Abstract
Quinolones are synthetic broad-spectrum antibiotics with good oral absorption and excellent bioavailability. Due to the chemical functions found on their nucleus (a carboxylic acid function at the 3-position, and in most cases a basic piperazinyl ring (or another N-heterocycle) at the 7-position, and a carbonyl oxygen atom at the 4-position) quinolones bind metal ions forming complexes in which they can act as bidentate, as unidentate and as bridging ligand, respectively. In the polymeric complexes in solid state, multiple modes of coordination are simultaneously possible. In strongly acidic conditions, quinolone molecules possessing a basic side nucleus are protonated and appear as cations in the ionic complexes. Interaction with metal ions has some important consequences for the solubility, pharmacokinetics and bioavailability of quinolones, and is also involved in the mechanism of action of these bactericidal agents. Many metal complexes with equal or enhanced antimicrobial activity compared to the parent quinolones were obtained. New strategies in the design of metal complexes of quinolones have led to compounds with anticancer activity. Analytical applications of complexation with metal ions were oriented toward two main directions: determination of quinolones based on complexation with metal ions or, reversely, determination of metal ions based on complexation with quinolones.
1. Introduction
The generic term “quinolone antibiotics” refers to a group of synthetic antibiotics with bactericidal effects, good oral absorption and excellent bioavailability [1,2]. Nalidixic acid (1-ethyl-1,4-dihydro-7-methyl-4-oxo-1,8-naphthyridine-3-carboxylic acid, Figure 1), the first compound of the series, was introduced in therapy in the 1960s [3].
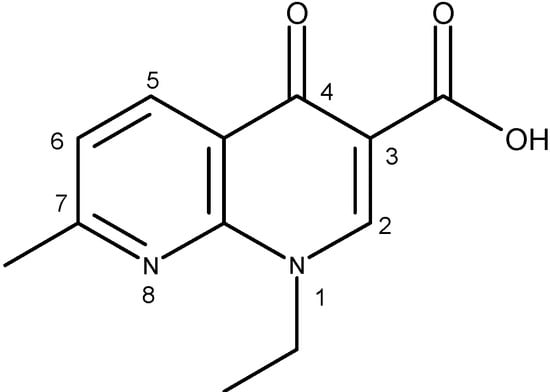
Figure 1.
Nalidixic acid.
The clinical use of nalidixic acid was limited by its narrow spectrum of activity. Several modifications were made on the basis nucleus in order to enlarge the antibacterial spectrum and to improve the pharmacokinetics properties, two of these considered as being major: introduction of a piperazine moiety or another N-heterocycles in the position 7 and introduction of a fluoride atom at the position 6. Thus, the new 4-quinolones, fluoroquinolones, have been discovered starting in the 1980s. Taking into account the chemical structure of the basis nucleus (Figure 2), the quinolone are classified in four groups (Table 1) [4,5,6].
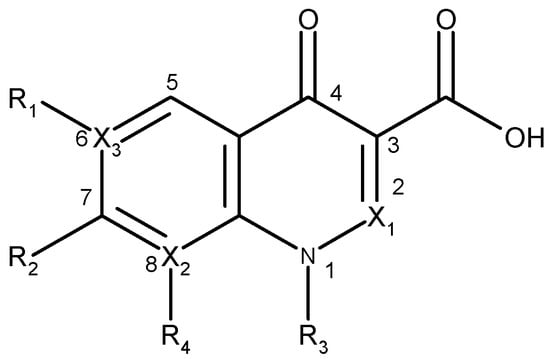
Figure 2.
The general structure of 4-quinolones.

Table 1.
Classes of quinolones based on chemical structure.
| Quinolone group/base heterocycle | X1 | X2 | X3 | R1 | R2 | R3 | R4 | Representatives | Generation |
|---|---|---|---|---|---|---|---|---|---|
| Naphthyridine (8-aza-4-quinolone) | CH | N | C | H | CH3 | C2H5 | - | Nalidixic acid | First |
| CH | N | C | F |  | C2H5 | - | Enoxacin | Second | |
| CH | N | C | F |  |  | - | Gemifloxacin | Third | |
| CH | N | C | F |  |  | - | Tosufloxacin | Third | |
| Pyridopyrimidine (6,8-diaza-4-quinolone) | CH | N | N | - |  | C2H5 | - | Pipemidic acid | First |
| CH | N | N | - |  | C2H5 | - | Piromidic acid | First | |
| Cinnoline (2-aza-4-quinolone) | N | C | C |  | C2H5 | H | Cinoxacin | First | |
| Quinoline (4-oxo-1,4-dihydroquinoline, 4-quinolone) | CH | C | C | H |  | C2H5 | H | Rosoxacin | First |
| CH | C | C |  | C2H5 | H | Oxolinic acid | First | ||
| CH | C | C | F | H |  | Flumequine | First | ||
| CH | C | C | F |  | C2H5 | H | Norfloxacin | Second | |
| CH | C | C | F |  | C2H5 | H | Pefloxacin | Second | |
| CH | C | C | F |  |  | H | Ciprofloxacin | Second | |
| CH | C | C | F |  |  | H | Enrofloxacin | Second | |
| CH | C | C | F | 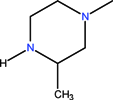 | C2H5 | F | Lomefloxacin | Second | |
| CH | C | C | F |  |  | Ofloxacin | Second | ||
| CH | C | C | F |  | 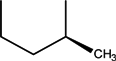 | Levofloxacin | Third | ||
| CH | C | C | F | 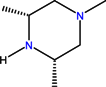 |  | F | Sparfloxacin * | Third | |
| CH | C | C | F | 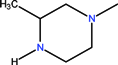 |  | OCH3 | Gatifloxacin | Third | |
| CH | C | C | F |  |  | OCH3 | Balofloxacin | Third | |
| CH | C | C | F |  |  | Cl | Clinafloxacin | Fourth | |
| CH | C | C | F |  |  | Cl | Sitafloxacin | Fourth | |
| CH | C | C | F |  |  | OCH3 | Moxifloxacin | Fourth | |
* possesses a - NH2 group in position 5.
Based on their antibacterial spectrum and their pharmacokinetic properties, the quinolones are classified in four generations [7,8,9] (Table 2).

Table 2.
Generations of quinolones based on their antibacterial spectrum and pharmacokinetic properties.
| Quinolone generation | Characteristic features |
|---|---|
| First | Active against Gram negative bacteria. |
| High protein binding. | |
| Short half life. | |
| Low serum and tissue concentrations. | |
| Uncomplicated urinary tract infection. | |
| Oral administration. | |
| Second | Class I (enoxacin, norfloxacin, lomefloxacin) |
| Enhanced activity against Gram negative bacteria. | |
| Protein binding (50%). | |
| Longer half life than the first generation. | |
| Moderate serum and tissue concentrations. | |
| Uncomplicated or complicated urinary tract infections. | |
| Oral administration. | |
| Class II (ofloxacin, ciprofloxacin) | |
| Enhanced activity against Gram negative bacteria. | |
| Atipical pathogens, Pseudomonas aeruginosa (ciprofloxacin). | |
| Protein binding (20%–50%). | |
| Moderate to long half life. | |
| Higher serum and tissue concentrations compared with class I. | |
| Complicated urinary infections, gastroenteritis, prostatitis, nosocomial infections. | |
| Oral and iv administration. | |
| Third | Active against Gram negative and Gram positive bacteria. |
| Similar pharmacokinetic profile as for second generation (class II). | |
| Similar indications and mode of administration. Consider for community aquired pneumonia in hospitalized patients. | |
| Fourth | Extended activity against Gram positive and Gram negative bacteria. |
| Active against anaerobes and atypical bacteria. | |
| Oral and i.v. administration. | |
| Consider for treatment of intraabdominal infections. |
Quinolones are bactericidal agents that inhibit the replication and transcription of bacterial DNA, causing rapid cell death [10,11]. They inhibit two antibacterial key-enzymes, DNA-gyrase (topoisomerase II) and DNA topoisomerase IV. DNA-gyrase is composed of two subunits encoded as GyrA and GyrB, and its role is to introduce negative supercoils into DNA, thereby catalyzing the separation of daughter chromosomes. DNA topoisomerase IV is composed of four subunits, two ParC and two ParE subunits and it is responsible for decatenation of DNA thereby allowing segregation into two daughter cells [12,13]. Quinolones interact with the enzyme-DNA complex, forming a drug-enzyme-DNA complex that blocks progression and the replication process [14,15].
Older quinolones have greater activity against DNA-gyrase than against topoisomerase IV in Gram negative bacteria and greater activity against topoisomerase IV than against DNA-gyrase in Gram positive bacteria. Newer quinolones equally inhibit both enzymes [16,17,18].
2. Chemical Properties of Quinolones Related to Complexation Process
Most quinolone molecules are zwitterionic, based on the presence of a carboxylic acid function at the 3-position and a basic piperazinyl ring (or another N-heterocycle) at the 7-position. Both functions are weak and give a good solubility for the quinolones in acidic or basic media.
Protonation equilibria of quinolones have been studied in aqueous solution using potentiometry, 1H- NMR spectrometry and UV spectrophotometry [19,20]. For a quinolone molecule with the general structure depicted in Figure 3, two proton-binding sites can be identified. In solution, such a molecule exists in four microscopic protonation forms, two of the microspecies being protonation isomers.
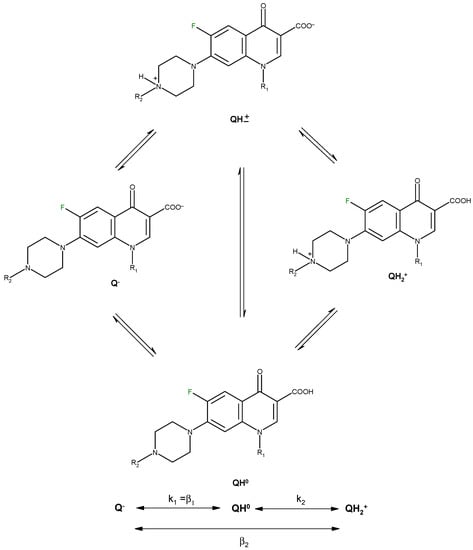
Figure 3.
Protonation scheme of a fluoroquinolone molecule with piperazine ring at the 7-position (adapted from [20,21,22]).
The microspeciation of drug molecules is used to depict the acid-base properties at the molecular level (macroconstants) and at the submolecular level (microconstants). The macroconstants quantify the overall basicity of the molecules. The values for pKa1, correlated with the acid function of carboxyl group, fall in the range 5.33–6.53, while the values for pKa2, correlated with the basic function of the piperazinic group, fall in the range 7.57–9.33. Table 3 contains the protonation constant values for norfloxacin and ofloxacin, two representative quinolones.

Table 3.
Protonation constant values for norfloxacin and ofloxacin.
| Compound | log β1 | log β2 = log Ka2 | log β1-log β2 = log Ka1 | Isoelectric point | Reference |
|---|---|---|---|---|---|
| Norfloxacin | 14.68 | 8.38 | 6.30 | 7.34 | [19] |
| 14.73 | 8.51 | 6.22 | 7.37 | [23] | |
| Ofloxacin | 14.27 | 8.22 | 6.05 | 7.14 | [19] |
| 13.94 | 8.25 | 5.69 | 6.97 | [23] |
The microconstants describe the proton binding affinity of the individual functional groups and are used in calculating the concentrations of different protonation isomers depending on the pH. The quinolones exist mainly in the zwitterionic form between pH 3 and 11. The positively charged form QH2+ is present in 99.9% at pH 1. At pH 7.4 all microspecies are present in commensurable concentrations.
Quinolone microspeciation has been correlated with bioavailability of quinolone molecules, serum protein binding and antibacterial activity [20]. The microspeciation is also important in the synthesis of metal complexes, the quinolone molecules acting as ligand in the deprotonated form (Q−) in basic conditions, and in the zwitterionic form (QH±) in neutral, slightly acidic or slightly basic medium. In strongly acidic medium, quinolones form ionic complexes in their cation form (QH2+).
Quinolones form metal complexes due to their capacity to bind metal ions. In their metal complexes, the quinolones can act as bidentate ligand, as unidentate ligand and as bridging ligand. Frequently, the quinolones are coordinated in a bidentate manner, through one of the oxygen atoms of deprotonated carboxylic group and the ring carbonyl oxygen atom [Figure 4(a)]. Rarely, quinolones can act as bidentate ligand coordinated via two carboxyl oxygen atoms [Figure 4(b)] or through both piperazinic nitrogen atoms [Figure 4(c)]. Quinolones can also form complexes as unidentate ligand coordinated to the metal ion through by terminal piperazinyl nitrogen [Figure 4(d)]. In the polymeric complexes in solid state, multiple modes of coordination are simultaneously possible. In strongly acidic conditions quinolones are protonated and appear as cations in the ionic complexes.
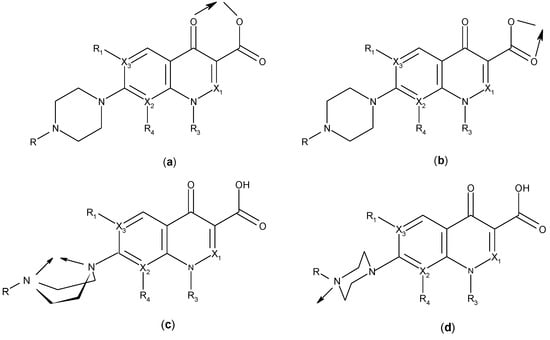
Figure 4.
Main coordination modes of quinolones.
3. Metal Complexes of Quinolones
3.1. Metal-Quinolone Chelates
The quinolone molecules possess two main sites of metal chelate formation [Figure 4(a,c)]. The first of these, represented by the carbonyl and carboxyl groups in neighboring positions, is the most common coordination mode in the quinolone chelates. Quinolones can bind divalent cations (Mg2+, Ca2+, Cu2+, Zn2+, Fe2+, Co2+ etc.), forming chelates with 1:1 or 1:2 (metal:ligand) stoichiometry or trivalent cations (A13+, Fe3+), forming chelates with 1:1, 1:2 or 1:3 (metal:ligand stoichiometry). A higher stoichiometry (1:4) is found in complexes with Bi3+. In Figure 5 is depicted the general structure of the chelates of quinolones with divalent cations with the 1:2 (metal:ligand) molar ratio. In a study of the Cu(II)-ciprofloxacin system it was observed that the number of coordinated ligands depends on the pH. Thus, in the more acidic region, a 1:1 complex is favoured, whereas a 1:2 complex is the main species at higher pH values [24].
Figure 5.
The general structure of 1:2 (metal:ligand) quinolone chelates with divalent cations.
It was found that quinolones have a similar affinity for the metal ions, forming chelates more stable with hard Lewis acids like the trivalent cations (Al3+, Fe3+). Chelates less stable are formed with the cations of group 2A (Mg2+, Ca2+, Ba2+). For instance, the formation constant values for ciprofloxacin chelates decrease in order: Al3+ > Fe3+ > Cu2+ > Zn2+ > Mn2+ > Mg2+ [25]. For norfloxacin chelates, the variation is quite similar: Fe3+ > Al3+ > Cu2+ > Fe2+ > Zn2+ > Mg2+ > Ca2+ [26].
The stability of chelates is greater in solvents with lower dielectric constant [26] and is pH dependent; the affinity of lomefloxacin for the Ca2+ and Mg2+ ions decreases in the order: anion>zwitterion>>cation [27].
Table 4, Table 5 and Table 6 present a selection of the chelates obtained in solid state with quinolone acting as bidentate ligand through the pyridone oxygen and one carboxylate oxygen, and the type of experiments carried out for investigating their biological activity. The tables include those chelates in which the quinolones are the only bidentate ligands; complexes with other bidentate co-ligands (e.g., 2, 2'-bipyridine, 1,10-phenantroline), and their biological activity are not discussed here.

Table 4.
Selected chelates of quinolones from first generation.
| Ligand | Metalion | Molar ratio M:L | General formulae of the complexes | Complex tested/investigated for | Reference |
|---|---|---|---|---|---|
| Pipemidic acid | VO2+ | 1:2 | [VO(PPA)2(H2O)] | DNA binding antimicrobial activity | [28] |
| Mn2+ | 1:2 | [Mn(PPA)2(H2O)2] | |||
| Fe3+ | 1:3 | [Fe(PPA)3] | |||
| Co2+ | 1:2 | [Co(PPA)2(H2O)2] | |||
| Ni2+ | 1:2 | [Ni(PPA)2(H2O)2] | |||
| Zn2+ | 1:2 | [Zn(PPA)2(H2O)2] | |||
| MoO22+ | 1:2 | [MoO2(PPA)2] | |||
| Cd2+ | 1:2 | [Cd(PPA)2(H2O)2] | |||
| UO22+ | 1:2 | [UO2(PPA)2] | |||
| Cu2+ | 1:2 | [Cu(PPA)2(H2O)] | DNA binding antimicrobial activity | [29] | |
| Fe3+ | 1:1 | [Fe (PPA)(HO)2(H2O)]2 | - | [30] | |
| Cinoxacin | Cu2+ | 1:2 | [Cu(Cx)2(H2O)]·3H2O | - | [31] |
| Ni2+ | [Ni(Cx)2(DMSO)2]·4H2O | ||||
| Cu2+ | 1:2 | [Cu(Cx)2]·2H2O | antimicrobial activity | [32] | |
| Co2+ | 1:3 | [Co(Cx)3]Na·10H2O | antimicrobial activity | [33] | |
| Cu2+ | 1:2 | [Cu(Cx)2]·2H2O Cu(Cx)(HCx)Cl·2H2O | |||
| Zn2+ | 1:2 | [Zn(Cx)2]·4H2O | |||
| Cd2+ | 1:1 | Cd(Cx)Cl·H2O | |||
| Cd2+ | 1:3 | Na2[(Cd(Cx)3)(Cd(Cx)3(H2O))] 12H2O | - | [34] | |
| Oxolinic acid | Cu2+ | 1:2 | [Cu(oxo)2(H2O)] | DNA binding antimicrobial activity | [35] |
| Ni2+ | 1:2 | [Ni(oxo)2(H2O)2] | DNA binding | [36] | |
| Zn2+ | 1:2 | [Zn(oxo)2(H2O)2] | DNA binding | [37] | |
| VO2+ | 1:2 | [VO(oxo)2(H2O)] | DNA binding | [38] | |
| Mn2+ | 1:2 | [Mn(oxo)2(H2O)2] | |||
| Fe3+ | 1:3 | [Fe(oxo)3] | |||
| Co2+ | 1:2 | [Co(oxo)2(H2O)2] | |||
| Ni2+ | 1:2 | [Ni(oxo)2(H2O)2] | |||
| Zn2+ | 1:2 | [Zn(oxo)2(H2O)2] | |||
| Cd2+ | 1:2 | [Cd(oxo)2(H2O)2] | |||
| MoO22+ | 1:2 | [MoO2(oxo)2] | DNA binding antimicrobial activity | [39] | |
| UO22+ | 1:2 | [UO2(oxo)2] | |||
| Flumequine | Cu2+ | 1:2 | [Cu(flmq)2(OH2)2] | - | [40] |
| Zn2+ | [Zn(flmq)2(OH2)2]·H2O | ||||
| Cu2+ | 1:2 | [Cu(flmq)2(H2O)] | DNA binding albumin binding | [41] | |
| Ni2+ | 1:2 | [Ni(flmq)2(H2O)2] | DNA binding albumin binding | [42] | |
| Zn2+ | 1:2 | [Zn(flmq)2(H2O)2] | DNA binding albumin binding | [43] |

Table 5.
