presence of hydroxyl at C-3 has a positive impact on the effectiveness of chelation of Fe2+ ions. This fact is demonstrated by comparative analysis of two series of flavone and flavone-3-ol derivatives:
The insertion of the hydroxyl group in the C-3 position of flavone results in chelating activity of the compound, which is due to the possibility of formation of a complex between the Fe
2+ atom, the hydroxyl at C-3 and the oxygen of the carbonyl group at C-4 [
21]. In the case of substitution of the hydroxyl at C-3, for example, by the residues of carbohydrates, the efficiency of the chelation of the compounds is reduced. Thus, the activity of kaempferol, quercetin and myricetin in comparison with their 3-O-glucosides was significantly higher:
The substitution of the hydroxyl at C-5 has little effect on the activity of the compounds. For example, the efficiency of azaleatin (5-methoxyquercetin) was 83.10% and was lower than that of quercetin (95.50%), whereas for fisetin (de-hydroxylated analogue of quercetin), it was 82.34%.
The importance of the hydroxyl group in the C-3' position in the processes of chelation previously identified for flavones was confirmed by the analysis of the flavone-3-ols. The activity of 4'-methoxy-quercetin (tamarixetin) was similar to that of 7-methoxyquercetin (rhamnetin) and exceeded the activity of the 3'-methoxy-quercetin (isorhamnetin) (71.38%, 73.41% and 59.67%, respectively).
It should be noted that for the 2'-hydroxy analogue of kaempferol, i.e., morin (3,5,7,2',4'-OH), relatively high activity was observed (69.65% vs. 57.76% for kaempferol), close to that of tamarixetin (3,5,7,3'-OH-4'-MeO; 71.38%). Moreover, datiscetin (3,5,7,2'-OH), which does not contain hydroxyl at C-4', also had a high capacity for chelating Fe2+ ions (61.38%). These findings suggest an important role of the 2'-hydroxy-substituted flavonoids in metal chelation.
The revealed patterns of influence of the structure of flavonoids on the effectiveness of the chelation of Fe
2+ ions are confirmed by the facts established earlier as a result of numerous studies by various research groups [
19,
23,
26,
27,
28,
29,
30,
31,
32,
33,
34]. In particular, among the major structural features that provide the primary influence on the ability of compounds to bind metal ions are the following:
the 2,3-double bond in conjugation with 4-keto-function;
the 3-hydroxy-4-keto- or 5-hydroxy-4-keto-functions;
the 5,7-di-hydroxy- and additionally, 3-hydroxy-functions (5,7-di-OH; 3,5,7-tri-OH);
the o-dihydroxyl (catechol) structure of the B-ring (3',4'-di-OH) and/or the A-ring (7,8-di-OH);
the galloyl structure of the B-ring (3',4',5'-tri-OH) and/or the A-ring (5,6,7-tri-OH).
All of the rules mentioned above are confirmed in the results of the present study. However, it should be noted that some issues concerning the structural features that have a positive influence on the process of chelation should be explored in more depth through additional studies.
In particular, the presence of 3,2'-dihydroxy function considered as an additional structural feature results in increased Fe-CA. One of the representatives of the compounds of this feature is morin (3,5,7,2',4'-pentahydroxyflavone), a known metal ion chelating agent. According to early data, morin can form complexes with bivalent metals such that the morin:metal ratio is equal to 2:1 [
35]. The detection of high chelating activity of datiscetin, which is a 4'-dehydroxylated analogue of morin, indicates the possibility of formation of complexes of the same type. This group is also found in the structure of other flavonoids, including datin (3,5,2'-trihydroxy-7-methoxyflavone), viscidulin I (3,5,7,2',6'-pentahydroxyflavone) and 5'-hydroxymorin [
24]. Additional data is necessary to explain why the substitution or deletion of a hydroxyl group at the C-3' position causes a significant reduction in the activity of the chelating compound.
2.3. Characterization of Fe-CA of Flavonoid Mixtures by HPLC Using a Prechromatographic Reaction with Fe2+ Ions
Given the fact that plants are a complex matrix containing different classes of compounds with diverse structural features, it is of interest to check the applicability of the developed approaches to the analysis of mixtures of flavonoids. An artificial mixture of flavonoids containing compounds with different levels of chelating action was composed. The mixture included components with high (baicalein), medium (quercetin, kaempferol, oroxylin A) and low binding activity (eupatorin, acacetin, galangin). Each sample contained an equal amount of flavonoids (0.3 mM) but had different contents of Fe
2+ (
Figure 3).
