Polyphenolic Composition, Antioxidant and Antibacterial Activities for Two Romanian Subspecies of Achillea distans Waldst. et Kit. ex Willd.
Abstract
:1. Introduction
2. Results and Discussion
2.1. HPLC Analysis of Polyphenols
| Polyphenolic compounds | m/z value | tR ± SD (min) | Achillea distans subsp. distans | Achillea distans subsp. alpina |
|---|---|---|---|---|
| Caffeic acid | 179 | 5.60 ± 0.04 | <0.2 | <0.2 |
| Chlorogenic acid | 353 | 5.62 ± 0.05 | <0.2 | <0.2 |
| Hyperoside | 463 | 18.60 ± 0.12 | NF | 2.05 ± 0.10 |
| Isoquercitrin | 463 | 19.60 ± 0.10 | NF | <0.2 |
| Rutin | 609 | 20.20 ± 0.15 | NF | <0.2 |
| Quercetin | 301 | 26.80 ± 0.15 | 1.44 ± 0.06 | 1.11 ± 0.09 |
| Luteolin | 285 | 29.10 ± 0.19 | 763.12 ± 1.88 | 52.65 ± 0.85 |
| Apigenin | 279 | 33.10 ± 0.15 | 264.84 ± 1.16 | 13.47 ± 0.53 |
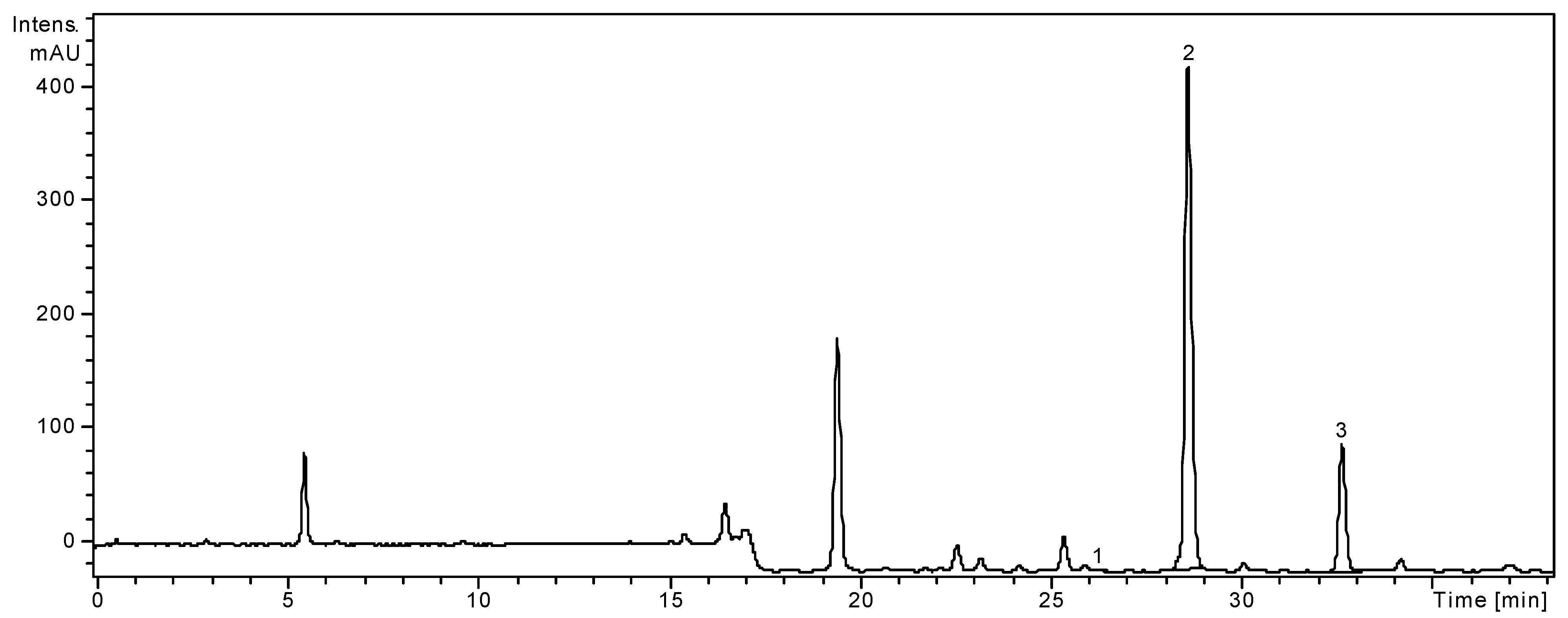
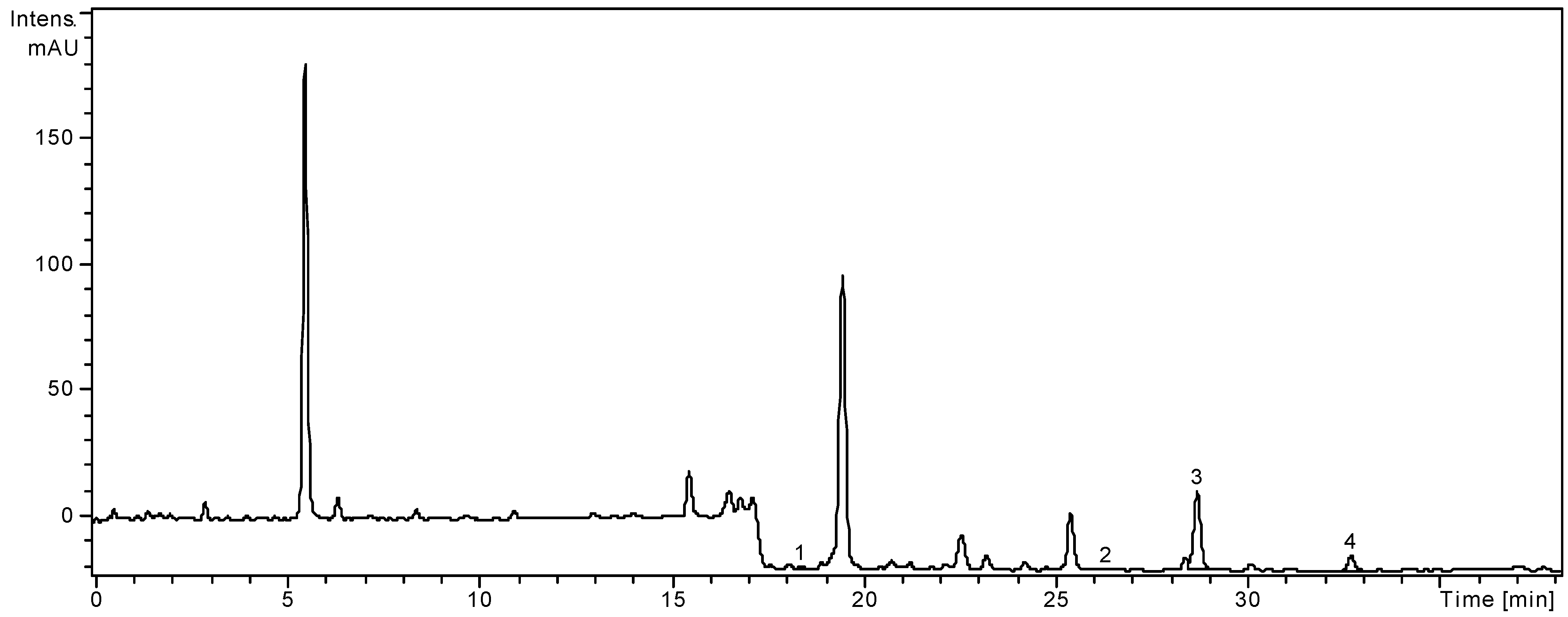
| Peak no. | Phenolic compounds | m/z | tR ± SD (min) |
|---|---|---|---|
| 1. | Caftaric acid | 311 | 3.54 ± 0.05 |
| 2. | Gentisic acid | 179 | 6.52 ± 0.04 |
| 3. | Caffeic acid | 179 | 5.60 ± 0.04 |
| 4. | Chlorogenic acid | 353 | 5.62 ± 0.05 |
| 5. | p-Coumaric acid | 163 | 9.48 ± 0.08 |
| 6. | Ferulic acid | 193 | 12.8 ± 0.10 |