Selected chelates of quinolones from second generation.
| Ligand | Metal ion | Molar ratio M:L | General formulae of the complexes | Complex tested/ investigated for | Reference |
|---|---|---|---|---|---|
| Enoxacin | Co2+ | 1:2 | [Co(HEx)2(ClO4)2]·3H2O [Co(HEx)2(NO3)2]·2H2O | antimicrobial activity DNA oxidative cleavage | [44] |
| Cu2+ Ni2+ | 1:2 | [M(Ex)2(H2O)2]·3H2O (M = CuII, NiII or MnII) | antimicrobial activity | [45] | |
| Mn2+ Fe3+ | [Fe(Ex)(H2O)2]Cl·4H2O | antiinflammatory activity | |||
| Ni2+ | 1:2 | Ni(Ex)2·2.5H2O | DNA binding | [46] | |
| Norfloxacin | Mg2+ | 1:2 | [M(Nf)2](ClO4)2·H2O | - | [47] |
| Ca2+ | M: Mg2+, Ca2+ (n = 4), | ||||
| Ba2+ | M: Ba2+ (n = 5) | ||||
| Al3+ | 1:3 | [(Nf·HCl)3Al] | solubility behavior | [48] | |
| Bi3+ | 1:4 | [Bi (C16H18FN3O3)4(H2O)2] | antimicrobial activity solubility behavior | [49] | |
| Bi3+ | 1:3 | [Bi(C16H17FN3O3)3(H2O)2] | antimicrobial activity, including Helicobacter pylori | [50] | |
| Mn2+ | 1:2 | [M(Nf)2]X2·8H2O | - | [51] | |
| Co2+ | (X = CH3COO-or SO42-). | ||||
| Fe3+ | 1:3 | [Fe(Nf)3]Cl3·12H2O | - | ||
| Co2+ | 1:2 | [Co(NfH-O,O’)2(H2O)2](NO3)2 | - | [52] | |
| Mn2+ Co2+ | 1:1 1:1 | [MnCl2(Nf)(H2O)2] [CoCl2(Nf)(H2O)2] | biological evaluation against Trypanosoma cruzi | [53] | |
| Ni2+ | 1:2 | [Ni(Nf)2]·6H2O | DNA binding | [46] | |
| Cu2+ | 1:2 | Cu(HNf)2·5H2O | - | [54] | |
| [Cu(HNf)2]Cl2·2H2O | - | ||||
| Cu(HNf)2(NO3)2·H2O | - | ||||
| 1:2 | [Cu(NfH)2]Cl2·6H2O | DNA binding albumin binding | [55] | ||
| Zn2+ | 1:2 | [Zn(Nf)2]·5H2O | - | [56] | |
| Zn2+ Cd2+ Hg2+ | 1:2 | [M(Nf)2]X2·nH2O [M = Zn(II), (X = Cl−, CH3COO−, Br− and I−), Cd(II), (X = Cl−, NO3− and SO42−) and Hg(II) (X = Cl−, NO3− and CH3COO−)] | antimicrobial activity | [57] | |
| ZrO2+ UO22+ | 1:2 1:3 | [ZrO(Nf)2Cl]Cl·15H2O [UO2(Nf)3](NO3)2·4H2O | antimicrobial activity | [58] | |
| W0 | [W(H2O)(CO)3(H-Nf)]· (H-Nf)·H2O | antimicrobial activity | [59] | ||
| Ru3+ | 1:2 | [Ru(Nf)2Cl2]·4H2O | - | [60] | |
| Pt2+ | 1:2 | [Pt(Nf)2] | DNA binding DNA cleavage ability antimicrobial activity | [61] | |
| Au3+ | 1:1 | [AuCl2(Nf)]Cl | DNA binding albumin binding cytotoxic activitycell cycle | [62] | |
| Y3+ Pd2+ | 1:2 1:2 | [Y(Nf)2(H2O)2]Cl3·10H2O [Pd(Nf)2]Cl2·3H2O | antimicrobial activity | [63] | |
| La3+ Ce3+ | 1:3 1:3 | [La(Nf)3]·3H2O [Ce(Nf)3]·3H2O | antimicrobial activity | [64] | |
| Ln= Nd(III) Sm(III) Ho(III) | 1:4 | [N(CH3)4][Ln(Nf)4]·6H2O | interaction with DNA and albumin | [65] | |
| Pefloxacin | Bi3+ | 1:3 | [Bi(C17H19FN3O3)3(H2O)2] | antimicrobial activity, including Helicobacter pylori | [50] |
| Zn2+ | 1:2 | [Zn (Pf)2(H2O)] ·2H2O | - | [66] | |
| Pt2+ | 1:2 | [Pt(Pf)2] | DNA binding DNA cleavage ability antimicrobial activity | [61] | |
| Ciprofloxacin | Mg2+ | 1:2 | [Mg(Cf)2]·2.5H2O | DNA binding | [67] |
| Mg2+ | 1:2 | [Mg(Cf)2(H2O)2]·2H2O | antimicrobial activity | [68] | |
| Mg2+ | 1:2 1:3 | [Mg(H2O)2(CfH)2](NO3)2·2H2O [Mg(CfH)3](SO4)·5H2O | - | [69] | |
| Mg2+ Ca2+ Ba2+ | 1:2 | [M(Cf)2](ClO4)2·H2O M: Mg2+(n = 6) M: Ca2+ (n = 4) M: Ba2+(n = 2) | - | [47] [70] | |
| Mg2+ Zn2+ Co2+ | 1:2 | [Mg(Cf)2(H2O)2]·2H2O [Zn(Cf)2]·3H2O [Co(Cf)2]·3H2O | - | [22] | |
| Al3+ | 1:3 | [(Cf·HCl)3Al] | [48] | ||
| Bi3+ | 1:3 | [Bi(C17H17FN3O3)3(H2O)2] | antimicrobial activity, including Helicobacter pylori | [50] | |
| VO2+ | 1:2 | [VO(Cf)2(H2O)] | - | [71] | |
| Mn2+ Co2+ Ni2+ Cu2+ Zn2+ Cd2+ | 1:1 | [Mn(Cf)(OAc)(H2O)2]·3H2O [Co(Cf)(OAc)(H2O)2]·3H2O [Ni(Cf)(OAc)]·6H2O [Cu(Cf)(OAc)(H2O)2]·3H2O [Zn(Cf)(OAc)]·6H2O [Cd(Cf)(OAc)(H2O)2]·3H2O | antimicrobial activity | [72] | |
| Mn2+ Fe3+, Co2+ Ni2+ MoO22+ | 1:2 for M2+ 1:3 for Fe3+ | [Mn(Cf)2(H2O)2] [Fe(Cf)3] [Co(Cf)2(H2O)2] [Ni(Cf)2(H2O)2] [MoO2(Cf)2] | DNA binding | [73] | |
| Co2+ Zn2+ Cd2+ Ni2+ Cu2+ | 1:2 | [Co(Cf)2(H2O)]·9H2O [Zn(Cf)2(H2O)2]·8H2O [Cd(HCf)2(Cl)2 ]·4H2O M(Cf)2·xH2O [M = Ni, Cu, Cd] | antimicrobial activity | [34] | |
| Co2+ | 1:2 | [Co(Cf)2]·3H2O | - | [22] | |
| Cu2+ | 1:2 | [Cu(HCf)2](NO3)2]·6H2O | - | [74] | |
| Cu2+ | 1:2 | [Cu(Cf)2]Cl2·11H2O | - | [75] | |
| Cu2+ | 1:2 | [Cu(Cf)2]Cl2·6H2O | - | [76] | |
| Cu2+ | 1:2 | [Cu(HCf)2(ClO4)2]·6H2O [Cu(HCf)2(NO3)2]·6H2 | antimicrobial activity | [44] | |
| 1:1 | [Cu(HCf)(C2O4)]·2H2O | DNA oxidative cleavage | [44] | ||
| Cu2+/ Cu+ | 3:2 | [CuII(Cf)2(CuICl2)2] | antimicrobial activity Gyrase inhibition DNA cleavage | [77] | |
| Ru3+ | 1:2 | [Ru(Cf)2Cl2]Cl·3H2O | - | [60] | |
| 1:3 | [Ru(Cf)3]·4H2O | DNA interaction | [78] | ||
| Pd2+ | 1:1 | [PdCl2(L)] | antitubercular activity | [79] | |
| Eu3+ | 1:2 | [Eu(CfH)(Cf)(H2O)4]Cl2·4.55H2O | - | [80] | |
| Lomefloxacin | Bi3+ | 1:3 | [Bi(C17H18F2N3O3)3(H2O)2] | antimicrobial activity, including H. pylori | [50] |
| Y3+ | 1:2 | [Y(LFX)2Cl2]Cl·12H2O | antimicrobial activity | [81] | |
| ZrO2+ | 1:2 | [ZrO(LFX)2Cl]Cl·15H2O | |||
| UO22+ | 1:3 | [UO2(LFX)3](NO3)2·4H2O | |||
| Cr3+ | 1:1 | [Cr(LFX)(H2O)4]Cl3 | antimicrobial, antifungal, and anticancer activity | [82] | |
| Mn2+ | 1:1 | [Mn(LFX)(H2O)4]Cl2 | |||
| Fe3+ | 1:1 | [Fe(LFX)(H2O)4]Cl3·H2O | |||
| Co2+ | 1:1 | [Co(LFX)(H2O)4]Cl2 | |||
| Ni2+ | 1:1 | [Ni(LFX)(H2O)4]Cl2·H2O | |||
| Cu2+ | 1:1 | [Cu(LFX)(H2O)4]Cl2·2H2O | |||
| Zn2+ | 1:1 | [Zn(LFX)(H2O)4]Cl2 | |||
| Th(IV) | 1:1 | [Th(LFX)(H2O)4]Cl4 | |||
| UO22+ | 1:1 | [UO2(LFX)(H2O)2](NO3)2 | |||
| Ofloxacin | Mg2+ | 1:2 | [Mg(R-oflo)(S-oflo)(H2O)2]·2H2O | antimicrobial activity | [83] |
| Ca2+ | 1:1 | Ca(oflo)Cl·2H2O | - | [84] | |
| Mg2+ | Mg(oflo)Cl·2H2O | ||||
| Ba2+ | Ba(oflo)Cl·2H2O | ||||
| Ni2+ | Ni(oflo)Cl·2H2O | ||||
| Co2+ | Co(oflo)Cl·2H2O | ||||
| Zn2+ | Zn(oflo)Cl·H2O | ||||
| Cu2+ | 1:2 | [CuII(ofloH)2][(CuICl2)2] | DNA binding albumin binding | [55] | |
| Co2+ Zn2+ | 1:2 | [M(oflo)2]·4H2O | - | [85] | |
| Cu2+ | 1:1 | M(oflo)Cl·2.5H2O | - | [86] | |
| Ni2+ | M(oflo)(SO4)0.5·2.5H2O | ||||
| M(oflo) (NO3)·2.5H2O | |||||
| 1:2 | [Cu(oflo)2·H2O]·2H2O | ||||
| Ni(oflo)2·3H2O | |||||
| Pd2+ | 1:1 | [PdCl2(L)] | antitubercular activity | [79] | |
| Pt2+ | 1:2 | [Pt(oflo)2] | DNA binding antimicrobial activity | [61] | |
| Bi3+ | 1:3 | [Bi(C17H17FN3O3)3(H2O)2] | antimicrobial activity, including Helicobacter pylori | [50] | |
| Pr3+ Nd3+ | 1:1 | [PrL(NO3)2(CH3OH)](NO3) [NdL(NO3)2(CH3OH)](NO3) | DNA binding DNA cleavage activity antioxidation properties | [87] | |
| Enrofloxacin | VO2+ | 1:2 | [VO(erx)2(H2O)] | antimicrobial activity DNA binding | [88] |
| MO22+ | 1:2 | [MoO2(erx)2] | antimicrobial activity DNA binding | [89] | |
| Mn2+ Fe3+ Co2+ Ni2+ Zn2+ Cd2+ UO22+ | 1:2 for M2+, 1:3 for Fe3+ | [Mn(erx)2(H2O)2] [Fe(erx)3] [Co(erx)2(H2O)2] [Ni(erx)2(H2O)2] [Zn(erx)2(H2O)2] [Cd(erx)2(H2O)2] [UO2(erx)2] | antimicrobial activity DNA binding | [90] | |
| Ni2+ | 1:2 | [Ni(erx)2(H2O)2] | DNA binding albumin binding | [91] | |
| Cu2+ | 1:2 | [Cu(erx)2]Cl | antimicrobial activity | [92] | |
| Cu2+ | 1:2 | [Cu(erx)2(H2O)] | DNA binding antimicrobial activity | [93] | |
| Cu2+ | 1:2 | [Cu(erx)2(H2O)2] | - | [94] | |
| Ru3+ | 1:2 | [Ru(erx)2Cl2]Cl·5H2O | - | [60] |

Table 6.
Selected chelates of quinolones from third and fourth generation.
| Ligand | Metal ion | Molar ratio M:L | General formulae of the complexes | Complex tested/investigated for | Reference |
|---|---|---|---|---|---|
| Sparfloxacin | Bi3+ | 1:3 | [Bi(C19H21F2N4O3)3(H2O)2] | antimicrobial activity, including Helicobacter pylori | [50] |
| Fe3+, VO2+ Mn2+ Ni2+ UO22+ | 1:3 1:2 for M2+ | [Fe(sf)3] [VO(sf)2(H2O)] [Mn(sf)2(H2O)2] [Ni(sf)2(H2O)2] [UO2(sf)2] | DNA binding Serum albumin binding | [95] | |
| Co2+ | 1:2 | [Co(sf)2(H2O)2] | antimicrobial activity DNA binding | [96] | |
| Cu2+ | 1:2 | [Cu(sf)2] | antimicrobial activity DNA binding | [97] | |
| Mn2+ Co2+ | 1:1 1:1 | [MnCl2(sf)(H2O)2] [CoCl2(sf)(H2O)2] | biological evaluation against Trypanosoma cruzi | [53] | |
| MO22+ | 1:2 | [MoO2(sf)2] | antimicrobial activity DNA binding | [89] | |
| Pd2+ | 1:1 | [PdCl2(L)] | antitubercular activity | [79] | |
| Pt2+ | 1:2 | [Pt(sf)2] | DNA bindingDNA cleavage abilityantimicrobial activity | [61] | |
| Au3+ | 1:1 | [AuCl2(sf)]Cl | DNA bindingalbumin bindingcytotoxic activitycell cycle | [62] | |
| Levofloxacin | Mg2+ | 1:2 | [Mg(S-oflo)2(H2O)2]·2H2O | antimicrobial activity | [83] |
| Mn2+ Co2+ Ni2+ Cu2+ Zn2+ | 1:2 | [M(levo)2(H2O)2]·nH2O (n = 2, excepting for Cu2+, n = 3) | antimicrobial activity immunomodulatory activity cytotoxicity | [98] | |
| Zn2+ | 1:2 | [Zn(levo)2(H2O)2] | DNA binding albumin binding | [99] | |
| Pd2+ | 1:1 | [PdCl2(L)] | antitubercular activity | [79] | |
| Pt2+ | 1:2 | [Pt(levo)2] | DNA binding DNA cleavage ability antimicrobial activity | [61] | |
| Au3+ | 1:1 | [AuCl2(levo)]Cl | DNA binding albumin binding cytotoxic activity cell cycle | [62] | |
| Gatifloxacin | Mg2+ Ca2+ Cr3+ Mn2+ Fe3+ Co2+ Ni2+ Cu2+ Zn2+ Cd2+ | 1:2 | [Mg(gat)2(H2O)2]Cl2·2H2O [Ca(gat)2(H2O)2]Cl2·2H2O [Cr(gat)2 Cl(H2O)2]Cl·2H2O [Mn (gat)2(H2O)2]·6H2O [Fe(gat)2Cl(H2O)2]Cl·2H2O [Co (gat)2(H2O)2]·4H2O [Ni (gat)2(H2O)2] Cl2·2H2O [Cu (gat)2(H2O)2]·H2O [Zn (gat)2(H2O)2]·2H2O [Cd (gat)2(H2O)2] Cl2·4H2O | antimicrobial activity antifungal activity antiiinflamatory | [100] |
| Zn2+ Ni2+ Co2+ | 1:2 | [M(gat)2(H2O)2]·4H2O | antimicrobial activity | [101] | |
| Bi3+ | 1:3 | [Bi(C19H21FN3O4)3(H2O)2] | antimicrobial activity, including Helicobacter pylori | [50] | |
| Pd2+ | 1:1 | [PdCl2(L)] | - | [79] | |
| Pt2+ | 1:2 | [Pt(gat)2] | DNA binding DNA cleavage ability antimicrobial activity | [61] | |
| Rh3+ | 1:1 | [X]+fac-[RhCl3(L)(gat)]- where L = H2O, Dimethylsulfoxide (DMSO), Tetramethylenesulfoxide (TMSO); gat = Gatifloxacin and X = Na or [H(DMSO)2]. | antimicrobial activity | [102] | |
| Moxifloxacin | Cu2+ | 1:1 | [Cu(MOX)(H2O)2Cl]BF4 | anti-proliferative and apoptosis-inducing activity | [103] |
| Pd2+ Y3+ Ti(IV) Ce(IV) | 1:2 1:2 1:2 1:2 | [Pd(MOX)2(H2O)2]Cl2·6H2O [Y(MOX)2Cl2]Cl·12H2O [Ti(MOX)2](SO4)2·7H2O [Ce(MOX)2](SO4)2·2H2O | antimicrobial activity | [104] | |
| VO2+ Zr(IV) UO22+ | 1:2 1:2 1:3 | [VO(MOX)2H2O]SO4·11H2O [ZrO(MOX)2Cl]Cl·15H2O [UO2(MOX)3](NO3)2·3H2O | antimicrobial activity | [105] |
The first review regarding the interactions of metal ions with quinolone was published ten years ago and discussed selected crystal structures of quinolone–metal compounds, different physico-chemical methods of characterization, as well as some results of bioactivity test [21]. The structural characteristics of a part of fluoroquinolone complexes and their biological activity were reviwed four years ago [106]. A recent comprehensive review [107] presented the structures and the biological activity of complexes of some quinolones with Cu(II), Ni(II), Co(II) and Zn(II) and analysed the influence of the second ligand on biological activity.