With increasing concentration of Fe2+ ions in the sample, a different rate of reduction of the areas of the chromatographic peaks for compounds with diverse effectiveness of chelation was observed. For example, at the concentration of 0.223 mM Fe2+ a pronounced decrease in the peak area of baicalein was typical, and at a concentration of 0.446 mM Fe2+, a complete disappearance of the peak of the compound was observed. A similar trend was found for quercetin and oroxylin A when the iron concentration in the sample was 1.786 mM. The disappearance of the kaempferol peak occurred at the Fe2+ ions concentration of 3.571 mM. The maximum fall of the peak areas of inactive compounds (eupatorin, acacetin, galangin) was only 20%–40% of the baseline, even in solutions with a high content of Fe2+ ions.
Figure 3.
(a) Chromatograms of flavonoid mixture before (0.000 mM) and after prechromatographic reaction with Fe2+ ions (0.223–3.571 mM). (b) Reduction of peak areas (S) of flavonoids in the mixture after prechromatographic reaction with different concentration of Fe2+ ions.
Figure 3.
(a) Chromatograms of flavonoid mixture before (0.000 mM) and after prechromatographic reaction with Fe2+ ions (0.223–3.571 mM). (b) Reduction of peak areas (S) of flavonoids in the mixture after prechromatographic reaction with different concentration of Fe2+ ions.
1. quercetin; 2. kaempferol; 3. baicalein; 4. oroxylin A; 5. eupatorin; 6. acacetin; 7. galangin.
Presenting the data as dependence “concentration of Fe2+ ions—the area of the chromatographic peak” for different compounds, one can notice the presence of a dose-dependent character of the chelation of Fe2+ ions. Moreover, the effectiveness of the chelation of Fe2+ ions by a compound is probably a function of the rate of the chelate-forming reaction between the compound and available chelating Fe2+ ions. For example, baicalein is an effective chelator because of structural features that allow it to bind Fe2+ ions with higher speed, unlike eupatorin, which is also capable of binding Fe2+ ions, but at a slower rate.
Thus, these studies have shown that the HPLC method is a useful tool for detection of compounds that are able to chelate Fe2+ ions. This method allows identifying potential chelators using a two-step procedure, namely, the chromatographic profiling of the sample at the first stage, and the analysing the sample after prechromatographic reaction with Fe2+ ions at the second stage.
2.4. Characterization of Fe-CA of Scutellaria baicalensis by HPLC and Spectrophotometry
To demonstrate the capabilities of the developed method, we have attempted to study the Fe-CA of a real plant,
Scutellaria baicalensis Georgi. (Lamiaceae family). This plant species contains compounds with high binding activity [
32,
36]. In spite of the known facts about the chelating properties of the individual flavonoids of
S. baicalensis, there is currently no scientific information about the comparative activity of the various organs of this species. This information allows evaluation of the prospect of application of various parts of the plant as a source of biologically active compounds. The information available concerns mainly the use of underground parts and information about the application of the aerial part, but is limited to a small number of works.
Chemical analysis of the different morphological groups (organs) of
S. baicalensis is necessary for the first stage of the investigation. Chromatographic analysis of the extracts from the flowers, stems, leaves and roots of
S. baicalensis was performed. It was revealed that flavonoids in
S. baicalensis distribute unevenly,
i.e., each part has a characteristic flavonoid profile (
Table 2).
Table 2.
Content of phenolic compounds in organs and extracts of S. baicalensis (mg·g−1 of dry plant/extract weight).
Table 2.
Content of phenolic compounds in organs and extracts of S. baicalensis (mg·g−1 of dry plant/extract weight).