In one report, norfloxacin acts as bidentate ligand through two carboxylate oxygen atoms (Figure 6) in complexes with Co(II) and Fe(III) ions [108]. A quite rare coordination mode of quinolones occurs in a bidentate fashion via the piperazine nitrogen atoms. This coordination was reported in complexes of general formula [PtCl2(L)] (Figure 7) formed by ciprofloxacin, levofloxacin, ofloxacin, sparfloxacin, and gatifloxacin with Pt(II) [109], and could be explained through the basicity both of N4 nitrogen from piperazine ring and of N1 nitrogen, the last one evidenced in recent studies [110].
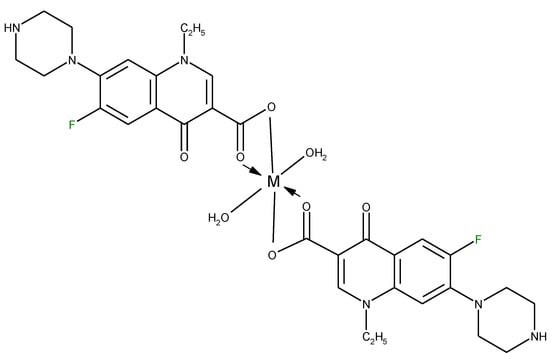
Figure 6.
The proposed structure of complexes of Fe(III)-Nf and Co(II)-Nf (adapted from [108]).
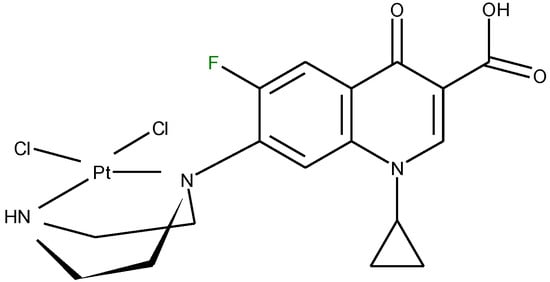
Figure 7.
Proposed structure for [PdCl2(L)] (adapted from [104]).
3.2. Chelates Introduced into the Polyoxometalates (POMs) Surface
Quinolone molecules are excellent multidentate ligands able to construct metal–organic polymers with medical applications, due to the higher electronic cloud density of oxygen and nitrogen atom [111]. Such hybrid organic-inorganic materials have been obtained by introducing a quinolone chelate into the surface of a polyoxometalate anion. The polyoxometalates (POMs) are known as anti-tumor, antiviral, and antibacterial inorganic medical agents, and the modifying of their surface with such compounds with biological activity is aimed to ameliorate their properties.
Generally, these complexes were obtained by hydrothermal reaction of a quinolone with a metal salt and a polyoxometalate (in the acidic form or as ammonium salt) with adjusting the pH.
One of the simplest compound of this series is V4O10(μ2-O)2[VO(H-Cf)2)]2·13H2O, with a structure consisting in one {V4O12} unit and two corner-sharing octahedral {VO6}-ciprofloxacin units linked through two μ2-O bridges [112].
Anions with α-Keggin structure (PW12O404-, SiW12O404-) were used as inorganic building blocks in compounds constructed from PW12 or SiW12 clusters and two M(Quin)2 chelates. The PW12 or SiW12 clusters and quinolone molecule as chelating bidentate organic ligands coordinate the metal ions together (Figure 8). The binuclear metal clusters are connected to the POM clusters, bound as unidentate or as bridging bi-dentate inorganic ligands, forming a 1D chain architecture, as shown in Figure 9.
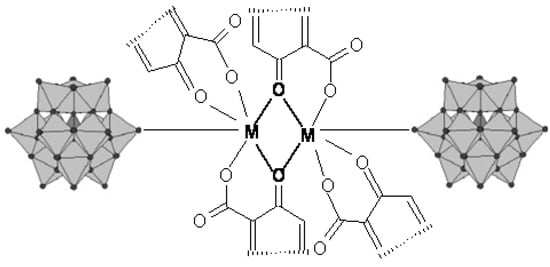
Figure 8.
A binuclear metallic cluster of quinolone bound to POM clusters.
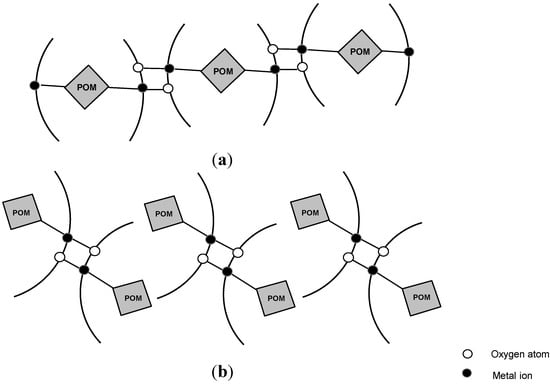
Figure 9.
Schematic representation of the 1D chain structure, constructed by POMs and M-quin binuclear clusters with POM bound as (a) bidentate bridging ligand or (b) unidentate ligand.
Starting to polyoxometalates (POMs) and the quinolone antibacterial drug pipemidic acid (HPPA), complexes as {[Co(PPA)2]H2[SiW12O40]}∙HPP∙3H2O [113], [Cu(PPA)2]2·[PW12O40]∙6H2O [114], {[Ni(PPA)2]H4[SiW12O40]}∙HPPA∙3H2O, and {[Zn(PPA)2]2H4[SiW12O40]}∙3H2O [115] were obtained. By introducing different quinolone antibacterial drugs into the octamolybdate POMs new compounds have been isolated, such as [CuII(L1)2(H2O)2]H2[β-Mo8O26]∙4H2O (1), [CuII2(L2)4][δ-Mo8O26]∙4H2O (2), [CuII2(L3)2(H2O)2][β-Mo8O26] (3), [CuII2(L4)2(H2O)4][β-Mo8O26]∙2H2O (4) (where L1 = enrofloxacin; L2 = pipemidic acid; L3 = norfloxacin; L4 = enoxacin) [111].
3.3. Metal Complexes with Quinolone Acting as Unidentate Ligand
The quinolones bearing a piperazinyl ring in the 7-position could form complexes where the terminal piperazinyl nitrogen (N4) is involved in the coordination to the metal ion. This coordination mode was reported for complexes with transition metals Ag(I), Au(III), and Ru(III). The structure proposed for the complex Ag2(Nf)2(NO3)2 [116] is presented in Figure 10.
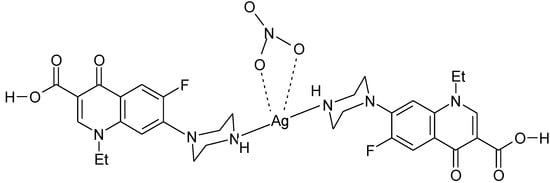
Figure 10.
Proposed structure for the complex Ag(H-Nf)2(NO3) [116].
By the reaction of Ag(I) and Au(III) with norfloxacin, a dinuclear complex Ag2(Nf)2(NO3)2 [Figure 11(a)], and a mononuclear complex [Au(Nf)2(H2O)2]Cl3 [Figure 11(b)] were obtained [117].
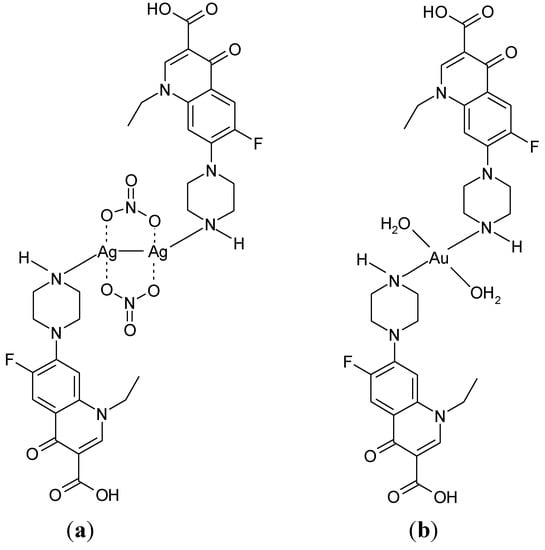
Figure 11.
Proposed structures for (a) Ag2(Nf)2(NO3)2, and (b) [Au(Nf)2(H2O)2]Cl3 [117].
In some complexes of Ru(III), formulated as Ru(L)2Cl3(DMSO)m∙xH2O (L: pipemidic acid, enoxacin, enrofloxacin, ciprofloxacin, norfloxacin, ofloxacin, levofloxacin), quinolones are bound as unidentate ligand coordinate through the N4 piperazinyl nitrogen [118,119].
3.4. Polymeric Complexes
Dimeric complexes [Mg2(H2O)6(HNf)2]Cl4·4H2O and [Ca2(Cl)(HNf)6]Cl3·10H2O [120] are formed with norfloxacin as bidentate bridging ligand bound through the pyridone oxygen and one carboxylate oxygen atom (unidentate bridging) (Figure 12).
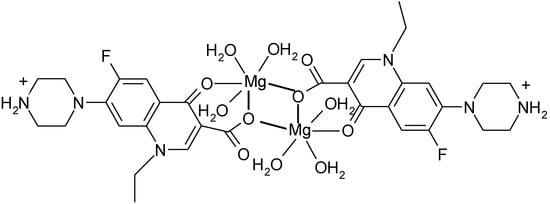
Figure 12.
Structure of the dimeric complex [Mg2(H2O)6(HNf)2]Cl4·4H2O (adapted from [120]).
A similar coordination it was found in the complex [Pb(H-Nf)(ONO2)2]2 (Figure 13) [121].
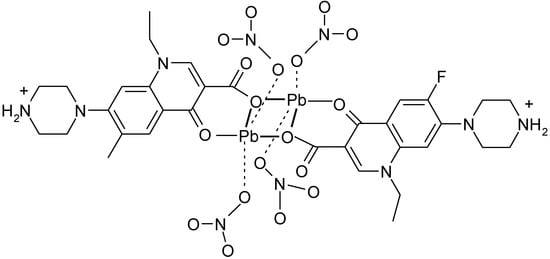
Figure 13.
Structure of the dimeric complex [Pb(H-Nf)(ONO2)2]2 (adapted from [121]).
X-ray determination of crystal structure of the dinuclear complexes [Cd2(Cx)4(H2O)2]·10H2O and [Cd2(Cx)4(DMSO)2]·2H2O revealed that the cadmium ion is heptacoordinated; the coordination environment consists in two cinoxacinate ions acting as tridentate chelate and bridging ligands, one as bidentate chelate ligand, and one water molecule [33].
In polymeric complexes, different modes of coordination are simultaneously possible. In the case of two Fe(II) complexes, norfloxacin adopts different modes of coordination depending on the synthesis conditions. In the structure of Fe(H-Nf)2(SO4)·2H2O, Fe(II) is surrounded by two norfloxacinate anions bound as bidentate ligand coordinated through the pyridone oxygen and one carboxyl carboxylate oxygen and two norfloxacin molecules coordinated as unidentate ligand by two oxygen atoms from two different carboxylate [Figure 14(a)]. In the other complex, Fe(Nf)2·4H2O, two molecules are bound as bidentate ligand, and two as unidentate ligand coordinated through piperazine nitrogen [Figure 14(b)] [122].
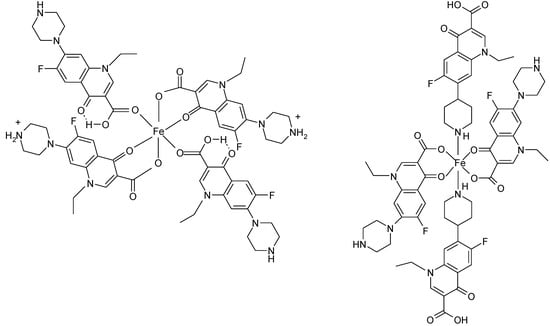
Figure 14.
Coordination modes of norfloxacin in (a) Fe(H-Nf)2(SO4)·2H2O and (b) Fe(Nf)2·4H2O (adapted from [122]).
In a 1D ladder-like silver(I) coordination polymer, {[Ag4(H-Cf)2(Cf)2(NO3)2]·4H2O}n [123] the pseudo-tetranuclear building blocks are constructed via unidentate ciprofloxacin coordinated through the N4 piperazine atom and tetradentate deprotonated ciprofloxacin ligands (Figure 15).
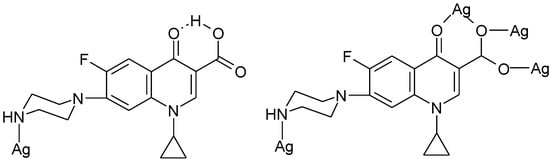
Figure 15.
Coordination modes of ciprofloxacin and its anion in {[Ag4(H-Cf)2(Cf)2(NO3)2]·4H2O}n [123].
3.5. Ionic Complexes
Based on the basic function of the N4 pyperazinyl atom, quinolones are protonated in acidic medium, forming ionic chlometalates, generally obtained by slow evaporation of an acidic solution of complex and metal salt. Most of these complexes were tested for their antimicrobial activity (see Subsection 4.3).
The chloroantimonates (III) obtained with nalidixium C12H13N2 (nalidixium cation) and ciprofloxacinium ions have the general formulae (C12H13N2O3)[SbCl4]·H2O [124], and (C17H19N3O3F) [SbCl5]·H2O (ciprofloxacinium cations (CfH3)2+) [125] respectively. Two types of chlorobismutates (III) were obtained with ciprofloxacin, (CfH2)(CfH)[BiCl6]·2H2O [126] and (CfH2)2[Bi2Cl10]·4H2O [127].
The tetrachlorocuprates (II) synthesized from norfloxacin, pefloxacin, and cinoxacin, were formulated as (NfH2)(NfH)[CuCl4]Cl·H2O [128], (C17H22FN3O3)2+[CuCl4]2− [129], and (CxH2)[CuCl4]·H2O [129], respectively.
Other chloromethalates, such as enrofloxacinium tetrachloroferate (II), (erxH2)[FeCl4]Cl [130], ciprofloxacinium tetrachlorozincate (II) dihydrate, [C17H19N3O3F]2[ZnCl4]·2H2O [131], ciprofloxacinium tetrachloroaurate (III) monohydrate, (CfH2)[AuCl4]· H2O [132] and ciprofloxacinium hexachlororuthenate (III) trihydrate, (CfH2+)3[RuCl6]·3H2O [78] were also reported.
4. Consequences and Applications of Metal-Quinolone Complexation
4.1. Pharmaceutical Aspects
Some chelates of quinolones with trivalent cations have shown an improved solubility compared to that of the free ligand, and this behaviour could be advantageous for pharmaceutical formulation. The hydrochlorides of the aluminium (III) complexes of ciprofloxacin and norfloxacin were reported [48,133]. Both complexes are more soluble than the antibiotics themselves. The complexes can be used for developing more dose-efficient formulations, such as compressed tablet dosage forms [48,134]. The pharmacodynamic properties of ciprofloxacin are not drastically affected upon complexation with aluminium. The complex [(HCl·Cf)3Al] showed a longer post-antibiotic effect (PAE) compared to that the free ciprofloxacin [135].
The solubility studies of a bismuth (III) complex of norfloxacin, [Bi(C16H18FN3O3)4(H2O)2] (BNC) in different pH buffers indicated that the solubility of the BNC was higher than that of norfloxacin until pH 6.5. Above this pH value, a significant decrease in the solubility of BNC was observed, while the solubility of norfloxacin did not change significantly. The increased solubility can be an advantage for the antibacterial activity of the bismuth complex [49].
4.2. Biopharmaceutical and Pharmacokinetic Implications
Reducing the oral bioavailability of quinolones in the presence of multivalent cations is the main consequence of the metal ions-quinolones interaction, and it was reported for the first time in 1985 [136]. A reduction in ciprofloxacin biavailability in healthy human subjects was observed at co-administration with ferrous salts and a combination of multi-vitamin and mineral preparation. In correlation with UV-Vis spectra features, the formation of a 1:3 ferric ion-ciprofloxain complex was proposed as the cause of the reduction in ciprofloxacin biovailability [137]. A strong correlation between the reduction in oral bioavailability of norfloxacin in the presence of divalent and trivalent cations and the magnitude of formation constants measured in vitro was established (Ca2+ < Mg2+ < Zn2+ ~ Fe2+ < Al3+). A marked difference between the effect of Zn2+ and Fe2+ was observed in vivo, namely a greater reduction in norfloxacin absorption with co-administration of Fe2+. The oxidation of Fe2+ to Fe3+ in gastrointestinal tract was proposed as possible explanation [138].
Several mechanisms were proposed in order to explain the decreased biovailability of quinolone in the presence of metal ions. The first hypothesis was that the reduction of quinolone absorption is due to the formation of insoluble and unabsorbable chelates in the gastrointestinal tract [139,140,141]. On the contrary, in other studies it was observed that the solubility of lomefloxacin increases in the presence of Ca2+, Mg2+, Al3+ şi Fe3+ ions [142]. This means that the reduction of the gastric absorption of lomefloxacin at co-administration with these metal ions, are not caused by the precipitation, but by a decrease of the octanol-water partition cofficient. Only for Bi3+, solubility and thus absorption of lomefloxacin, decresed as a result of formation of species with low solubility [143]. The permeability through intestinal mucosa of fluoroquinolone alone and in the presence of metal ions was studied in vitro. The effect of Ca2+, Mg2+, Fe2+ was tested with ciprofloxacin, while the effect of Al3+ was tested with ciprofloxacin, norfloxacin and ofloxacin. The experimental data revealed that the fluoroquinolone-metal ion combinations resulted in a reduced intestinal permeability compared to that of the corresponding fluoroquinolone, leading to a reduction of fluoroquinolone bioavailability [144].
4.3. Mechanism of Action of Quinolones
The DNA-binding capacity of quinolone complexes was studied in relation with the mechanism of action of quinolones. Experimental data suggested an interaction of quinolone-Mg2+ complex with DNA and gyrase and not a direct interaction of free quinolones with DNA, and a model for the ternary complex was proposed. In this model, Mg2+ acts as a bridge between the phosphate groups of the nucleic acid and the carbonyl and carboxyl moieties of norfloxacin, with additional stabilization arising from stacking interactions between the condensed rings of the drug and DNA bases [145].
Interaction of an oligonucleotide duplex and ciprofloxacin in the absence and in the presence of Mg2+ was studied and a model of the ternary Cf–Mg2+–duplex adduct orientation was proposed. Docking carried out on this model sustained the orientation of the CFX–Mg2+ in the minor groove of DNA [146].
Interaction with calf thymus DNA was investigated in vitro using different associations between quinolone and divalent metal ions: norfloxacin-Cu2+ [147], ciprofloxacin-Mg2+, -Cu2+ [148,149], levofloxacin-Cu2+ [150], gatifloxacin- Mg2+, - Cu2+ [149,151], -Co2+, -Cd2+ [151], fleroxacin- Mg2+, -Cu2+ [146], sparfloxacin-Mg2+ [149,152], -Cu2+ [149], -Cd2+ [152], -Cr(III), -Cr(VI) [153], pazufloxacin-Cu2+ [154].
From the experimental results, it was concluded that the metal ion plays an intermediary role in the interaction between quinolone and DNA, and the metal complex of quinolone can interact with DNA by an intercalative binding model [155,156]. In vitro experiments demonstrated the hypothesis that, on the one hand, DNA gyrase cannot bind quinolones in the absence of DNA, and on the other hand, the quinolone-gyrase-DNA complex is formed in the presence of Mg2+.
Magnesium and related metal ions affect the stability and function of topoisomerases: they reduce the stability of protein thus increasing the structural flexibility required for the structural changes involved in catalytic cycle [157,158]. On the other side, the divalent metal ions (especially Mg2+) might play a role in enzyme poisoning due to their ability to bind the topoisomerase II-directed drugs, including quinolones [158]. The coordination environment proposed for Mg2+ bound to topoisomerase IV consists in two C3/C4 oxygen atoms from a quinolone molecule chelated and four water molecules. Two of these water molecules are involved in hydrogen bonds with serine side chain hydroxyl group and with serine glutamic acid side chain carboxyl group. It was suggested that the interaction between quinolone and topoisomerases is mediated by this water-metal ion “bridge” [159]. Mutations of one of both amino acid residues that disrupt the bridge function partially or total, and thus the protein-quinolone interaction, are the most common causes of quinolone resistance [160].
4.4. Metal Complexes with Biological Activity
4.4.1. Antimicrobial Activity
The consequence of interaction with metal ions on the biological activity of quinolones was approached in the first instance as a negative phenomenon, and some evidences of reduction in the antimicrobial activity of quinolones in the presence of metal ions [161,162] support this assumption. Two possible mechanisms were proposed for explaining the reduction of ciprofloxacin activity by metal cations. First of these, especially valid for chelates with 1:1 stoichiometry, could be a decreased permeation of the antibiotic into bacterial cells, while the second one is the formation of an inactive chelate [25].
However, for many chelates of quinolones obtained in solid state, an equal or superior activity was observed compared to that of parent drugs. Selected results expressed as minimal inhibitory concentration (MIC, μg mL−1) or as the inhibition diameter zone (mm) are presented in Table 7 and Table 8. Increased biological activity of metal chelates was explained by the overtone concept of cell permeability and chelation theory. Upon chelation, the polarity of a metal ion is reduced due to the partial sharing of positive charge with the donor groups of ligand and as a consequence of overlap with the ligand orbitals. Chelation increases the delocalization of π electrons over the whole chelate ring and thus increases the lipophilic nature of the central ion. This increased in lipophilicity enhances the passage of complex through the lipid membranes and the penetration in cells [163,164,165].

Table 7.
Minimal inhibitory concentration (MIC, μg mL−1) of the drugs for some assayed bacteria.
| Compound | Bacterial strain | Ref | ||||||
|---|---|---|---|---|---|---|---|---|
| Gram (+) | Gram (-) | |||||||
| S. aureus | B. subtilis | E. faecalis | E. coli | P. aeruginosa | K.pneumoniae | S.typhimurium | ||
| Pipemidic acid | 16.0 | - | - | 64.0 | 64.0 | - | - | [29] |
| [Cu(PPA)2(H2O)] | 16.0 | - | - | 8.0 | 8.0 | - | - | |
| [VO(PPA)2(H2O)] | 16.0 | - | - | 64.0 | 64.0 | - | - | [28] |
| [Mn(PPA)2(H2O)2] | 16.0 | - | - | 64.0 | 64.0 | - | - | |
| [Fe(PPA)3] | 32.0 | - | - | 64.0 | 64.0 | - | - | |
| [Co(PPA)2(H2O)2] | 32.0 | - | - | 64.0 | 64.0 | - | - | |
| [Ni(PPA)2(H2O)2] | 32.0 | - | - | 64.0 | 32.0 | - | - | |
| [Zn(PPA)2(H2O)2] | 32.0 | - | - | 32.0 | 32.0 | - | - | |
| [MoO2(PPA)2] | 16.0 | - | - | 64.0 | 64.0 | - | - | |
| [Cd(PPA)2(H2O)2] | 16.0 | - | - | 64.0 | 64.0 | - | - | |
| [UO2(PPA)2] | 8.0 | - | - | 8.0 | 8.0 | - | - | |
| Cinoxacin | > 64 | - | > 64 | 4.0 | > 64 | 8.0 | 4.0 | [33] |
| [Cu(Cx)2]·2H 2O | > 64 | - | > 64 | 4.0 | > 64 | 8.0 | 4.0 | |
| [Co(Cx)3]Na·10H2O | > 64 | - | > 64 | 2.0 | > 64 | 2.0* | 2.0 | |
| Cu(Cx)(HCx)Cl·2H2O | > 64 | - | > 64 | 4.0 | > 64 | 8.0* | 8.0 | |
| [Zn(Cx)2]·4H2O | > 64 | - | > 64 | 4.0 | > 64 | 4.0* | 4.0 | |
| Cd(Cx)Cl·H2O | > 64 | - | 64 | 4.0 | > 64 | 8.0* | 8.0 | |
| [Cd2(Cx)4(DMSO)2]·2H2O | > 64 | - | 64 | 8.0 | > 64 | 8.0* | 8.0 | |
| [Cd2(Cx)4(H2O)2]·10H2O | > 64 | - | 64 | 4.0 | > 64 | 4.0* | 4.0 | |
| Oxolinic acid | 16 | - | - | 1 | 16 | - | - | [35] |
| [Cu(oxo)2(H2O)] | 64 | - | - | 64 | 32 | - | - | |
| Enoxacin | 1 | 0.25 | 4 | 0.12 | 0.12 | 0.12 | 0.12 | [44] |
| [Co(HEx)2(ClO4)2]·3H2O | 2 | 0.5 | 8 | 0.25 | 0.25 | 0.25 | 0.12 | |
| [Co(HEx)2(NO3)2]·2H2O | 1 | 0.25 | 8 | 0.25 | 0.25 | 0.25 | 0.12 | |
| Norfloxacin | 0.060 | - | - | 0.050 | - | 0.075 | - | [49] |
| [Bi(C16H18FN3O3)4(H2O)2] | 0.045 | - | - | 0.025 | - | 0.060 | - | |
| Ciprofloxacin | 1 | 0.12 | 1 | 0.03 | 0.5 | 0.03 | 0.016 | [44] |
| [Cu(HCf)2(NO3)2]·6H2O | 0.5 | 0.12 | 0.5 | 0.03 | 1 | 0.06 | 0.03 | |
| [Cu(HCf)(C2O4)]·2H2O | 0.5 | 0.12 | 2 | 0.06 | 1 | 0.06 | 0.06 | |
| Ciprofloxacin | 0.25 | 0.03 | 1 | 0.016 | 0.12 | 0.03 | 0.016 | [34] |
| [Co(Cf)2(H2O)]·9H2O | 0.25 | 0.06 | 1 | 0.004 | 0.12 | 0.016 | 0.008 | |
| [Zn(Cf)2(H2O)2]·8H2O | 0.25 | 0.03 | 1 | 0.004 | 0.12 | 0.03 | 0.016 | |
| Ni(Cf)2· 10H2O | 0.5 | 0.03 | 1 | 0.12 | 0.12 | 0.03 | 0.016 | |
| Cu(Cf)2· 6H2O | 0.25 | 0.03 | 1 | 0.004 | 0.12 | 0.03 | 0.008 | |
| Ofloxacin | 0.75 ** | 0.5 | 10 | 0.2 | 7 | 0.7 | 0.75 *** | [83] |
| [Mg(R-oflo)(S-oflo)(H2O)2]·2H2O | 1 ** | 0.8 | 15 | 0.25 | 10 | 1 | 1 *** | |
| Levofloxacin | 0.3 ** | 0.3 | 4 | 0.15 | 3 | 0.25 | 0.5 *** | |
| [Mg(S-oflo)2(H2O)2]·2H2O | 0.6 ** | 0.5 | 4 | 0.15 | 5 | 0.5 | 0.75 *** | |
| Enrofloxacin | 8 | - | - | 1 | 1 | - | - | [93] |
| [Cu(erx)2(H2O) | 32 | - | - | 0.125 | 0.125 | - | - | |
| erx | 0.012 | - | - | - | - | - | - | [92] |
| [Cu(erx)2]Cl | 0.0085 | - | - | - | - | - | - | |
| Herx | 8 | - | - | 1 | 1 | - | - | |
| [VO(erx)2(H2O)] | 8 | - | - | 4 | 4 | - | - | |
| [Cu(erx)2(H2O)] | 4 | - | - | 0.125 | 0.125 | - | - | |
| [MO2(erx)2] | 4 | - | - | 1 | 1 | - | - | |
Abbreviations: S. aureus, Staphylococcus aureus; B. subtilis, Bacillus subtilis; E. faecalis, Enterococcus (Streptococcus) faecalis; E. coli, Escherichia coli; P. aeruginosa, Pseudomonas aeruginosa; K. Pneumoniae, Klebsiella pneumoniae; S. thyphimurium, Salmonella typhimurium; * Klebsiella spp; ** S. epidermidis; *** S. enteriditis.

Table 8.
The inhibition diameter zone values (mm) for norfloxacin and some of its complexes.
| Compound | Bacterial strain | Reference | ||
|---|---|---|---|---|
| Staphylococcus aureus | Escherichia coli | Pseudomonas aeruginosa | ||
| Norfloxacin | 12 | 25 | 13 | [63] |
| [Y(NOR)2(H2O)2]Cl3∙10H2O | 31 | 39 | 47 | |
| [Pd(NOR)2]Cl2∙3H2O | 27 | 26 | 28 | |
| [La(nor)3]∙3H2O | 12 | 10 | 9 | [64] |
| [Ce(nor)3]∙3H2O | 12 | 11 | 10 | |
In fact, many more factors should be considered for metal complexes with antimicrobial activity: (i) the nature of the metal ion; (ii) the nature of the ligands; (iii) the chelate effect; (iv) the total charge of the complex; (iv) the nature of the ion neutralizing the ionic complex; and (vi) the nuclearity of the metal center in the complex [28,29,89,90,91,107]. A detailed comment of the effect of these factors on the biological activity of metal-quinolone complexes was made in a recent review [107].
The results obtained in some particular bacterial strains (Mycobacterium tuberculosis and Helycobacter pylori), which have not been included in Table 7 and Table 8, are worth emphasizing distinctively. Fluoroquinolones have been used successfully in helping cure multidrug-resistant tuberculosis, and studies in mice suggest that they can be considered as first line drugs to shorten the duration of therapy [166]. The main drawback with these agents is the high level of resistance, mainly associated with mutation at gyrA or gyrB genes [167,168]. Metal coordination to quinolones can be used not only as strategy to enhance their activity, but also to overcome the drug resistance. The complex of Cu(II) with ciprofloxacin having general formula [Cu(Cf)2(BF4)2]·6H2O exhibited a significant enhancement in the antitubercular activity comparing to ciprofloxacin alone [169]. A series of Pd(II) and Pt(II) complexes with general formula [MCl2(L)] (where L = ciprofloxacin, levofloxacin, ofloxacin, sparfloxacin, and gatifloxacin) were evaluated against Mycobacterium tuberculosis virulent strain H37Rv. The Pd(II) and Pt(II) complexes with sparfloxacin and the Pt(II) complex with gatifloxacin were the most active within each series in inhibiting bacterial growth, while the least active complexes of the series were the Pd(II) complex with ciprofloxacin and the Pt(II) complex with ofloxacin. Complexes have not shown better antitubercular activity than free gatifloxacin, but their activity was good and, except the complex of Pd(II) with ciprofloxacin, all of them were more active than rifampicin [79]. The results are in agreement with the in vitro activities of the parent drugs against M. tuberculosis isolated: ciprofloxacin < or = ofloxacin < sparfloxacin < gatifloxacin [170].
Fluoroquinolones from new generations, like levofloxacin, moxifloxacin, gatifloxacin or sitafloxacin have demonstrated efficacy in Helicobacter pylori eradication, in third-line or second-line triple therapy, in combination with a proton pump inhibitor (PPI) and amoxicilin [171,172]. Bismuth-containing quadruple therapy (omeprazole, bismuth, metronidazole and tetracycline) is an alternative first choice treatment for H. pylori [173]. Good results were also obtained with quadruple therapy of bismuth subcytrate-moxifloxacine-tetracycline-lansoprazole (BMTL) with high eradication rate and relatively mild side effects [174]. Starting from these premises, a series of bismuth-fluoroquinolone complexes [Bi(Flq)3(H2O)2] (Flq: norfloxacin, ofloxacin, ciprofloxacin, sparfloxacin, lomefloxacin, pefloxacin, gatifloxacin) were evaluated for their anti-H. pylori activity, and were found to be more potent against all strains of H. pylori used, comparing to the parent FLQs. Moreover, the synthesized complexes also showed high potency against some fluoroquinolone-resistant strains of H. pylori. [50].
4.4.2. Antifungal and Antiparasitic Activity
Altough quinolones themselves does not exhibit antifungal activity some of complexes generated by newer fluoroquinolones act not only as antimicrobial agents, but have also shown antifungal activity. Complexes with 1:1 stoichiometry of levofloxacin with Cr(III), Fe(III), Co(II), Ni(II), Cu(II), Th(IV), Mn(II), Zn(II) and UO2(II) have proved an antifungal effect higher than the free ligand against Candida albicans [82]. Complexes of gatifloxacin with Ni(II), Cu(II), Zn(II), Cd(II), Fe(III), Ca(II), Mg(II),Cr(III), Mn(II) and Co(II) having a stoichiometry 1:2 (metal: ligand) have excellent activity as compared to standard drug toward the fungi Trichophyton rubrum, Candida albicans and Fusarium solani [100].
The complexes [MnCl2(sf)(H2O)2] and [CoCl2(sf)(H2O)2] displayed a considerable antiparasitic activity against Trypanosoma cruzi. The corresponding complexes of norfloxacin have a differentiated activity: the Mn(II) complex did not improve the anti-parasitic effect of the free norfloxacin, while the Co(II) complex displayed a 4-fold higher activity than norfloxacin ligand [53].
A new field of research was opened starting to the synthesis of organometallic ruthenium complexes of some quinolone antibacterial agents. The organometallic ruthenium complex of ofloxacin [(η6-p-cymene)RuCl(O,O-oflo)]·2.8H2O has a “piano-stool” structure with quinolone acting as bidentate ligand coordinated to the metal through the ring carbonyl and one of the carboxylic oxygen atoms [175]. The complex interacts with DNA and provokes DNA shrinkage. It is moderately active against Trypanosoma brucei rhodesiense, Trypanosoma cruzi and Plasmodium falciparum.
4.4.3. Anticancer Activity
The anticancer activity of fluoroquinolones has been explored in the last years [176,177,178,179] based on their ability to block topoisomerase II, thus inhibiting the DNA repair activity. It is not surprising that numerous studies concerning the biological activity of quinolone metal complexes include their ability to interact with DNA, as a premise for anticancer activity (see Table 4).
Some complexes of lomefloxacin, [Co(LFX)(H2O)4]∙Cl2 and [Zn(LFX)(H2O)4]∙Cl2 were found to be very active against the breast cancer cell line MCF7 [82]. The anti-proliferative activities of the complex [Cu(mox)(H2O)2Cl]BF4 and of other congeneric complexes with mixed ligands were evaluated against four breast cancer cell lines (MCF-7, T47D, MDA-MB-231 and BT-20), along with the normal breast epithelial MCF-10A cell line, comparing to the parent drug, moxifloxacin. Both the parent ligand as well as its copper complex did not significantly inhibit the proliferation of non-tumorogenic MCF-10A breast epithelial cells. Moxifloxacin did not exhibit anti-proliferative effect against any of the breast cancer cell lines examined, instead, the Co(II) complexes showed differential anti-proliferative activity against the tested breast cancer cell lines [103].
The gold (III) complexes with general formula [AuCl2L]Cl (L = norfloxacin, levofloxacin, sparfloxacin) were tested against A20 (murine lymphoma), B16-F10 (murine melanoma) and K562 (human myeloid leukemia) tumor cell lines comparing to the normal cell lines L919 (murine lung fibroblasts) and MCR-5 (human lung fibroblasts). The free ligands did not showed significant activity in the tumor or normal cell lines, whereas the complexes are more active than the parent drugs, and they have with a similar cytotoxic activity [62].
Recent research has focused on increasing the antitumor activity of polyoxometalates (POMs) by introduction of medicine molecules into the POM surface [180], and such molecules could be quinolone chelates. The first compound obtained by modifying the surface of a POM with a quinolone chelate was the complex {[Co(PPA)2]H2[SiW12O40]}∙HPPA∙3H2O. The inhibitory effect against MCF-7 cells lines showed that the complex and pipemidic acid have shown high antitumor activity to MCF-7, whereas the parent compound SiW12 exhibits no antitumor activity to MCF-7. Furthermore, the antitumor activity of complex was higher that that of its parent compounds, and this superiority could be explained from the synergism of POMs and Co-PPA [113]. Other complexes of pipemidic acid, [Cu(PPA)2]2∙[PW12O40]∙6H2O), [HPPA]5∙[PW11CdO39]∙2H2O, and [HPPA]3∙[PW12O40]∙2H2O showed a stronger antitumor activity than that of the parent anion against PC-3, Hela and HepG2 cells [114].
It was found that antitumor activity depends on the binding mode of the polyoxoanion. Thus, the complex {[Ni(PPA)2]H4[SiW12O40]}∙HPPA∙3H2O, with a SiW12 polyoxoanion acting as a mono-dentate inorganic ligand covalently linked to the nickel ions, showed no antitumor activity, whereas {[Zn(PPA)2]2H4[SiW12O40]}∙3H2O, with a SiW12 polyoxoanion acting as a bi-dentate inorganic ligand covalently linked to the two zinc ions, exhibited higher antitumor activities than its parent compound against MCF-7 lines [115]. The type of polyoxoanion also affects the antitumor activity. This effect was observed for complexes [CuII(L1)2(H2O)2]H2[β-Mo8O26]∙4H2O (1), [CuII2(L2)4][δ-Mo8O26]∙4H2O (2), [CuII2(L3)2(H2O)2][β-Mo8O26] (3), [CuII2(L4)2(H2O)4][β-Mo8O26]∙2H2O (4) (where L1 = enrofloxacin; L2 = pipemidic Acid; L3 = norfloxacin; L4 = enoxacin). The complexes 1, 3, and 4 exhibited a higher effect against SGC7901 lines comparing to the parent compound, while compound 2 showed no anti-SGC7901 activity [111].