| Compound | Flowers | Leaves | Stems | Roots |
|---|
| Buds | Beginning of flowering | Mass flowering | Young leaves of the main stem | Mature leaves of the main stem | Leaves of the lateral stem | Main stems | Lateral stems | Main root | Adventi-tious root |
|---|
| Organs |
| A7G | 14.10 | 21.57 | 21.30 | 2.23 | 2.21 | 2.54 | 0.52 | 1.70 | tr. | tr. |
| B | tr. | tr. | tr. | tr. | tr. | tr. | tr. | tr. | 4.44 | 20.50 |
| B7G | 26.06 | 7.26 | 4.89 | 3.80 | 4.11 | 4.52 | 0.17 | 0.43 | 195.57 | 105.41 |
| C | tr. | tr. | tr. | tr. | tr. | tr. | n.d. | n.d. | 0.42 | 1.10 |
| C7G | 67.37 | 45.11 | 31.61 | 37.55 | 23.38 | 44.61 | 0.26 | 1.22 | n.d. | n.d. |
| CC1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 7.50 | 5.84 |
| CC2 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 5.26 | 3.64 |
| DB7G | tr. | tr. | 0.48 | 0.23 | 0.14 | 0.24 | tr. | tr. | 2.62 | 1.54 |
| DS7G | tr. | tr. | tr. | 40.21 | 69.21 | 66.18 | 6.86 | 20.14 | tr. | tr. |
| Fl7G | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 4.17 | 2.26 |
| IS7G | 0.75 | tr. | n.d. | 0.65 | 0.28 | 0.69 | 0.25 | 0.41 | tr. | tr. |
| L7G | 23.49 | 12.17 | 10.28 | tr. | tr. | tr. | tr. | tr. | tr. | tr. |
| N7G | 4.84 | 0.75 | 0.61 | 0.28 | 0.17 | 0.32 | 0.14 | 0.12 | 5.54 | 5.69 |
| O7G | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 7.93 | 3.74 |
| S7G | 14.73 | 2.43 | 2.32 | 3.77 | 2.82 | 5.43 | 2.27 | 5.61 | tr. | tr. |
| W | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.48 | 2.29 |
| W7G | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 41.59 | 26.47 |
| Extracts |
| A7G | 23.94 | 38.48 | 40.59 | 7.43 | 6.02 | 9.72 | 2.38 | 7.84 | 1.12 | tr. |
| B | 1.04 | 0.93 | 0.60 | 0.53 | 0.47 | 0.86 | 0.15 | 0.39 | 10.45 | 41.62 |
| B7G | 45.24 | 11.39 | 9.08 | 12.68 | 14.52 | 16.20 | 0.81 | 2.43 | 460.16 | 214.98 |
| C | 4.16 | 2.18 | 1.12 | 1.16 | 1.12 | 0.83 | n.d. | n.d. | 1.04 | 2.29 |
| C7G | 117.86 | 82.14 | 63.28 | 127.03 | 75.82 | 150.32 | 1.37 | 5.63 | n.d. | n.d. |
| CC1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 17.69 | 12.82 |
| CC2 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 11.38 | 7.42 |
| DB7G | 1.83 | 1.86 | 0.84 | 0.82 | 0.52 | 0.81 | tr. | tr. | 6.53 | 3.10 |
| DS7G | 2.10 | 1.12 | 0.57 | 135.30 | 223.96 | 221.48 | 32.84 | 92.93 | 0.64 | 0.39 |
| Fl7G | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 10.48 | 5.06 |
| IS7G | 1.29 | 0.83 | n.d. | 1.89 | 0.73 | 2.32 | 1.19 | 1.58 | 1.86 | 0.93 |
| L7G | 40.78 | 20.66 | 20.69 | 0.32 | 0.16 | 1.78 | tr. | 0.24 | 2.09 | 0.52 |
| N7G | 8.23 | 1.44 | 1.17 | 1.03 | 0.53 | 1.73 | 0.63 | 0.60 | 14.89 | 10.02 |
| O7G | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 19.96 | 7.83 |
| S7G | 24.09 | 4.38 | 4.45 | 14.07 | 9.04 | 18.29 | 10.63 | 26.43 | 0.96 | 1.12 |
| W | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 1.10 | 4.65 |
| W7G | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 99.60 | 53.78 |
The ability of flowers to accumulate chrysin-7-O-glucuronide and luteolin-7-O-glucuronide was marked in all periods of growth (from buds to the phase of mass flowering). It should be noted that the content of these compounds was reduced in flowers during their growth, as was the total flavonoid content. High concentrations of baicalin, scutellarin and norwogonoside were typical for buds. The concentration of apigenin-7-O-glucuronide was higher in adult flowers than in the buds. The leaves of S. baicalensis are used for storage of dihydroscutellarin and chrysin-7-O-glucuronide, and the concentrations of which are higher in mature leaves. Apigenin-7-O-glucuronide, baicalin, norwogonoside and scutellarin are minor compounds of this organ. The predominant flavonoid of S. baicalensis stems is dihydroscutellarin, the highest concentration of which is in the lateral stems. In general, the composition of flavonoids of stems is close to that of the leaves, except for the minor content of chrysin-7-O-glucuronide. The roots have a special chemical feature consisting of a high concentration of baicalin and wogonoside, which has previously has been shown in many studies. However, a high concentration of aglycone of baicalin (baicalein) was observed for adventitious roots (ca. fivefold more than in the main root).