The organometallic ruthenium complexes chlorido(η6-p-cymene)(nalidixicato-κ2O,O)ruthenium(II) and chlorido(η6-p-cymene)(cinoxacinato-κ2O,O)ruthenium(II) were investigated as anticancer agents in human A549 (nonsmall cell lung carcinoma), CH1 (ovarian carcinoma), and SW480 (colon carcinoma) cells by means of the colorimetric MTT assay and compared to the tumor-inhibiting properties of the respective ligands. Even though the compounds were shown to be mostly non-cytotoxic to the various cell lines, the complexes and all the ligands are inactive in the three cell lines [181].
4.5. Analytical Applications
4.5.1. Determination of Quinolones Based on Complexation with Metal Ions
The capacity to form complexes with different metal ions has been applied in the analysis of quinolones in pharmaceutical formulations or in biological samples through spectrophotometric, spectroflurimetric and atomic absorption spectrometric methods. Most of the spectrophotometric methods developed for analysis of quinolones are based on the formation of yellow or orange-yellow chelates with Fe3+ in acid medium. The structure of such a complex is depicted in Figure 16. Generally, these methods are simple, rapid, efficient and inexpensive.
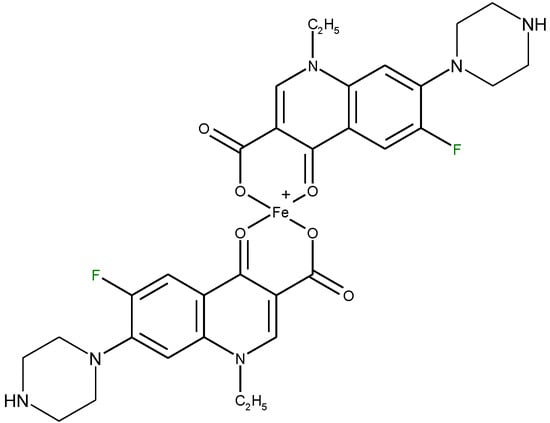
Figure 16.
Structure of a 1:2 (metal: ligand) chelate of norfloxacin with Fe3+.
Ciprofloxacin, ofloxacin and norfloxacin have been determined colorimetrically in tablets based on their amber coloured complex with Fe(III) that exhibited a maximum at 370 nm [182]. The complex with Fe3+ showing a maximum absorption of 435 nm allowed spectrophotometric determination of ciprofloxacin in tablets and in solution for infusion [183]. Based on the complexation with Fe3+, some flow injection (FI) spectrophotometric methods for determination of norfloxacin in drug formulations were developed. The coloured Fe(III) complexes absorb at 430 nm [184] or 440 nm [185]. Ofloxacin has been also determined by a flow-injection spectrophotometric method by measuring the absorbance of its complex with Fe3+ at 420 nm. The method was applied for analysis of ofloxacin in pharmaceuticals and human urine [186]. Ciprofloxacin formed with Fe(III) a brown-red complex whose absorbance was monitored at 447 nm, and the developed method was used for determination of ciprofloxacin in drug formulations [187]. A sequential injection spectrophotometric method was developed for analysis of ciprofloxacin and norfloxacin by measuring the absorbance of the corresponding complexes at 447 nm and 430 nm, respectively [188].
Chelates with Fe(II) and Cu(II) with maximum absorptions placed below 400 nm were also applied in the spectrophotometric analysis of quinolones. Based on the yellow-coloured chelate with Fe(II) with absorbance at 358 nm, norfloxacin has been determined both in pure form and in tablet form [189]. Norfloxacin, ciprofloxacin and sparfloxacin have been determined in formulations and spiked biological fluids (plasma and urine) via their Cu(II) complexes [190].
Coloured ion-association complexes were applied in developing the new visible spectrophotometric methods for determination of quinolones. Ciprofloxacin and norfloxacin have been determined in pharmaceutical tablets via formation of a ternary complex with eosin and palladium (II) which showed an absorption maximum at 545 nm [191]. Ofloxacin generates with Al(III) and erythrosin an ion-association complex between {AlIII(OFX)} cation and (ERY) anion. The ternary complex has an effective molar absorptivity at 555 nm, allowed spectrophotometrically determination of ofloxacin and other quinolone antibiotics (norfloxacin, enoxacin and levofloxacin) in pharmaceutical preparation [192]. Ion-association complexes formed with [Cr(NCS)4(NH3)2]- (Reineckate anion) displaying a maximum absorption at 524 nm were used for determination of ofloxacin [193] and norfloxacin [194].
A spectrophotometric method related to the interaction of quinolones with metal ions was developed based on the oxidation of quinolones with ammonium vanadate in sulphuric acid medium, followed by the development of a greenish blue colour measured at 766 nm, which has been attributed to vanadium(IV). The method was applied for determination of amifloxacin, ciprofloxacin, difloxacin, enoxacin, enrofloxacin, lomefloxacin, levofloxacin, norfloxacin, ofloxacin and pefloxacin in pharmaceutical dosage forms [195].
Modification of the fluorescent properties of quinolones in the presence of different metal ions has attracted the interest for studying the interaction of quinolones with antacids [196] and for development of spectrofluorimetric methods, applied in determination of quinolones in bulk, in biological fluids and in pharmaceutical formulations.
Determination of quinolones by spectrofluorimetric methods is based on: (i) the enhancement of quinolone fluorescence in the presence of metal ions (i.e., Al3+, Cu2+, Au3+ etc.); (ii) fluorescence sensitization of Tb3+ or Eu3+ in the presence of quinolone or (iii) quenching the fluorescence of a Tb3+ chelate after the addition of quinolone.
Interaction of a series of quinolones (sparfloxacin, oxolinic acid, flumequine and enrofloxacin) with Al3+ was used to analyse them in pharmaceutical dosage forms or in biological fluids [197]. Norfloxacin has been also determined as its fluorescent complex with Al3+ in serum [198] and in pharmaceutical preparations [199].
Formation of Y(III) fluorescent complexes underlying spectrofluorimetric methods for determination of norfloxacin in eye drops [200] and enrofloxacin in pharmaceutical formulations and its residue in milk [201].
The enhancement of luminescent properties of Tb(III) sorbates with ciprofloxacin and norfloxacin in zeolite was used for determination of these quinolones in biological fluids [202]. The enhancement effect of some quinolones on the fluorescence intensity of Tb(III)-sodium dodecylbenzenesulfonate system allowed the determination of enoxacin in pharmaceutical samples [203] and danofloxacin in milk [204]. Based on the sensitized fluorescence of Tb(III) enhanced by silver nanoparticles ciprofloxacin was dosed in pharmaceutical formulations [205], whereas pipemidic acid and lomefloxacin have been determined in pharmaceutical forms, urine and serum samples [206]. Europium (III)-sensitized fluorescence in the presence of quinolones was also applied for determination of quinolones ciprofloxacin, norfloxacin and gatifloxacin in pharmaceutical and serum samples [207] as for determination of ulifloxacin, the active metabolite of prulifloxacin in human serum and urine [208]. An optical sensor using Tb(III) and Eu(III) was constructed for analysis of norfloxacin and gatifloxacin in pharmaceutical and serum samples [209].
Quencing the fluorescence of an Eu(III)-β-diketone complex in micellar solution after the addition of pefloxacin underlying a time-resolved fluorimetric method for determination of pefloxacin in serum [210].
Apart from the main analytical applications in determination of quinolones in pharmaceutical forms and in biological samples, the fluorescent complexes were used also for other purposes. In this regard, fluorescence studies of Au(III)-norfloxacin system were carried out in order to study the association of Au3+ ions with cationic, zwitterionic and anionic forms of the drug [211]. Cu(II)-ofloxacin interaction, studied by means of ofloxacin fluorescence quenching experiments in the presence of Cu(II), was evaluated for its environmental impact [212].
Forming the metal complexes was the basis of some indirect methods for analysis of quinolones using atomic absorption spectrometry (AAS). Flow injection-fast atomic absorption spectroscopy (FI-AAS) was applied for determination of norfloxacin based on the complexation reaction with Fe(III), via measuring the absorbance of Fe3+ [213]. The formation of ion associated in the presence of cobalt sulphate was used for AAS determination of some fluoroquinolones in pharmaceutical dosage forms and biological fluids [195]. Ion-pair complexes formed with Reineckate anion allowed AAS determination of gatifloxacin, moxifloxacin and sparfloxacin in pharmaceutical formulations [214].
4.5.2. Determination of Metal Ions Based on Complexation with Quinolones
Spectrophotometric and spectroflurimetric methods were developed for determination of metal ions based on their complexation with quinolones. Formation of a coloured chelate with norfloxacin, which exhibits an absorption maximum at 377 nm, was used for development of a spectophotometric method for determination of trace amounts of Fe(III) [215].
Norfloxacin was used as reagent for determination of neodymium, holmium and erbium in mixed rare earth through a derivative spectrophotometric method, based on the enhancement of absorption at 575 nm for neodymium, 450 nm for holmium, and 523 for erbium, respectively [216].
The complex between europium(III) and gatifloxacin in a co-luminiscence system Eu3+-La3+-gatifloxacin-sodium dodecylbenzene sulfonate was used for the determination of trace amounts of Eu3+ in rare earth samples [217]. Quencing fluorescence of a terbium chelates in the presence of Hg2+ was used for development of a highly sensitive and specific detection method of trace Hg2+ in trace Hg2+ in biological samples (urine) and environmental water [218].
4.5.3. Quinolone Metal Complexes as Labels or Probes for Various Purposes
The luminescent properties of Tb(III) and Eu(III) chelates of some quinolones (nalidixic acid, oxolinic acid, pipemidic acid, pefloxacin, norfloxacin, ofloxacin, ciprofloxacin and lomefloxacin) were characterized and the obtained reagents were proposed as labels for immunofluorimetric assay [219]. Based on the enhancement of the fluorescence intensity of the enoxacin-Tb3+ complex, an environmentally friendly probe for determination of DNA (both single-stranded and double-stranded) was developed [220].
5. Conclusions
The metal ion - quinolone complexation represents a research field of increasing progress, having in view the consequences and applications of this process. Pharmaceutical profiles of quinolones can be improved by obtaining complexes with enhanced solubility. On the other side, pharmacokinetic interactions can occur at oral co-administration of quinolones and metal ions from mineral supplements and antacids. At the target site of their action, a quinolone-gyrase-DNA complex is formed in the presence of Mg2+ ions.
Many metal ion—quinolone complexes obtained in the solid state have shown various biological effects: antimicrobial activity (sometimes equal or better than that of the parent quinolone), anticancer activity, and, in some cases, antifungal and antiparasitic activity.
Complexation with metal ions was harnessed in the development of spectrophotometric, spectroflurimetric and atomic absorbtion spectrometric methods for the determination of quinolones in pharmaceutical preparations or in biological samples. Conversely, trace metal ions can be determined using quinolones as complexing agents. It must be noted that the progresses in the field of quinolone complexes and their applications parallel the development of the newer fluoroquinolones with enlarged biological activity.
Acknowledgments
This work was supported by a grant of the Romanian National Authority for Scientific Research, CNDI–UEFISCDI, project number 136/2012. I would like to express my special gratitude to the National Electronic Access to Scientific Research Literature (ANELiS) Project financed by the European Regional Development Fund who gave me the opportunity to do this work, which also helped me in doing a lot of research and I came to know about so many new things. I am really thankful to them.
Conflicts of Interest
The author declares no conflict of interest.
References
- Appelbaum, P.C.; Hunter, P.A. The fluoroquinolone antibacterials: Past, present and future perspectives. Int. J. Antimicrob. Agents 2000, 16, 5–15. [Google Scholar] [CrossRef]
- Hooper, D.C. Clinical application of quinolones. Biochim. Biophys. Acta-Gene Struct. Express. 1998, 1400, 45–61. [Google Scholar] [CrossRef]
- Buchbinder, M.; Webb, J.C.; Anderson, L.V.; McCabe, W.R. Laboratory studies and clinical pharmacology ofnalidixic acid (WIN 18, 320). Antimicrob. Agents Chemother. 1962, 2, 308–317. [Google Scholar]
- Brighty, K.E.; Gootz, T.D. Chemistry and Mechanism of Action of the Quinolone Antibacterials. In The Quinolones, 3rd ed.; Andriole, V.T., Ed.; Academic Press: San Diego, CA, USA, 2000; pp. 33–97. [Google Scholar]
- Senf, H.J. Fluorochinolone (Gyrasehemmer). Pharmazie 1988, 43, 444–447. [Google Scholar]
- Smith, J.T.; Lewin, C.S. Chemistry and Mechanisms of Action of the Quinolone Antibacterials. In The Quinolones; Andriole, V.T., Ed.; Academic Press: London, UK, 1988; pp. 23–81. [Google Scholar]
- Oliphant, C.M.; Green, G.M. Quinolones: A comprehensive review. Am. Fam. Phys. 2002, 65, 455–464. [Google Scholar]
- King, D.E.; Malone, R.; Lilley, S.H. New classification and update on the quinolone antibiotics. Am. Fam. Phys. 2000, 61, 2741–1748. [Google Scholar]
- Zhanel, G.G.; Walkty, A.; Vercaigne, L.; Karlowsky, J.A.; Embil, J.; Gin, A.S.; Hoban, D.J. The new fluoroquinolones: A critical review. Can. J. Infect. Dis. 1999, 10, 207–238. [Google Scholar]
- Cozzarelli, N.R. DNA gyrase and the supercoiling of DNA. Science 1980, 207, 953–960. [Google Scholar]
- Mitscher, L.A. Bacterial topoisomerase inhibitors: Quinolone and pyridone antibacterial agents. Chem. Rev. 2005, 105, 559–592. [Google Scholar]
- Blondeau, J.M. Fluoroquinolones: Mechanism of action, classification, and development of resistance. Surv. Ophthalmol. 2004, 49, S73–S78. [Google Scholar] [CrossRef]
- Drlica, K.; Zhao, X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol. Mol. Biol. Rev. 1997, 61, 377–392. [Google Scholar]
- Hooper, D.C. Mechanisms of action and resistance of older and newer fluoroquinolones. Clin. Infect. Dis. 2000, 31, S24–S28. [Google Scholar] [CrossRef]
- Maxwell, A. The molecular basis of quinolone action. J. Antimicrob. Chemother. 1992, 30, 409–414. [Google Scholar] [CrossRef]
- Schaumann, R.; Rodloff, A.C. Activities of quinolones against obligately anaerobic bacteria. Anti-Infective Agents Med. Chem. 2007, 6, 49–56. [Google Scholar] [CrossRef]
- Shen, L.L.; Chu, D.T.W. Type II DNA topoisomerases as antibacterial targets. Curr. Pharm. Des. 1996, 2, 195–208. [Google Scholar]
- Peterson, L.R. Quinolone molecular structure-activity relationships: What we have learned about improving antimicrobial activity. Clin. Infect. Dis. 2001, 33, S180–S186. [Google Scholar] [CrossRef]
- Ross, D.; Riley, C. Physicochemical properties of the fluoroquinolone antimicrobials. II. Acid ionization constants and their relationship to structure. Int. J. Pharmaceut. 1992, 83, 267–272. [Google Scholar] [CrossRef]
- Takacs-Novak, K.; Noszal, B.; Hermecz, I.; Kereszturi, G.; Podanyi, B.; Szasz, G. Protonation equilibria of quinolone antibacterials. J. Pharm. Sci. 1990, 79, 1023–1028. [Google Scholar] [CrossRef]
- Turel, I. The interactions of metal ions with quinolone antibacterial agents. Coord. Chem. Rev. 2002, 232, 27–47. [Google Scholar] [CrossRef]
- Zupančič, M.; Cerc Korošec, R.; Bukovec, P. The thermal-stability of ciprofloxacin complexes with magnesium (II), zinc (II) and cobalt (II). J. Therm. Anal. Calorim. 2001, 63, 787–795. [Google Scholar]
- Sasz, G.; Takacs-Novak, K.; Budvari-Barany, S.; Hermecz, J.; Jozan, M.; Lore, A.; Noszal, B. Correlation between the structures and physicochemical properties of chemoterapeutic fluoroquinolone agents. Acta Pharm. Hung. 1993, 63, 105–114. [Google Scholar]
- Turel, I.; Bukovec, N.; Farkas, E. Complex formation between some metals and a quinolone family member (ciprofloxacin). Polyhedron 1996, 15, 269–275. [Google Scholar] [CrossRef]
- Ma, H.; Chiu, F.; Li, R. Mechanistic investigation of the reduction in antimicrobial activity of ciprofloxacin by metal cations. Pharm. Res. 1997, 14, 366–370. [Google Scholar] [CrossRef]
- El-Roudi, A.M.; Soliman, E.M.; Refaiy, S.A. Effect of substituent and solvent composition on the stability of the metal complexes of 2-quinolone derivatives. Afinidad 1989, 420, 154–156. [Google Scholar]
- Ross, D.; Riley, C. Physicochemical properties of the fluoroquinolone antimicrobials. V. Effect of fluoroquinolones structure and pH on the complexation of various fluoroquinolones with magnesium and calcium ions. Int. J. Pharmaceut. 1993, 93, 121–129. [Google Scholar] [CrossRef]
- Efthimiadou, E.K.; Sanakis, Y.; Katsaros, N.; Karaliota, A.; Psomas, G. Transition metal complexes with the quinolone antibacterial agent pipemidic acid: Synthesis, characterization and biological activity. Polyhedron 2007, 26, 1148–1158. [Google Scholar] [CrossRef]
- Efthimiadou, E.K.; Katsaros, N.; Karaliota, A.; Psomas, G. Mononuclear copper(II) complexes with quinolones and nitrogen-donor heterocyclic ligands: Synthesis, characterization, biological activity and interaction with DNA. Inorg. Chim. Acta 2007, 360, 4093–4102. [Google Scholar] [CrossRef]
- Skrzypek, D.; Szymanska, B.; Kovala-Demertzi, D.; Wiecek, J.; Talik, E.; Demertzis, M.A. Synthesis and spectroscopic studies of iron (III) complex with a quinolone family member (pipemidic acid). J. Phys. Chem. Solids 2006, 67, 2550–2558. [Google Scholar] [CrossRef]
- Ruíz, M.; Ortiz, R.; Perelló, L.; Latorre, J.; Server-Carrio, J. Potentiometric and spectroscopic studies of transition-metal ions complexes with a quinolone derivative (cinoxacin). Crystal structures of new Cu (II) and Ni (II) cinoxacin complexes. J. Inorg. Biochem. 1997, 65, 87–96. [Google Scholar] [CrossRef]
- Ruíz, M.; Perelló, L.; Ortiz, R.; Castineiras, A.; Maichle-Mossmer, C.; Canton, E. Synthesis, characterization and crystal structure of [Cu(Cinoxacinate)2].2H2O complex: A square planar CuO4 cromophore. Antibacterial studies. J. Inorg. Biohem. 1995, 59, 801–810. [Google Scholar] [CrossRef]
- Ruíz, M.; Perelló, L.; Server-Carrio, J.; Ortiz, R.; Garcia-Granda, S.; Diaz, M.R.; Canton, E. Cinoxacin complexes with divalent metal ions. Spectroscopic characterization. Crystal structure of a new dinuclear Cd (II) complex having two chelate-bridging carboxylate groups. Antibacterial studies. J. Inorg. Biochem. 1998, 69, 231–239. [Google Scholar] [CrossRef]
- Lopez-Gresa, M.P.; Ortiz, R.; Perelló, L.; Latorre, J.; Liu-González, M.; García-Granda, S.; Pérez-Priede, M.; Canton, E. Interaction of metal ions with two quinolone antimicrobial agents (cinoxacin and ciprofloxacin). Spectroscopic and X-ray structural characterization. Antibacterial studies. J. Inorg. Biochem. 2002, 92, 65–74. [Google Scholar] [CrossRef]
- Psomas, G.; Tarushi, A.; Efthimiadou, E.K.; Sanakis, Y.; Raptopoulou, C.P.; Katsaros, N. Synthesis, structure and biological activity of copper(II) complexes with oxolinic acid. J. Inorg. Biochem. 2006, 100, 1764–1773. [Google Scholar] [CrossRef]
- Skyrianou, K.C.; Perdih, F.; Turel, I.; Kessissoglou, D.P.; Psomas, G. Nickel–quinolones interaction. Part 2 – Interaction of nickel(II) with the antibacterial drug oxolinic acid. J. Inorg. Biochem. 2010, 104, 161–170. [Google Scholar] [CrossRef]
- Tarushi, A.; Psomas, G.; Raptopoulou, C.P.; Kessissoglou, D.P. Zinc complexes of the antibacterialdrug oxolinic acid: Structure and DNA-binding properties. J. Inorg. Biochem. 2009, 103, 898–905. [Google Scholar] [CrossRef]
- Tarushi, A.; Christofis, P.; Psomas, G. Synthesis, characterization and interaction with DNA of mononuclear metal complexes with oxolinic acid. Polyhedron 2007, 26, 3963–3972. [Google Scholar] [CrossRef]
- Tarushi, A.; Efthimiadou, E.K.; Christofis, P.; Psomas, G. Neutral mononuclear dioxomolybdenum(VI) and dioxouranium(VI)complexes of oxolinic acid: Characterization and biological evaluation. Inorg. Chim. Acta 2007, 360, 3978–3986. [Google Scholar] [CrossRef]
- Perez-Guaita, D.; Boudesocque, S.; Sayen, S.; Guillon, E. Cu(II) and Zn(II) complexes with a fluoroquinolone antibiotic: Spectroscopic and X-ray absorption characterization. Polyhedron 2011, 30, 438–443. [Google Scholar] [CrossRef]
- Chalkidou, E.; Perdih, F.; Turel, I.; Kessissoglou, D.P.; Psomas, G. Copper(II) complexes with antimicrobial drug flumequine: Structure and biological evaluation. J. Inorg. Biochem. 2012, 113, 55–65. [Google Scholar] [CrossRef]
- Skyrianou, K.C.; Perdih, F.; Turel, I.; Kessissoglou, D.P.; Psomas, G. Nickel–quinolones interaction Part 3 — Nickel(II) complexes of the antibacterial drug flumequine. J. Inorg. Biochem. 2010, 104, 740–749. [Google Scholar] [CrossRef]
- Tarushi, A.; Kljun, J.; Turel, I.; Pantazaki, A.A.; Psomas, G.; Kessissoglou, D.P. Zinc(II) complexes with the quinolone antibacterial drug flumequine: Structure, DNA- and albumin-binding. New J. Chem. 2013, 37, 342–355. [Google Scholar] [CrossRef]
- Jiménez-Garrido, N.; Perelló, L.; Ortiz, R.; Alzuet, G.; González-Álvarez, M.; Cantón, E.; Liu-González, M.; García Granda, S.; Pérez-Priede, M. Antibacterial studies, DNA oxidative cleavage, and crystal structures of Cu(II) and Co(II) complexes with two quinolone family members, ciprofloxacin and enoxacin. J. Inorg. Biochem. 2005, 99, 677–689. [Google Scholar] [CrossRef]
- Arayne, S.; Sultana, N.; Haroon, U.; Mesaik, M.A. Synthesis, characterization, antibacterial and anti-inflammatory activities of enoxacin metal complexes. Bioinorg. Chem. Appl. 2009. [Google Scholar] [CrossRef]
- Sha, J.-Q.; Li, X.; Qiu, H.-B.; Zhang, Y.-H.; Yan, H. Nickel complexes of the different quinolone antibacterial drugs: Synthesis, structure and interaction with DNA. Inorg. Chim. Acta 2012, 383, 178–184. [Google Scholar] [CrossRef]
- Al-Mustafa, J. Magnesium, calcium and barium perchlorate complexes of ciprofloxacin and norfloxacin. Acta Chim. Slov. 2002, 49, 457–466. [Google Scholar]
- Breda, S.A.; Jimenez-Kairuz, A.F.; Manzo, R.H.; Olivera, M.E. Solubility behavior and biopharmaceutical classification of novel high-solubility ciprofloxacin and norfloxacin pharmaceutical derivatives. Int. J. Pharmaceut. 2009, 371, 106–113. [Google Scholar] [CrossRef]
- Shaikh, A.R.; Giridhar, R.; Yadav, M.R. Bismuth-norfloxacin complex: Synthesis, physicochemical and antimicrobial evaluation. Int. J. Pharmaceut. 2007, 332, 24–30. [Google Scholar] [CrossRef]
- Shaikh, A.R.; Giridhar, R.; Megraud, F.; Yadav, M.R. Metalloantibiotics: Synthesis, characterization and antimicrobial evaluation of bismuth-fluoroquinolone complexes against Helicobacter Pylori. Acta Pharm. 2009, 59, 259–271. [Google Scholar] [CrossRef]
- Sadeek, S.A. Synthesis, thermogravimetric analysis, infrared, electronic and mass spectra of Mn(II), Co(II) and Fe(III) norfloxacin complexes. J. Mol. Struct. 2005, 753, 1–12. [Google Scholar] [CrossRef]
- Golovnev, N.N.; Kirik, S.D.; Golovneva, I.I. Synthesis of norfloxacin compounds with cobalt(II), zinc(II), cadmium(II), and mercury(II). Russ. J. Inorg. Chem. 2009, 54, 223–225. [Google Scholar] [CrossRef]
- Batista, D.G.J.; da Silva, P.B.; Stivanin, L.; Lachter, D.R.; Silva, R.S.; Felcman, J.; Louro, S.R.W.; Teixeira, L.R.; de Nazare C. Soeiro, M. Co(II), Mn(II) and Cu(II) complexes of fluoroquinolones: Synthesis, spectroscopical studies and biological evaluation against Trypanosoma cruzi. Polyhedron 2011, 30, 1718–1725. [Google Scholar] [CrossRef]
- Ruíz, P.; Ortiz, R.; Perelló, L.; Alzuet, G.; González-Álvarez, M.; Liu-González, M.; Sanz-Ruíz, F. Synthesis, structure, and nuclease properties of several binary and ternary complexes of copper(II) with norfloxacin and 1,10 phenantroline. J. Inorg. Biochem. 2007, 101, 831–840. [Google Scholar] [CrossRef]
- Živec, P.; Perdih, F.; Turel, I.; Giester, G.; Psomas, G. Different types of copper complexes with the quinolone antimicrobial drugs ofloxacin and norfloxacin: Structure, DNA- and albumin-binding. J. Inorg. Biochem. 2012, 117, 35–47. [Google Scholar] [CrossRef]
- Zhang, J.J.; Ge, L.G.; Zhang, X.L.; Dai, Y.J.; Chen, H.L.; Mo, L.P. Thermal decomposition kinetics of the Zn(II) complex with norfloxacin in static air atmosphere. J. Therm Anal. Calorim. 1999, 58, 269–278. [Google Scholar] [CrossRef]
- Refat, M.S.; Mohamed, G.G.; de Farias, R.F.; Powell, A.K.; El-Garib, M.S.; El-Korashy, S.A.; Hussien, M.A. Spectroscopic, thermal and kinetic studies of coordination compounds of Zn(II), Cd(II) and Hg(II) with norfloxacin. J. Therm. Anal. Calorim. 2010, 102, 225–232. [Google Scholar] [CrossRef]
- Sadeek, S.A.; El-Did Amony, A.M.; El-Shwiniy, W.H.; Zordok, W.A. Uranium (VI) and zirconium (IV) of the second -generation quinolone antimicrobial drug norfloxacin: Structure and biological activity. J. Argent. Chem. Soc. 2009, 97, 51–76. [Google Scholar]
- Chen, X.-B.; Ye, Q.; Wu, Q.; Song, Y.-M.; Xiong, R.-G.; You, X.-Z. The first organometallic carbonyl tungsten complex of antibacterial drug norfloxacin. Inorg. Chem. Commun. 2004, 7, 1302–1305. [Google Scholar] [CrossRef]
- Uivarosi, V.; Badea, M.; Olar, R.; Marinescu, D.; Nicolescu, T.O.; Nitulescu, G.M. Thermal degradation behavior of some ruthenium complexes with fluoroquinolone derivatives as potential antitumor agents. J. Therm. Anal. Calorim. 2011, 105, 645–650. [Google Scholar] [CrossRef]
- Patel, M.N.; Gandhi, D.S.; Parmar, P.A. DNA interaction and in-vitro antibacterial studies of fluoroquinolone based platinum(II) complexes. Inorg. Chem. Commun. 2012, 15, 248–251. [Google Scholar] [CrossRef]
- Gouvea, L.R.; Garcia, L.S.; Lachter, D.R.; Nunes, P.R.; de Castro Pereira, F.; Silveira-Lacerda, E.P.; Louro, S.R.W.; Barbeira, P.J.S.; Teixeira, L.R. Atypical fluoroquinolone gold(III) chelates as potential anticancer agents: Relevance of DNA and protein interactions for their mechanism of action. Eur. J. Med. Chem. 2012, 55, 67–73. [Google Scholar] [CrossRef]
- Sadeek, S.A.; El-Shwiniy, W.H.; Zordok, W.A.; El-Didamony, A.M. Synthesis, spectroscopic, thermal and biological activity investigation of new Y(ΙΙΙ) and Pd(ΙΙ) norfloxacin complexes. J. Argent. Chem. Soc. 2009, 97, 128–148. [Google Scholar]
- Refat, M.S.; El-Hawary, W.F.; Mohamed, M.A. Study of the chemical chelates and anti-microbial effect of some metal ions in nanostructural form on the efficiency of antibiotic therapy “norfloxacin drug”. J. Mol. Struct. 2012, 1013, 45–54. [Google Scholar]
- Li, S.; Wang, Y.; Lin, Q.; Liu, W.; Ding, J.; Wang, Y. Synthesis, crystal structures of novel complexes of rare earth with norfloxacin, interaction with DNA and BSA. J. Rare Earths 2012, 30, 460–466. [Google Scholar] [CrossRef]
- Qi, W.; Huang, J.; An, Z. Aquabis[1-ethyl-6-fluoro-7-(4-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylato]zinc(II) dihydrate. Acta Crystallogr. 2008, 64, m302. [Google Scholar]
- Drevenšek, P.; Poklar Ulrih, N.; Majerle, A.; Turel, I. Synthesis, characterization and DNA binding of magnesium–ciprofloxacin (cfH) complex [Mg(cf)2]·2.5H2O. J. Inorg. Biochem. 2006, 100, 1705–1713. [Google Scholar] [CrossRef]
- Turel, I.; Šonc, A.; Zupančič, M.; Sepčić, K.; Turk, T. Biological activity of some magnesium(II) complexes of quinolones. Met. Based Drugs 2000, 7, 101–104. [Google Scholar] [CrossRef]
- Turel, I.; Živec, P.; Pevec, A.; Tempelaar, S.; Psomas, G. Compounds of antibacterial agent ciprofloxacin and magnesium – crystal structures and molecular modeling calculations. Eur. J. Inorg. Chem. 2008, 23, 3718–3727. [Google Scholar]
- Al-Mustafa, J.; Taha, Z.A. Thermodynamics of the complexation of ciprofloxacin with calcium and magnesium perchlorate. Thermochim. Acta 2011, 521, 9–13. [Google Scholar] [CrossRef]
- Turel, I.; Golobič, A.; Klavžar, A.; Pihlar, B.; Buglyó, P.; Tolis, E.; Rehder, D.; Sepčić, K. Interactions of oxovanadium(IV) and the quinolone family member—ciprofloxacin. J. Inorg. Biochem. 2003, 95, 199–207. [Google Scholar] [CrossRef]
- Anacona, J.R.; Toledo, C. Synthesis and antibacterial activity of metal complexes of ciprofloxacin. Trans. Met. Chem. 2001, 26, 228–231. [Google Scholar] [CrossRef]
- Psomas, G. Mononuclear metal complexes with ciprofloxacin: Synthesis, characterization and DNA-binding properties. J. Inorg. Biochem. 2008, 102, 1798–1811. [Google Scholar] [CrossRef]
- Hernandez-Gil, J.; Perello, L.; Ortiz, R.; Alzuet, G.; Gonzalez-Alvarez, M.; Liu-Gonzalez, M. Synthesis, structure and biological properties of several binary and ternary complexes of copper(II) with ciprofloxacin and 1,10 phenanthroline. Polyhedron 2009, 28, 138–144. [Google Scholar] [CrossRef]
- Wallis, S.C.; Gahan, L.R.; Charles, B.G.; Hambley, T.W.; Duckworth, P.A. Copper (II) complexes of the fluoroquinolone antimicrobial ciprofloxacin: Synthesis, X-ray structural characterization and potentiometric study. J. Inorg. Biochem. 1996, 62, 1–16. [Google Scholar] [CrossRef]
- Turel, I.; Leban, I.; Bukovec, N. Synthesis, characterization, and crystal structure of a copper(II) complex with quinolone family member (ciprofloxacin): Bis(1)-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-piperazin-1ylquinoline-3-carboxylate) copper(II) chloride hexahydrate. J. Inorg. Biochem. 1994, 56, 273–282. [Google Scholar] [CrossRef]
- Drevenšek, P.; Zupančič, T.; Pihlar, B.; Jerala, R.; Kolitsch, U.; Plaper, A.; Turel, I. Mixed-valence Cu(II)/Cu(I) complex of quinolone ciprofloxacin isolated by a hydrothermal reaction in the presence of L-histidine: Comparison of biological activities of various copper–ciprofloxacin compounds. J. Inorg. Biochem. 2005, 99, 432–442. [Google Scholar] [CrossRef]
- Tanimoto, M.; Dias, K.; Dovidauskas, S.; Nikolaou, S. Tuning the reaction products of ruthenium and ciprofloxacin for studies of DNA interactions. J. Coord Chem. 2012, 65, 1504–1517. [Google Scholar] [CrossRef]
- Vieira, L.M.M.; de Almeida, M.V.; Lourenço, M.C.S.; Bezerra, F.A.F.M.; Fontes, A.P.S. Synthesis and antitubercular activity of palladium and platinum complexes with fluoroquinolones. Eur. J. Med. Chem. 2009, 44, 4107–4111. [Google Scholar] [CrossRef]
- Čurman, D.; Živec, P.; Leban, I.; Turel, I.; Polishchuk, A.; Klika, K.D.; Karaseva, E.; Karasev, V. Spectral properties of Eu(III) compound with antibacterial agent ciprofloxacin (cfqH). Crystal structure of [Eu(cfqH)(cfq)(H2O)4]Cl2· 4.55H2O. Polyhedron 2008, 27, 1489–1496. [Google Scholar] [CrossRef]
- Sadeek, S.A.; El-Shwiniy, W.H. Preparation, structure and microbial evaluation of metal complexes of the second generation quinolone antibacterial drug lomefloxacin. J. Mol. Struct. 2010, 98, 130–138. [Google Scholar] [CrossRef]
- Abd El-Halim, H.F.; Mohamed, G.G.; El-Dessouky, M.M.I.; Mahmoud, W.H. Ligational behaviour of lomefloxacin drug towards Cr(III), Mn(II), Fe(III), Co(II), Ni(II), Cu(II), Zn(II), Th(IV) and UO2(VI) ions: Synthesis, structural characterization and biological activity studies. Spectrochim. Acta A 2011, 82, 8–19. [Google Scholar] [CrossRef]
- Drevenšek, P.; Košmrlj, J.; Giester, G.; Skauge, T.; Sletten, E.; Sepčić, K.; Turel, I. X-ray crystallographic, NMR and antimicrobial activity studies of magnesium complexes of fluoroquinolones – racemic ofloxacin and its S-form, levofloxacin. J. Inorg. Biochem. 2006, 100, 1755–1763. [Google Scholar] [CrossRef]