Based on the identified features, a variety of Fe-CA levels in extracts from different organs is expected. Extracts were obtained from all the above-listed parts and investigated for their Fe-CA. Chromatographic analysis showed that the composition of the extracts is similar to that of producing parts of them (
Table 2).
The developed method was applied to study Fe-CA of extracts obtained from various parts of
S. baicalensis. Some chromatograms of roots, leaves, flowers and stems of
S. baicalensis are presented in
Figure 4.
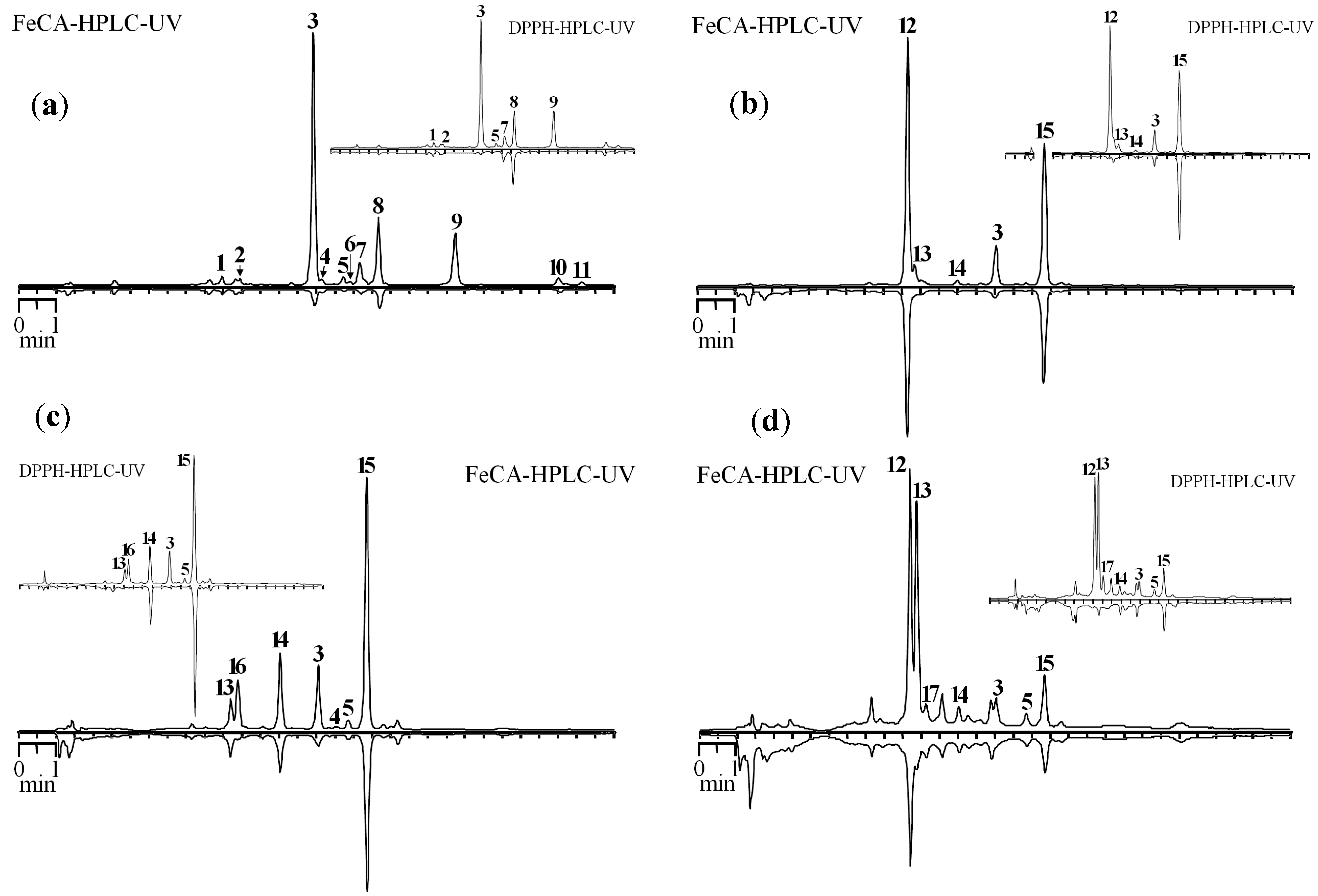
Figure 4.