- Sagdinc, S.; Bayari, S. Spectroscopic studies on the interaction of ofloxacin with metals. J. Mol. Struct. 2004, 691, 107–113. [Google Scholar] [CrossRef]
- Macias, B.; Villa, M.V.; Sastre, M.; Castiñeiras, A.; Borras, J. Complexes of Co(II) and Zn(II) with ofloxacin. Crystal structure of [Co(oflo)2(MeOH)2]·4MeOH. J. Pharm. Sci. 2002, 91, 2416–2423. [Google Scholar] [CrossRef]
- Macias, B.; Villa, M.; Rubio, I.; Castineiras, A.; Borras, J. Complexes of Ni (II) and Cu (II) with ofloxacin. Crystal structure of a new Cu (II) ofloxacin complex. J. Inorg. Biochem. 2001, 84, 163–170. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, Y.-C.; Xu, Z.-H.; Zeng, Z.-Z. Crystal structure, biological studies of water-soluble rare earth metal complexes with an ofloxacin derivative. Inorg. Chim. Acta 2012, 384, 324–332. [Google Scholar] [CrossRef]
- Efthimiadou, E.K.; Katsaros, N.; Karaliota, A.; Psomas, G. Synthesis, characterization, antibacterial activity, and interaction with DNA of the vanadyl-enrofloxacin complex. Bioorg. Med. Chem. Lett. 2007, 17, 1238–1242. [Google Scholar]
- Efthimiadou, E.K.; Karaliota, A.; Psomas, G. Mononuclear dioxomolybdenum(VI) complexes with the quinolones enrofloxacin and sparfloxacin: Synthesis, structure, antibacterial activity and interaction with DNA. Polyhedron 2008, 27, 349–356. [Google Scholar] [CrossRef]
- Efthimiadou, E.K.; Karaliota, A.; Psomas, G. Mononuclear metal complexes of the second-generation quinolone antibacterial agent enrofloxacin: Synthesis, structure, antibacterial activity and interaction with DNA. Polyhedron 2008, 27, 1729–1738. [Google Scholar] [CrossRef]
- Skyrianou, K.C.; Psycharis, V.; Raptopoulou, C.P.; Kessissoglou, D.P.; Psomas, G. Nickel–quinolones interaction. Part 4 — Structure and biological evaluation of nickel(II)–enrofloxacin complexes compared to zinc(II) analogues. J. Inorg. Biochem. 2011, 105, 63–74. [Google Scholar] [CrossRef]
- Saraiva, R.; Lopes, S.; Ferreira, M.; Novais, F.; Pereira, E.; Feio, M.J.; Gameiro, P. Solution and biological behaviour of enrofloxacin metalloantibiotics: A route to counteract bacterial resistance? J. Inorg. Biochem. 2010, 104, 843–850. [Google Scholar] [CrossRef]
- Efthimiadou, E.K.; Sanakis, Y.; Katsarou, M.; Raptopoulou, C.P.; Karaliota, A.; Katsaros, N.; Psomas, G. Neutral and cationic mononuclear copper(II) complexes with enrofloxacin: Structure and biological activity. J. Inorg. Biochem. 2006, 100, 1378–1388. [Google Scholar] [CrossRef]
- Ftouni, H.; Sayen, S.; Boudesocque, S.; Dechamps-Olivier, I.; Guillon, E. Structural study of the copper(II)-enrofloxacin metallo–antibiotic. Inorg. Chim. Acta 2012, 382, 186–190. [Google Scholar] [CrossRef]
- Efthimiadou, E.K.; Karaliota, A.; Psomas, G. Metal complexes of the third-generation quinolone antimicrobial drug sparfloxacin: Structure and biological evaluation. J. Inorg. Biochem. 2010, 104, 455–466. [Google Scholar] [CrossRef]
- Efthimiadou, E.K.; Karaliota, A.; Psomas, G. Structure, antimicrobial activity and DNA-binding properties of the cobalt(II)–sparfloxacin complex. Bioorg. Med. Chem. Lett. 2008, 18, 4033–4037. [Google Scholar] [CrossRef]
- Efthimiadou, E.K.; Sanakis, Y.; Raptopoulou, C.P.; Karaliota, A.; Katsaros, N.; Psomas, G. Crystal structure, spectroscopic, and biological study of the copper(II) complex with third-generation quinolone antibiotic sparfloxacin. Bioorg. Med. Chem. Lett. 2006, 16, 3864–3867. [Google Scholar]
- Sultana, N.; Arayne, M.S.; Rizvi, S.B.S.; Haroon, U.; Mesaik, M.A. Synthesis, spectroscopic, and biological evaluation of some levofloxacin metal complexes. Med. Chem. Res. 2013, 22, 1371–1377. [Google Scholar]
- Tarushi, A.; Polatoglou, E.; Kljun, J.; Turel, I.; Psomas, G.; Kessissoglou, D.P. Interaction of Zn(II) with quinolone drugs: Structure and biological evaluation. Dalton Trans. 2011, 40, 9461–9473. [Google Scholar] [CrossRef]
- Sultana, N.; Naz, A.; Arayne, M.S.; Ahmed Mesaik, M. Synthesis, characterization, antibacterial, antifungal and immunomodulating activities of gatifloxacin–metal complexes. J. Mol. Struct. 2010, 969, 17–24. [Google Scholar]
- Li, Z.-Q.; Wu, F.-J.; Gong, Y.; Hu, C.-W.; Zhang, Y.-H.; Gan, M.-Y. Synthesis, characterization and activity against Staphylococcus of metal(II)-gatifloxacin complexes. Chin. J. Chem. 2007, 25, 1809–1814. [Google Scholar] [CrossRef]
- Mehrotra, R.; Shukla, S.N.; Gaur, P.; Dubey, A. Identification of pharmacophore in bioactive metal complexes: Synthesis, spectroscopic characterization and application. Eur. J. Med. Chem. 2012, 50, 149–153. [Google Scholar] [CrossRef]
- Patitungkho, S.; Adsule, S.; Dandawate, P.; Padhye, S.; Ahmad, A.; Sarkar, F.H. Synthesis, characterization and anti-tumor activity of moxifloxacin–copper complexes against breast cancer cell lines. Bioorg. Med. Chem. Lett. 2011, 21, 1802–1806. [Google Scholar] [CrossRef]
- Sadeek, S.A.; El-Shwiniy, W.H.; El-Attar, M.S. Synthesis, characterization and antimicrobial investigation of some moxifloxacin metal complexes. Spectrochim. Acta Part A 2011, 84, 99–110. [Google Scholar] [CrossRef]
- Sadeek, S.A.; El-Shwiniy, W.H.; Zordok, W.A.; Kotb, E. Spectroscopic studies, thermal analyses and biological evaluation of new V(IV), Zr(IV) and U(VI) moxifloxacin complexes. J. Mol. Struct. 2011, 1006, 192–209. [Google Scholar] [CrossRef]
- Serafin, A.; Stanczak, A. The complexes of metal ions with fluoroquinolones. Russ. J. Coord. Chem. 2009, 35, 81–95. [Google Scholar] [CrossRef]
- Psomas, G.; Kessissoglou, D.P. Quinolones and non-steroidal antiinflammatory drugs interacting with copper(II), nickel(II), cobalt(II) and zinc(II): Structural features, biological evaluation and perspectives. Dalton Trans. 2013, 42, 6252–6276. [Google Scholar] [CrossRef]
- Gao, F.; Yang, P.; Xie, J.; Wang, H. Synthesis, characterization and antibacterial activity of novel Fe(III), Co(II), and Zn(II) complexes with norfloxacin. J. Inorg. Biochem. 1995, 60, 61–67. [Google Scholar] [CrossRef]
- Vieira, L.M.M.; de Almeida, M.V.; de Abreu, H.A.; Duarte, H.A.; Grazul, R.M.; Fontes, A.P.S. Platinum(II) complexes with fluoroquinolones: Synthesis and characterization of unusual metal–piperazine chelates. Inorg. Chim. Acta 2009, 362, 2060–2064. [Google Scholar] [CrossRef]
- Rusu, A.; Tóth, G.; Szőcs, L.; Kökösi, J.; Kraszni, M.; Gyéresi, A.; Noszál, B. Triprotic site-specific acid-base equilibria and related properties of fluoroquinolone antibacterials. J. Pharm. Biomed. Anal. 2012, 66, 50–57. [Google Scholar] [CrossRef]
- Sha, J.-Q.; Liang, L.-Y.; Yan, P.-F.; Li, G.-M.; Wang, C.; Ma, D.-Y. Study on ligation of copper complexes of the quinolone antibacterial drugs and octamolybdates POMs. Polyhedron 2012, 31, 422–430. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Chen, Z.-F.; Shi, S.-M.; Luo, H.-S.; Zhong, D.-C.; Zou, H.-L.; Liang, H. Synthesis, crystal structure of polyoxovanadate complex of ciprofloxacin: V4O10(μ2-O)2[VO(H-Ciprof)2]2 · 13H2O by hydrothermal reaction. Inorg. Chem. Commun. 2007, 10, 1269–1272. [Google Scholar] [CrossRef]
- Li, C.; Lu, J.; Tu, F.; Chen, J.; Li, Y. Study of the first antibacterial agent pipemidic acid modifying Keggin polyoxometalate. Inorg. Chem. Commun. 2011, 14, 1192–1195. [Google Scholar] [CrossRef]
- Sha, J.-Q.; Liang, L.-Y.; Li, X.; Zhang, Yu.; Yan, H.; Chen, G. Ligation of the quinolone antibacterial agent pipemidic acid to Keggin polyoxotungstates. Polyhedron 2011, 30, 1657–1662. [Google Scholar] [CrossRef]
- Sha, J.-Q.; Li, X.; Zhou, Y.-H.; Yan, P.-F.; Li, G.-M.; Wang, C. The introduction of antibacterial drug pipemidic acid into the POM field: Syntheses, characterization and antitumor activity. Solid State Sci. 2011, 13, 1972–1977. [Google Scholar] [CrossRef]
- Li, Y.-X.; Chen, Z.-F.; Xiong, R.-G.; Xue, Z.; Ju, H.-X.; You, X.-Z. A mononuclear complex of norfloxacin with silver(I) and its properties. Inorg. Chem. Commun. 2003, 6, 819–822. [Google Scholar] [CrossRef]
- Refat, M.S. Synthesis and characterization of norfloxacin-transition metal complexes (group 11, IB): Spectroscopic, thermal, kinetic and biological activity. Spectrochim. Acta Part A 2007, 5, 1393–1405. [Google Scholar]
- Badea, M.; Olar, R.; Marinescu, D.; Uivarosi, V.; Iacob, D. thermal decomposition of some biologically active complexes of ruthenium (III) with quinolone derivatives. J. Therm. Anal. Calorim. 2009, 97, 735–739. [Google Scholar] [CrossRef]
- Badea, M.; Olar, R.; Marinescu, D.; Uivarosi, V.; Nicolescu, T.O.; Iacob, D. Thermal study of some new quinolone ruthenium(III) complexes with potential cytostatic activity. J. Therm. Anal. Calorim. 2010, 99, 829–834. [Google Scholar] [CrossRef]
- Chen, Z.F.; Xiong, R.G.; Zuo, J.; Guo, Z.; You, X.; Fun, H.K. X-ray crystal structures of Mg2+ and Ca2+ dimers of the antibacterial drug norfloxacin. J. Chem. Soc. Dalton Trans. 2000, 22, 4013–4014. [Google Scholar]
- Chen, Z.F.; Zhou, H.L.; Liang, H.; Li, Y.; Xiong, R.G.; You, X.Z. Crystallographic report: Bis(norfloxacin)dilead(II) tetranitrate, [Pb2(H-Norf)2(ONO2)4]. Appl. Organomet. Chem. 2003, 17, 883–884. [Google Scholar] [CrossRef]
- Qu, Z.-R.; Zhao, H.; Xing, L.-X.; Wang, X.-S.; Chen, Z.-F.; Yu, Z.; Xiong, R.-G.; You, X.-Z. Two polymeric complexes of norfloxacin with iron(II) and their magnetic properties. Eur. J. Inorg. Chem. 2003, 16, 2920–2923. [Google Scholar]
- Chen, Z.-F.; Yu, L.-C.; Zhong, D.-C.; Liang, H.; Zhu, X.-H.; Zhu, Z.-Y. An unprecedented 1D ladder-like silver (I) coordination polymer with ciprofloxacin. Inorg. Chem. Commun. 2006, 9, 839–843. [Google Scholar] [CrossRef]
- Gerasimenko, A.V.; Polishchuk, A.V.; Volkova, L.M.; Karaseva, E.T.; Karasev, V.E. Synthesis and structure of nalidixium tetrachloroantimonate monohydrate, (C12H13N2O3)SbCl4· H2O. Russ. J. Coord. Chem. 2008, 34, 8–13. [Google Scholar] [CrossRef]
- Gerasimenko, A.V.; Polishchuk, A.V.; Karaseva, E.T.; Karasev, V.E. Crystal Structure and Spectroscopic Properties of Ciprofloxacinium Pentachloroantimonate(III) Monohydrate (C17H19N3O3F)SbCl5· H2O. Russ. J. Coord. Chem. 2008, 34, 647–652. [Google Scholar] [CrossRef]
- Turel, I.; Leban, I.; Bukovec, N. Crystal structure and characterization of the bismuth (III) compound with quinolone family member (ciprofloxacin). Antibacterial study. J. Inorg. Biochem. 1997, 66, 241–245. [Google Scholar] [CrossRef]
- Turel, I.; Golić, L.; Bukovec, P.; Gubina, M. antibacterial tests of bismuth(III)-quinolone (ciprofoxacin, cf ) compounds against Helicobacter pylori and some other bacteria. Crystal structure of (cfH2)2[Bi2Cl10]·4H2O. J. Inorg. Biochem. 1998, 71, 53–60. [Google Scholar] [CrossRef]
- Turel, I.; Guber, K.; Leban, I.; Bukovec, N. Synthesis, crystal structure, and characterization of tree novel compounds of quinolone family member (norfloxacin). J. Inorg. Biochem. 1996, 61, 197–212. [Google Scholar] [CrossRef]
- Vasiliev, D.; Golovnev, N.N. Synthesis and Structure of C17H22FN3O32+CuCl42-. J. Struct. Chem. 2010, 51, 177–180. [Google Scholar] [CrossRef]
- Turel, I.; Leban, I.; Klinchar, G.; Bukovec, N.; Zalar, S. Synthesis, crystal structure and characterization of two metal-quinolone compound. J. Inorg. Biochem. 1997, 66, 77–82. [Google Scholar] [CrossRef]
- Zupančič, M.; Turel, I.; Bukovec, P.; White, A.J.P.; Williams, D.J. Synthesis and characterization of 2 novel zinc (II) complexes with ciprofloxacin. Crystal-structure of [C17H19N3O3F]2·[ZnCl4]·2H2O. Croat. Chem. Acta 2001, 74, 61–74. [Google Scholar]
- Polishchuk, A.V.; Karaseva, E.T.; Cherednichenko, A.I.; Gerasimenko, A.V.; Karasev, V.E. Crystal structure and X-ray photoelectron spectroscopy of ciprofloxacinium tetrachloroaurate monohydrate. Russ. J. Coord. Chem. 2011, 37, 215–222. [Google Scholar] [CrossRef]
- Olivera, M.E.; Mazzieri, M.R.; Manzo, R.H. New pharmaceutical fluoroquinolone derivatives hydrochloride of aluminum complexes of ciprofloxacin and norfloxacin. STP Pharma Sci. 2000, 10, 251–256. [Google Scholar]
- Olivera, M.E.; Allemandi, D.A.; Manzo, R.H. Intrinsic dissolution rate and intestinal permeability of metallic complexes of norfloxacin and ciprofloxacin in relation to their formulation. Acta Farm. Bonaerense 2000, 19, 185–191. [Google Scholar]
- Alovero, F.L.; Olivera, M.E.; Manzo, R.H. In vitro pharmacodynamic properties of a fluoroquinolone pharmaceutical derivative: Hydrochloride of ciprofloxacin–aluminium complex. Int. J. Antimicrob. Agents 2003, 21, 446–451. [Google Scholar] [CrossRef]
- Höffken, G.; Borner, K.; Glatzel, P.D.; Koeppe, P.; Lode, H. Reduced enteral absorption of ciprofloxacin in the presence of antacids (letter). Eur. J. Clin. Microbiol. 1985, 4, 345. [Google Scholar]
- Kara, M.; Hassinoff, B.B.; Mckay, D.N.; Campbell, N.R.C. Clinical and chemical interactions between iron preparations and ciprofloxacin. Brit. J. Clin. Pharmacol. 1991, 31, 257–261. [Google Scholar] [CrossRef]
- Wallis, S.C.; Charles, B.G.; Gahan, L.R.; Filippich, L.J.; Bredhauer, M.G.; Duckworth, P.A. Interaction of norfloxacin with divalent and trivalent pharmaceutical cations, In vitro complexation and in vivo pharmacokinetic studies in the dog. J. Pharm. Sci. 1996, 85, 803–809. [Google Scholar] [CrossRef]
- Davies, M.; Maesen, F.P.V. Drug interactions with quinolones. Rev. Infect. Dis. 1989, 2, S1083–S1090. [Google Scholar] [CrossRef]
- Nix, D.E.; Watson, W.A.; Handy, L.; Frost, R.W.; Rescott, D.L.; Goldstein, H.R. The effect of sucralfate pretreatment on the pharmacokinetics of ciprofloxacin. Pharmacotherapy 1989, 9, 377–380. [Google Scholar]
- Polk, R.E.; Healey, D.P.; Sahai, J.; Drwal, L.; Racht, E. Effect of ferrous sulphate and multivitamins with zinc on absorption of ciprofloxacin in normal volunteers. Antimicrob. Agents Chemother. 1989, 33, 1841–1844. [Google Scholar] [CrossRef]
- Ross, D.; Riley, C. Physicochemical properties of the fluoroquinolone antimicrobials. III. Complexation of lomefloxacin with various metal ions and the effect of metal ion complexation on aqueous solubility. Int. J. Pharmaceut. 1992, 87, 203–213. [Google Scholar] [CrossRef]
- Ross, D.; Elkinton, S.; Knaub, S.; Riley, C. Physicochemical properties of the fluoroquinolone antimicrobials. VI. Effect of metal-ion complexation on octanol-1-ol-water partitioning. Int. J. Pharmaceut. 1993, 93, 131–138. [Google Scholar] [CrossRef]
- akelj, S.; Berginc, K.; Uršič, D.; Veber, M.; Kristl, A. Metal cation-fluoroquinolone complexes do not permeate through the intestinal absorption barrier. J. Pharm. Biomed. Anal. 2010, 53, 655–659. [Google Scholar] [CrossRef]
- Pallù, G.; Valisena, S.; Ciarrocchi, G.; Gatto, B.; Palumbo, M. Quinolone binding to DNA is mediated by magnesium ions. Proc. Natl. Acad. Sci. USA 1992, 89, 9671–9675. [Google Scholar] [CrossRef]
- Skauge, T.; Turel, I.; Sletten, E. Interaction between ciprofloxacin and DNA mediated by Mg2+-ions. Inorg. Chim. Acta 2002, 339, 239–247. [Google Scholar] [CrossRef]
- Song, G.; Yan, Q.; He, Y. Studies on interaction of norfloxacin, Cu2+ and DNA by spectral methods. J. Fluoresc. 2005, 15, 673–678. [Google Scholar] [CrossRef]
- Drevenšek, P.; Turel, I.; Poklar Ulrih, N. Influence o copper (II) and magnesium (II) ions on the ciprofloxacin binding to DNA. J. Inorg. Biochem. 2003, 96, 407–415. [Google Scholar] [CrossRef]
- Guo, D.-S.; Jing, B.Y.; Yuan, X.-Y. Influence of Mg2+ and Cu2+ on the interaction between quinolone and calf thymus DNA. J. Fluoresc. 2011, 21, 113–118. [Google Scholar] [CrossRef]
- Song, G.; He, Y.; Cai, Z. The interaction between levofloxacine hydrochloride and DNA mediated by Cu2+. J. Fluoresc. 2004, 14, 705–710. [Google Scholar] [CrossRef]