HPLC chromatograms of S. baicalensis extracts before (top chromatogram) and after prechromatographic reaction with Fe2+ ions (bottom chromatogram) (FeCA-HPLC-UV). Extracts: (a) main roots; (b) mature leaves of main stem; (c) flowers, mass flowering period; (d) main stem. On cuts—HPLC chromatograms of the same extracts before (top chromatogram) and after prechromatographic reaction with DPPH• radicals (bottom chromatogram) (DPPH-HPLC-UV).
Figure 4.
HPLC chromatograms of S. baicalensis extracts before (top chromatogram) and after prechromatographic reaction with Fe2+ ions (bottom chromatogram) (FeCA-HPLC-UV). Extracts: (a) main roots; (b) mature leaves of main stem; (c) flowers, mass flowering period; (d) main stem. On cuts—HPLC chromatograms of the same extracts before (top chromatogram) and after prechromatographic reaction with DPPH• radicals (bottom chromatogram) (DPPH-HPLC-UV).
1. chrysin-6-C-arabinoside-8-C-glucoside; 2. chrysin-6-C-glucoside-8-C-arabinoside; 3. baicalin; 4. dihydrobaicalin; 5. norwogonoside; 6. 5,7,8-trihydroxy-6-methoxyflavone-7-O-glucuronide; 7. oroxyloside A; 8. wogonoside; 9. baicalein; 10. wogonin; 11. chrysin; 12. dihydroscutellarin; 13. scutellarin; 14. apigenin-7-O-glucuronide; 15. chrysin-7-O-glucuronide; 16. luteolin-7-O-glucuronide; 17. isoscutellarin.
The largest decrease in the peak area was observed for baicalin, baicalein and wogonoside in the roots. It should be noted that minor 5,7,8-trihydroxy-6-methoxyflavone-7-O-glucuronide, oroxyloside A and norwogonoside may be characterized as good chelators. Dihydroscutellarin and chrysin-7-O-glucuronide, predominant compounds of the flower extract, have a weak Fe-CA, while scutellarin and baicalin are main chelators of this extract.
The dominance of chrysin-7-O-glucuronide, which exhibits weak chelating properties, is also observed in the extract from the leaves. Luteolin-7-O-glucuronide and baicalin are compounds that are responsible for the existence of Fe-CA. Dihydroscutellarin and scutellarin are the main constituents of the extract from the stems. However, Fe-CA of the former compound is significantly lower than that of the latter, the presence of which determines the existence of the effect in the extract from this organ.
Presentation of the results in the form of coupled chromatograms, one of which is inverted, allows initial evaluation in a more convenient form. For comparison of the capabilities of chromatographic techniques using prechromatographic treatment of the sample, we conducted an investigation by applying the DPPH-HPLC method. Despite the proximity of the structural features determining the presence of antiradical action with those for Fe
2+-chelating activity, similar chromatographic profiles for DPPH-HPLC and FeCA-HPLC methods would be expected, but sometimes profiles may differ. In particular, a significant decrease in the peak area of dihydroscutellarin after the treatment of the extract from the flowers by the DPPH radical is observed. The presence of a single peak (chrysin-7-glucuronide) in the inverted chromatogram is the result of this. However, this compound showed weak Fe
2+-chelating properties that, in the case of FeCA-HPLC, resulted in two peaks on Fe
2+-treated chromatogram. A similar phenomenon was observed for the extract from the stems, whose also contain a significant amount of dihydroscutellarin. The main reason of these differences is the presence of 2,3-saturated bond in dihydroscutellarin resulted in high antiradical action but pronounced reduction of Fe
2+-chelating properties [
34]. Thus, the simultaneous use of DPPH-HPLC and FeCA-HPLC for characterization of plants (extracts) obtains a more complete picture of the active compounds and features of their antioxidant action.
In order to quantify the degree of Fe-CA in extracts from the parts of
S. baicalensis, we defined the indicators of Fe-CA using a spectrophotometric method. It was found that the values of Fe-CA ranged from 10.09 μM Fe
2+ g
−1 for flowers in a phase of mass flowering to 471.70 μM Fe
2+ g
−1 for the main root (
Table 3).
Table 3.
Fe2+-Chelating activity (Fe-CA), reduction of total peaks areas (ΔSt) and chemical parameters of extracts from different S. baicalensis organs.
Table 3.
Fe2+-Chelating activity (Fe-CA), reduction of total peaks areas (ΔSt) and chemical parameters of extracts from different S. baicalensis organs.
| Sample | Fe-CA, μM Fe2+ g−1 *,** | ΔSt, % | Total flavonoid content, mg·g−1 ** | “Active” flavonoid content, mg·g−1 ** |
|---|
| Flowers |
| Buds | 102.63 ± 2.36 f | 48.07 ± 1.11 j | 270.56 | 119.38 |
| beginning of flowering | 33.29 ± 0.76 de | 35.79 ± 0.79 hi | 165.41 | 38.80 |
| mass flowering | 30.02 ± 0.69 de | 33.22 ± 0.70 h | 142.39 | 35.99 |
| Leaves |
| young leaves of the main stem | 20.92 ± 0.48 c | 27.46 ± 0.63 g | 302.26 | 28.63 |
| mature leaves of the main stem | 18.64 ± 0.41 bc | 22.86 ± 0.57 ig | 332.89 | 24.72 |
| leaves of the lateral stem | 32.67 ± 0.75 de | 34.37 ± 0.79 h | 424.34 | 38.86 |
| Stems |
| main stem | 10.14 ± 0.24 a | 18.20 ± 0.43 i | 50.00 | 12.22 |
| lateral stems | 27.16 ± 0.59 d | 30.42 ± 0.69 gh | 138.07 | 30.09 |
| Roots |
| main root | 471.70 ± 12.26 h | 85.57 ± 1.97 l | 659.95 | 518.99 |
| adventitious root | 253.08 ± 6.07 g | 59.19 ± 1.30 k | 366.53 | 281.15 |
Flowers, except buds, were characterized by a relatively low Fe-CA and intermediate rates were revealed for the extract from leaves, while the extract from the roots was the most active. The different flavonoid content in these parts is the reason for this phenomenon, and it should be noted that various flavonoids found in S. baicalensis possess different Fe2+-chelating activities, so baicalein, baicalin, 5,7,8-trihydroxy-6-methoxyflavone-7-O-glucuronide, luteolin-7-O-glucuronide, norwogonoside, oroxyloside A and scutellarin can be referred to as “active” flavonoids. The remaining compounds are characterized as weak chelators.
Therefore, to describe the extracts, we used two measures: total flavonoid content and “active” flavonoid content. The value of total flavonoid content is calculated as a sum of all identified flavonoid compounds (HPLC) and “active” flavonoid content is a sum of most active compound, including baicalein, baicalin, 5,7,8-trihydroxy-6-methoxyflavone-7-
O-glucuronide, luteolin-7-
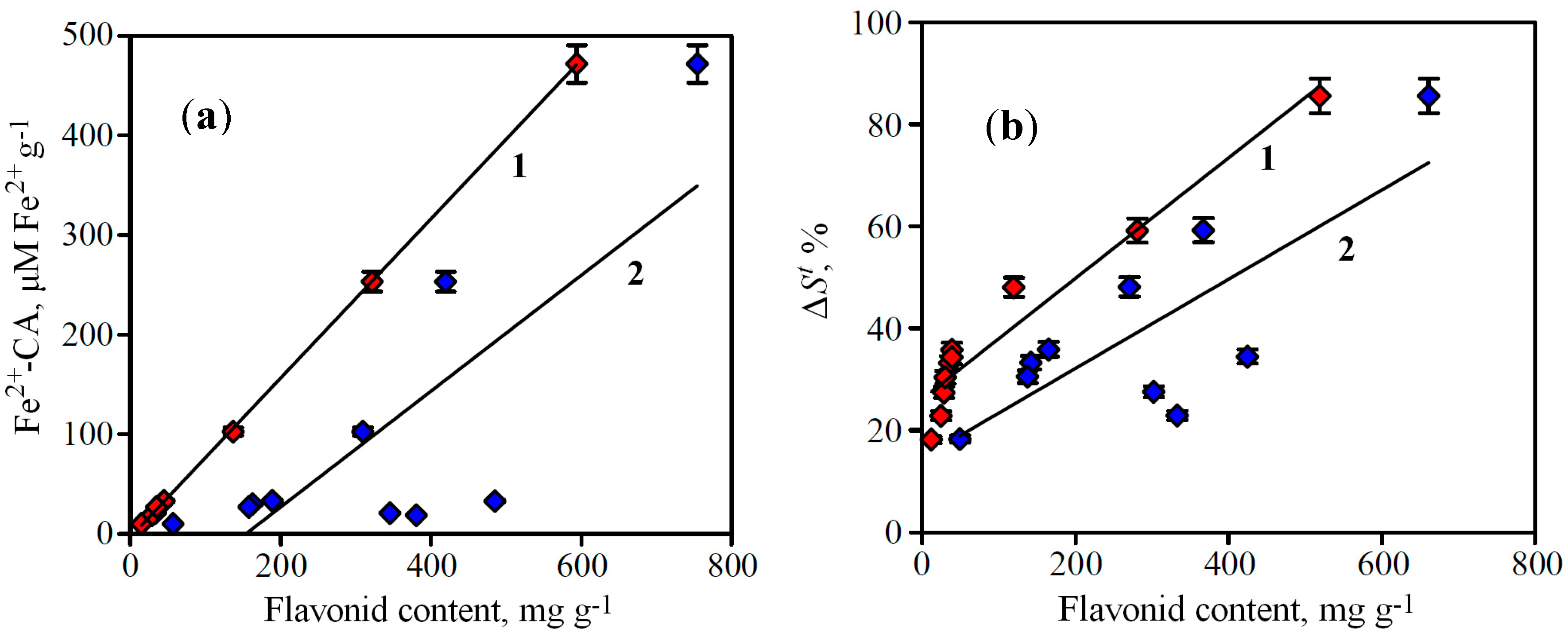
O-glucuronide, norwogonoside, oroxyloside A and scutellarin. The use of regression analysis revealed the presence of linear correlations between flavonoid content in
S. baicalensis extracts and their Fe-CA (
Figure 5a). The value of the regression coefficients indicates that the rates of flavonoid content and Fe-CA depend on each other,