- Yuan, X.-Y.; Qin, J.; Lu, L.-L. Influence of metal ions on the interaction between gatifloxacin and calf thymus DNA. Spectrochim. Acta A 2010, 75, 520–524. [Google Scholar] [CrossRef]
- Yuan, X.-Y.; Guo, D.-S.; Wang, L.L. Influence of Mg2+ and Cd2+ on the interaction between sparfloxacin and calf thymus DNA. Spectrochim. Acta A 2008, 69, 1130–1135. [Google Scholar] [CrossRef]
- Guo, D.-S.; Yuan, X.-Y.; Wu, J.-B. Influence of Cr(III) and Cr(VI) on the interaction between sparfloxacin and calf thymus DNA. J. Inorg. Biochem. 2007, 101, 644–648. [Google Scholar] [CrossRef]
- Zhang, G.; Fu, X.; Liu, Q.; Wang, G. Interaction between pazufloxacin and DNA mediated by copper(II) ions. J. Fluoresc. 2008, 18, 701–706. [Google Scholar] [CrossRef]
- Sissi, C.; Andreolli, M.; Cecchetti, V.; Fravolini, A.; Gatto, B.; Palumbo, M. Mg2+-mediated binding of 6-Substituted quinolones to DNA: Relevance to biological activity. Bioorg. Med. Chem. 1998, 6, 1555–1561. [Google Scholar] [CrossRef]
- Robles, J.; Martin-Polo, J.; Avarez-Valtierra, L.; Hinojosa, L.; Mendoza-Diaz, G. A theoretical-experimental study on the structure and activity of certain quinolones and the interaction of their Cu(II)-complexes on a DNA model. Met. Based Drugs 2000, 7, 301–311. [Google Scholar] [CrossRef]
- Sissi, C.; Marangon, E.; Chemello, A.; Noble, C.G.; Maxwell, A.; Palumbo, M. The effects of metal ions on the structure and stability of the DNA gyrase B protein. J. Mol. Biol. 2005, 353, 1152–1160. [Google Scholar] [CrossRef]
- Sissi, C.; Palumbo, M. Effects of magnesium and related divalent metal ions in topoisomerase structure and function. Nucleic Acids Res. 2009, 37, 702–711. [Google Scholar] [CrossRef]
- Wohlkonig, A.; Chan, P.F.; Fosberry, A.P.; Homes, P.; Huang, J.; Kranz, M.; Leydon, V.R.; Miles, T.J.; Pearson, N.D.; Perera, R.L.; et al. Structural basis of quinolone inhibition of type IIA topoisomerases and target-mediated resistance. Nat. Struct. Mol. Biol. 2010, 17, 1152–1153. [Google Scholar] [CrossRef]
- Aldred, K.J.; McPherson, S.A.; Turnbough, C.L., Jr.; Kerns, R.J.; Osheroff, N. Topoisomerase IV-quinolone interactions are mediated through a water-metal ion bridge: Mechanistic basis of quinolone resistance. Nucleic Acids Res. 2013, 41, 4628–4639. [Google Scholar] [CrossRef]
- Lecomte, S.; Baron, M.H.; Chenon, M.T.; Compry, C.; Moreau, N.J. Effect of magnesium complexation by fluoroquinolones on their antibacterial properties. Antimicrob. Agents Chemother. 1994, 38, 2810–2816. [Google Scholar] [CrossRef]
- Alkaysi, H.N.; Abdel-Hay, M.H.; Sheikh Salem, M.; Gharaibeh, A.M.; Na’was, T.E. Chemical and biological investigations of metal ion interaction with norfloxacin. Int. J. Pharmaceut. 1992, 87, 73–77. [Google Scholar] [CrossRef]
- Tumer, M.; Koksal, H.; Sener, M.K.; Serin, S. Antimicrobial activity studies of the binuclear metal complexes derived from tridentate schiff base ligands. Transit. Met. Chem. 1999, 24, 414–420. [Google Scholar] [CrossRef]
- Imran, M.; Iqbal, J.; Iqbal, S.; Ijaz, N. In vitro antibacterial studies of ciprofloxacin-imines and their complexes with Cu(II), Ni(II), Co(II), and Zn(II). Turk. J. Biol. 2007, 31, 67–72. [Google Scholar]
- Patel, N.H.; Parekh, H.M.; Patel, M.N. Synthesis, physicochemical characteristics, and biocidal activity of some transition metal mixed-ligand complexes with bidentate (NO and NN) Schiff bases. Pharm. Chem. J. 2007, 41, 78–82. [Google Scholar] [CrossRef]
- Takiff, H.; Guerrero, E. Current prospects for the fluoroquinolones as first-line tuberculosis therapy. Antimicrob. Agents. Chemother. 2011, 55, 5421–5429. [Google Scholar] [CrossRef]
- Chang, K.C.; Yew, W.W.; Chan, R.C. Rapid assays for fluoroquinolone resistance in Mycobacterium tuberculosis: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2010, 65, 1551–1561. [Google Scholar] [CrossRef]
- Ahmad, S.; Mokaddas, E. Recent advances in the diagnosis and treatment of multidrug-resistant tuberculosis. Respir. Med. 2009, 103, 1777–1790. [Google Scholar] [CrossRef]
- Saha, D.K.; Padhye, S.; Anson, C.E.; Powell, A.K. Hydrothermal synthesis, crystal structure, spectroscopy, electrochemistry and antimycobacterial evaluation of the copper (II) ciprofloxacin complex: [Cu(cf)2(BF4)2]·6H2O. Inorg. Chem. Commun. 2002, 5, 1022–1027. [Google Scholar] [CrossRef]
- Sulochana, S.; Rahman, F.; Paramasivan, C.N. In vitro activity of fluoroquinolones against Mycobacterium tuberculosis. J. Chemother. 2005, 17, 169–173. [Google Scholar]
- Nishizawa, T.; Suzuki, H.; Hibi, T. Quinolone-based third-line therapy for Helicobacter pylori eradication. J. Clin. Biochem. Nutr. 2009, 44, 119–124. [Google Scholar] [CrossRef]
- Berning, M.; Krasz, S.; Miehlke, S. Should quinolones come first in Helicobacter pylori therapy? Ther. Adv. Gastroenterol. 2011, 4, 103–114. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Bazzoli, F.; Delchier, J.C.; Celiñski, K.; Giguère, M.; Rivière, M.; Mégraud, F. Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: A randomised, open-label, non-inferiority, phase 3 trial. Lancet 2011, 377, 905–913. [Google Scholar] [CrossRef]
- Ergül, B.; Koçak, E.; Taş, A.; Filik, L.; Köklü, S. Bismuth, moxifloxacin, tetracycline, lansoprazole quadruple first line therapy for eradication of H. pylori: A prospective study. Clin. Res. Hepatol. Gastroenterol. 2013. [Google Scholar] [CrossRef]
- Turel, I.; Kljun, J.; Perdih, F.; Morozova, E.; Bakulev, V.; Kasyanenco, N.; Byl, J.A.W.; Osheroff, N. First ruthenium organometallic complex of antibacterial agent ofloxacin. Crystal structure and interactions with DNA. Inorg. Chem. 2010, 49, 10750–10752. [Google Scholar] [CrossRef]
- Herold, C.; Ocker, M.; Ganslmayer, M.; Gerauer, H.; Hahn, E.G.; Schuppan, D. Ciprofloxacin induces apoptosis and inhibits proliferation of human colorectal carcinoma cells. Br. J. Cancer 2002, 86, 443–448. [Google Scholar] [CrossRef]
- Sissi, C.; Palumbo, M. The quinolone family: From antibacterial to anticancer agents. Curr. Med. Chem. Anticancer Agents. 2003, 3, 439–450. [Google Scholar] [CrossRef]
- Thadepalli, H.; Salem, F.; Chuah, S.K.; Gollapudi, S. Antitumor activity of trovafloxacin in an animal model. In Vivo 2005, 19, 269–276. [Google Scholar]
- Ahmed, A.; Daneshtalab, M. Nonclassical Biological Activities of Quinolone Derivatives. J. Pharm. Pharmaceut. Sci. 2012, 15, 52–72. [Google Scholar]
- Rhule, J.T.; Hill, C.L.; Judd, D.A.; Schinazi, R.F. Polyoxometalates in medicine. Chem. Rev. 1998, 98, 327357. [Google Scholar]
- Kljun, J.; Bytzek, A.K.; Kandioller, W.; Bartel, C.; Jakupec, M.A.; Hartinger, C.G.; Keppler, B.K.; Turel, I. Physicochemical studies and anticancer potency of ruthenium η-p-cymene complexes containing antibacterial quinolones. Organometallics 2011, 30, 2506–2512. [Google Scholar] [CrossRef]
- Eboka, C.J.; Aigbavboa, S.O.; Akerele, J.O. Colorimetric determination of the fluoroquinolones. J. Antimicrob. Chemother. 1997, 39, 639–641. [Google Scholar] [CrossRef]
- Fratini, L.; Schapoval, E.E.S. Ciprofloxacin determination by visible light spectrophotometry using iron(III) nitrate. Int. J. Pharmaceut. 1996, 127, 279–282. [Google Scholar] [CrossRef]
- Sultan, S.M.; Suliman, F.-E.O. Chemometric optimization and flow injection method for the determination of norfloxacin in drug formulations. Analyst 1993, 118, 573–576. [Google Scholar] [CrossRef]
- Al-Momani, I.F.; Haj-Hussein, A.T.; Tahtamouni, A.N. Flow injection spectrophotometric and chromatographic determination of ciprofloxacin and norfloxacin in pharmaceutical formulations. J. Flow Inject. Anal. 2008, 25, 151–155. [Google Scholar]
- García, M.S.; Albero, M.I.; Sánchez-Pedreño, C.; Abuherba, M.S. Flow injection spectrophotometric determination of ofloxacin in pharmaceuticals and urine. Eur. J. Pharm. Biopharm. 2005, 61, 87–93. [Google Scholar] [CrossRef]
- Sultan, S.M.; Suliman, F.-E.O. Flow injection spectrophotometric determination of the antibiotic ciprofloxacin in drug formulations. Analyst 1992, 117, 1523–1526. [Google Scholar] [CrossRef]
- Suliman, F.E.O.; Sultan, S.M. Sequential injection technique employed for stoichiometric studies, optimization and quantitative determination of some fluoroquinolones antibiotics complexed with iron (III) in sulfuric acid media. Talanta 1996, 43, 559–568. [Google Scholar] [CrossRef]
- El Khateeb, S.Z.; Razek, S.A.; Amer, M.M. Stability-indicating methods for the spectrophotometric determination of norfloxacin. J. Pharm. Biomed. Anal. 1998, 17, 829–840. [Google Scholar] [CrossRef]
- Rizk, M.; Belal, F.; Ibrahim, F.; Ahmed, S.; Sheribah, Z.A. Derivative spectrophotometric analysis of 4-quinolone antibacterials in formulations and spiked biological fluids by their Cu (II) complexes. J. AOAC Int. 2001, 84, 368–375. [Google Scholar]
- El Walily, A.F.M.; Belal, S.F.; Bakry, R.S. Spectrophotometric and spectrofluorimetric estimation of ciprofloxacin and norfloxacin by ternary complex formation with eosin and palladium(II). J. Pharm. Biomed. Anal. 1996, 14, 561–569. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Nakao, M.; Nakahara, R.; Nishioka, Y.; Ikeda, C.; Fujita, Y. Spectrophotometric determination of quinolone antibiotics by an association complex formation with aluminum(III) and erythrosine. Anal. Sci. 2009, 25, 125–128. [Google Scholar] [CrossRef]
- Uivarosi, V.; Monciu, C.M. The gravimetric and spectrophotometric assay of ofloxacin using ammonium reineckate. Rev. Chim. 2005, 56, 726–730. [Google Scholar]
- Uivarosi, V.; Monciu, C.M. Studies on the gravimetric and spectrophotometric analysis of norfloxacin using ammonium reineckate. Rev. Chim. 2009, 60, 132–136. [Google Scholar]
- Salem, H. Spectrofluorimetric, atomic absorption spectrometric and spectrophotometric determination of some fluoroquinolones. Am. J. Appl. Sci. 2005, 2, 719–729. [Google Scholar] [CrossRef]
- Cordoba-Diaz, M.; Cordoba-Borrego, M.; Cordoba-Diaz, D. modification of fluorescent properties of norfloxacin in the presence of certain antacids. J. Pharm. Biomed. Anal. 1998, 18, 565–571. [Google Scholar] [CrossRef]
- Rizk, M.; Belal, F.; Ibrahim, F.; Ahmed, S.; el-Enany, N. Spectrofluorimetric analysis of certain 4-quinolone in pharmaceuticals and biological fluids. Pharm. Acta Helv. 2000, 74, 371–377. [Google Scholar] [CrossRef]
- Djurdjević, P.T.; Jelikić-Stankov, M.; Stankov, D. Fluorescence reaction and complexation equilibria between norfloxacin and aluminium (III) ion in chloride medium. Anal. Chim. Acta 1995, 300, 253–259. [Google Scholar] [CrossRef]
- Pérez-Ruiz, T.; Martínez-Lozano, C.; Tomás, V.; Carpena, J. Determination of norfloxacin in real samples by different pectrofluorimetric techniques. Analyst 1997, 122, 705–708. [Google Scholar] [CrossRef]
- Han, Y.; Wu, X.; Yang, J.; Sun, S. The fluorescence characteristic of the yttrium–norfloxacin system and its analytical application. J. Pharm. Biomed. Anal. 2005, 38, 528–531. [Google Scholar] [CrossRef]
- Tong, C.; Zhuo, X.; Liu, W.; Wu, J. Synchronous fluorescence measurement of enrofloxacin in the pharmaceutical formulation and its residue in milks based on the yttrium (III)-perturbed luminescence. Talanta 2010, 82, 1858–1863. [Google Scholar] [CrossRef]
- Beltyukova, S.; Teslyuk, O.; Egorova, A.; Tselik, E. Solid-phase luminescence determination of ciprofloxacin and norfloxacin in biological fluids. J. Fluoresc. 2002, 12, 269–271. [Google Scholar] [CrossRef]
- Tong, C.; Xiang, G. Sensitive determination of enoxacin by its enhancement effect on the fluorescence of terbium(III)–sodium dodecylbenzene sulfonate and its luminescence mechanism. J. Luminesc. 2007, 126, 575–580. [Google Scholar] [CrossRef]
- Kaur, K.; Singh Saini, S.; Malik, A.K.; Singh, B. Micelle enhanced and terbium sensitized spectrofluorimetric determination of danofloxacin in milk using molecularly imprinted solid phase extraction. Spectrochim. Acta A 2012, 96, 790–795. [Google Scholar] [CrossRef]
- Zhao, H.C.; Ding, F.; Wang, X.; Ju, H.; Li, A.; Jin, L.P. A study on silver nanoparticles-sensitized fluorescence and second-order scattering of the complexes of Tb(III) with ciprofloxacin and its applications. Spectrochim. Acta Part A 2008, 70, 332–336. [Google Scholar] [CrossRef]
- Ding, F.; Zhao, H.; Jin, L.; Zheng, D. Study of the influence of silver nanoparticles on the second-order scattering and the fluorescence of the complexes of Tb(III) with quinolones and determination of the quinolones. Anal. Chim. Acta 2006, 566, 136–143. [Google Scholar] [CrossRef]
- Attia, M.S.; Essawy, A.A.; Youssef, A.O. Europium-sensitized and simultaneous pH-assisted spectrofluorimetric assessment of ciprofloxacin, norfloxacin and gatifloxacin in pharmaceutical and serum samples. J. Photochem. Photobiol. A 2012, 236, 26–34. [Google Scholar] [CrossRef]
- Dong, P.; Na, X.; Fu, B.; Wang, L. Rapid europium-sensitized fluorescent determination of ulifloxacin, the active metabolite of prulifloxacin, in human serum and urine. J. Pharm. Anal. 2011, 1, 46–50. [Google Scholar]
- Attia, M.S.; Youssef, A.O.; Essawy, Amr A.; Abdel-Mottaleb, M.S.A. A highly luminescent complexes of Eu(III) and Tb(III) with norfloxacin and gatifloxacin doped in sol–gel matrix: A comparable approach of using silica doped Tb(III) and Eu(III) as optical sensor. J. Luminesc. 2012, 132, 2741–2746. [Google Scholar] [CrossRef]
- Jelikić-Stankov, M.; Stankov, D.; Djurdjević, P. Determination of pefloxacin in serum by time-resolved fluorimetry. Pharmazie 1999, 54, 73–74. [Google Scholar]
- Luiz, F.C.L.; Garcia, L.S.; Goes Filho, L.S.; Teixeira, L.R.; Louro, S.R.W. Fluorescence studies of gold(III)-norfloxacin complexes in aqueous solutions. J. Fluoresc. 2011, 21, 1933–1940. [Google Scholar] [CrossRef]
- Pan, B.; Han, X.; Wu, M.; Peng, H.; Zhang, D.; Li, H.; Xing, B. Temperature dependence of ofloxacin fluorescence quenching and complexation by Cu(II). Environ. Pollut. 2012, 171, 168–173. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Jiang, Y.C. Flow injection flame atomic spectrometry for the indirect analysis of norfloxacin. Atom. Spectroscop. 2001, 22, 429–432. [Google Scholar]
- Al-Ghannam, S.M. Atomic absorption spectroscopic, conductometric and colorimetric methods for determination of some fluoroquinolone antibacterials using ammonium reineckate. Spectrochim. Acta A 2008, 69, 1188–1194. [Google Scholar] [CrossRef]
- Issopoulos, B.P. spectrophotometric determination of norfloxacin in pharmaceutical formulations. Analyst 1989, 114, 627–630. [Google Scholar] [CrossRef]
- Wang, N.-X.; Wang, L.; Jiang, W.; Ren, Y.-Z.; Si, Z.-K.; Qiu, X.-X.; Du, G.-Y.; Qi, P. Determination of neodymium, holmium and erbium in mixed rare earths by norfloxacin. Fresenius J. Anal. Chem. 1998, 361, 821–824. [Google Scholar] [CrossRef]
- Guo, C.; Lang, A.; Wang, L.; Jiang, W. The co-luminescence effect of a europium (III)–lanthanum (III)–gatifloxacin–sodium dodecylbenzene sulfonate system and its application for the determination of trace amount of europium(III). J. Luminesc. 2010, 130, 591–597. [Google Scholar] [CrossRef]
- Tan, H.; Zhang, Y.; Chen, Y. Detection of mercury ions (Hg2+) in urine using a terbium chelate fluorescent probe. Sens. Actuators B 2011, 156, 120–125. [Google Scholar] [CrossRef]
- Beltyukova, S.V.; Egorova, A.V.; Teslyuk, O.I. Europium(III) and terbium(III) chelates of quinolonecarboxylic acid derivatives as labels for immunofluorimetric assay. J. Anal. Chem. 2000, 55, 682–685. [Google Scholar] [CrossRef]
- Tong, C.; Hu, Z.; Liu, W. Enoxacin–Tb3+ complex as an environmentally friendly fluorescence probe for DNA and its application. Talanta 2007, 71, 816–821. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).