i.e., while the flavonoid content is high, the iron-chelating activity is also high.
Figure 5.
(a) Correlations between flavonoid content in S. baicalensis extracts and their Fe-CA determined by spectrophotometry (Fe2+-CA). (b) Correlations between flavonoid content in S. baicalensis extracts and their Fe-CA determined by FeCA-HPLC (ΔSt).
Figure 5.
(a) Correlations between flavonoid content in S. baicalensis extracts and their Fe-CA determined by spectrophotometry (Fe2+-CA). (b) Correlations between flavonoid content in S. baicalensis extracts and their Fe-CA determined by FeCA-HPLC (ΔSt).
1. “active” flavonoid content, 2. total flavonoid content. (a) r2 = 0.9999 for “active” flavonoid content and r2 = 0.6167 for total flavonoid content. (b) r2 = 0.9329 for “active” flavonoid content and r2 = 0.6005 for total flavonoid content.
However, the dispersion of r2-values for relationship “active” flavonoid content—Fe-CA is much smaller than for total flavonoid content—Fe-CA one. This phenomenon confirms the predominant role of active flavonoids in availability of iron-chelating activity, so the use of this criterion for the correlation investigations is more informative.
To compare the efficiency of the chromatographic method of determination of Fe
2+-chelating activity to that of the traditionally used spectrophotometric method, we investigated the presence of correlations between the results of both methods. Parameter reduction of total peaks areas (Δ
St) was proposed as a quantitative criterion for assessing Fe
2+-chelating activity by the FeCA-HPLC method. This parameter is the ratio of the sum of areas of all chromatographic peaks after treatment of the sample to that before treatment (in per cent):
where
Safter is the total area of all chromatographic peaks after Fe
2+ treatment, and
Sbef°re is the total area of all chromatographic peaks before Fe
2+ treatment. The calculation of this parameter for the
S. baicalensis extracts showed that the rate varied from 18.20% (extract from the stems, the least active sample) to 85.57% (extract from the roots, the most active sample) (
Table 3). This parameter is in good correlation with both indices of flavonoid content in extracts, “active” flavonoid content and total flavonoid content. As in the case of Fe-CA determined by spectrophotometry the dispersion of
r2-values in for relationship “active” flavonoid content—Δ
St is much smaller than for total flavonoid content—Δ
St (
Figure 5b).
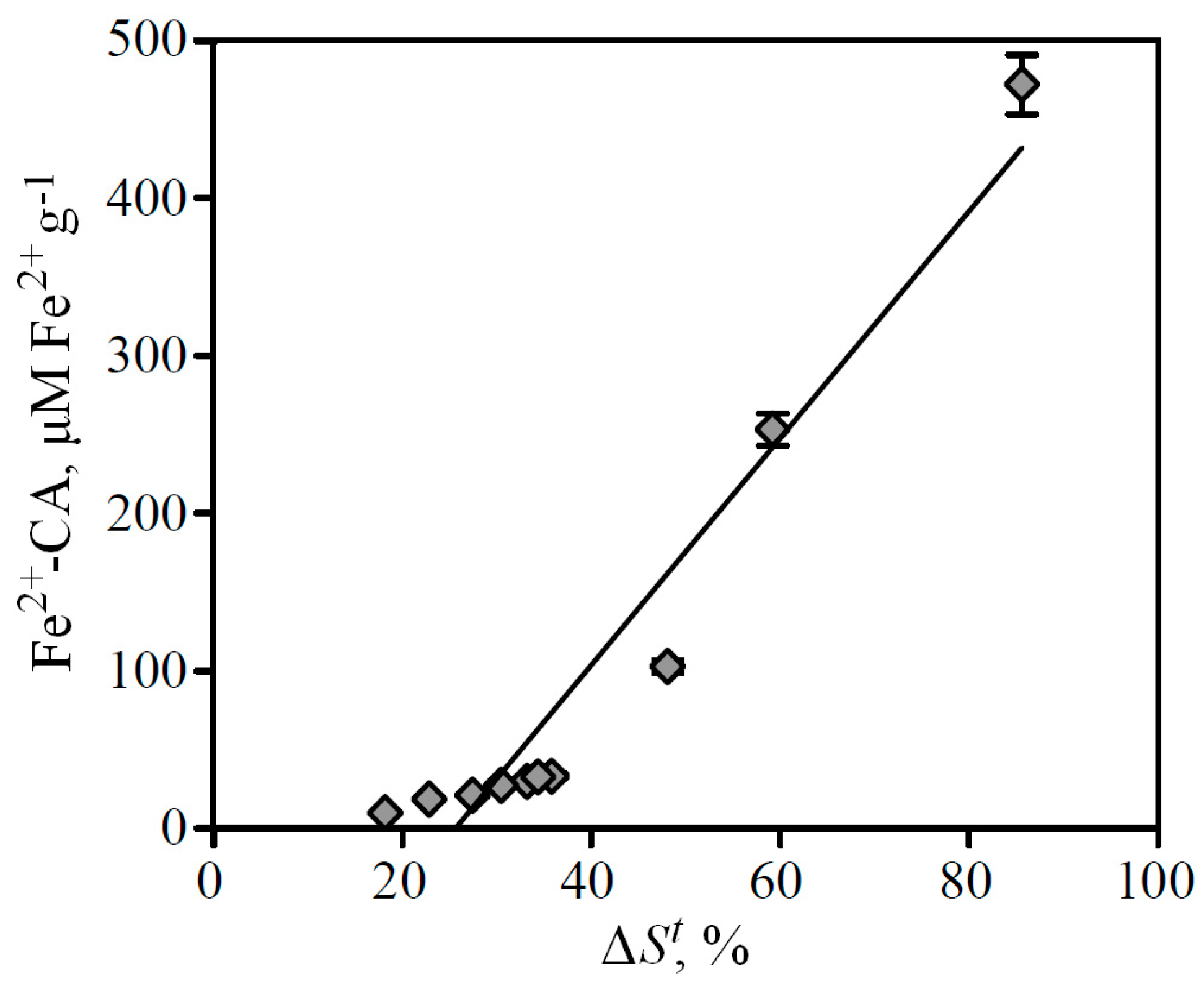
Comparative analysis of the two methods' data showed that the correlation between values of reduction of total peaks areas (Δ
St) in FeCA-HPLC and Fe-CA index in spectrophotometry is characterized by a high correlation coefficient (
r2 = 0.9307), which indicates a strong bond of both parameters (
Figure 6).
Figure 6.
Correlation between values of Fe-CA determined by FeCA-HPLC (ΔSt) and spectrophotometry (Fe2+-CA).
Figure 6.
Correlation between values of Fe-CA determined by FeCA-HPLC (ΔSt) and spectrophotometry (Fe2+-CA).
Our studies have shown the possibility of combining the advantages of the HPLC method and prechromatographic derivatization of samples by Fe2+ ions for investigation of Fe2+-chelating activity of natural compounds. The economic feasibility of this method is obvious because it does not require additional chemicals and is rapid. This method is sensitive because it uses an HPLC technique for detection of quantitative and qualitative changes in the sample after Fe2+ treatment. Undoubtedly, it is not possible to transfer the results of this method to processes in the human organism. However, obtained results may give an idea of the Fe2+ chelating efficacy of different plant metabolites.