| 7. | Sinapic acid | 223 | 15.00 ± 0.10 |
| 8. | Cichoric acid | 473 | 15.96 ± 0.13 |
| 9. | Hyperoside | 463 | 18.60 ± 0.12 |
| 10. | Isoquercitrin | 463 | 19.60 ± 0.10 |
| 11. | Rutin | 609 | 20.20 ± 0.15 |
| 12. | Myricetin | 317 | 21.13 ± 0.12 |
| 13. | Fisetin | 285 | 22.91 ± 0.15 |
| 14. | Quercitrin | 447 | 23.64 ± 0.13 |
| 15. | Quercetin | 301 | 26.80 ± 0.15 |
| 16. | Patuletin | 331 | 29.41 ± 0.12 |
| 17. | Luteolin | 285 | 29.10 ± 0.19 |
| 18. | Kaempferol | 285 | 32.48 ± 0.17 |
| 19. | Apigenin | 279 | 33.10 ± 0.15 |
2.2. Polyphenolic and Flavonoid Contents of the Extracts
| Samples | TPC (mg GAE/g plant material) | Flavonoids (mg RE/g plant material) |
|---|---|---|
| A. distans subsp. distans | 101.61 ± 1.24 | 37.26 ± 0.71 |
| A. distans subsp. alpina | 174.75 ± 1.47 | 33.18 ± 0.60 |
2.3. Antioxidant Activity
| Samples | DPPH (% decolorization) | IC50 (µg·mL−1) | TEAC (µmol Trolox/mg plant material) | HAPX (%) |
|---|---|---|---|---|
| A. distans subsp. distans | 52.73 ± 0.77 | 204.85 ± 2.93 | 42.14 ± 0.48 | 4.91 ± 1.24 |
| A. distans subsp. alpina | 93 ± 1.47 | 83.80 ± 1.20 | 46.72 ± 0.35 | 10.27 ± 1.81 |
| Quercetin | - | 5.60 ± 0.35 | - | - |
| BHT | - | 16 ± 0.54 | - | - |
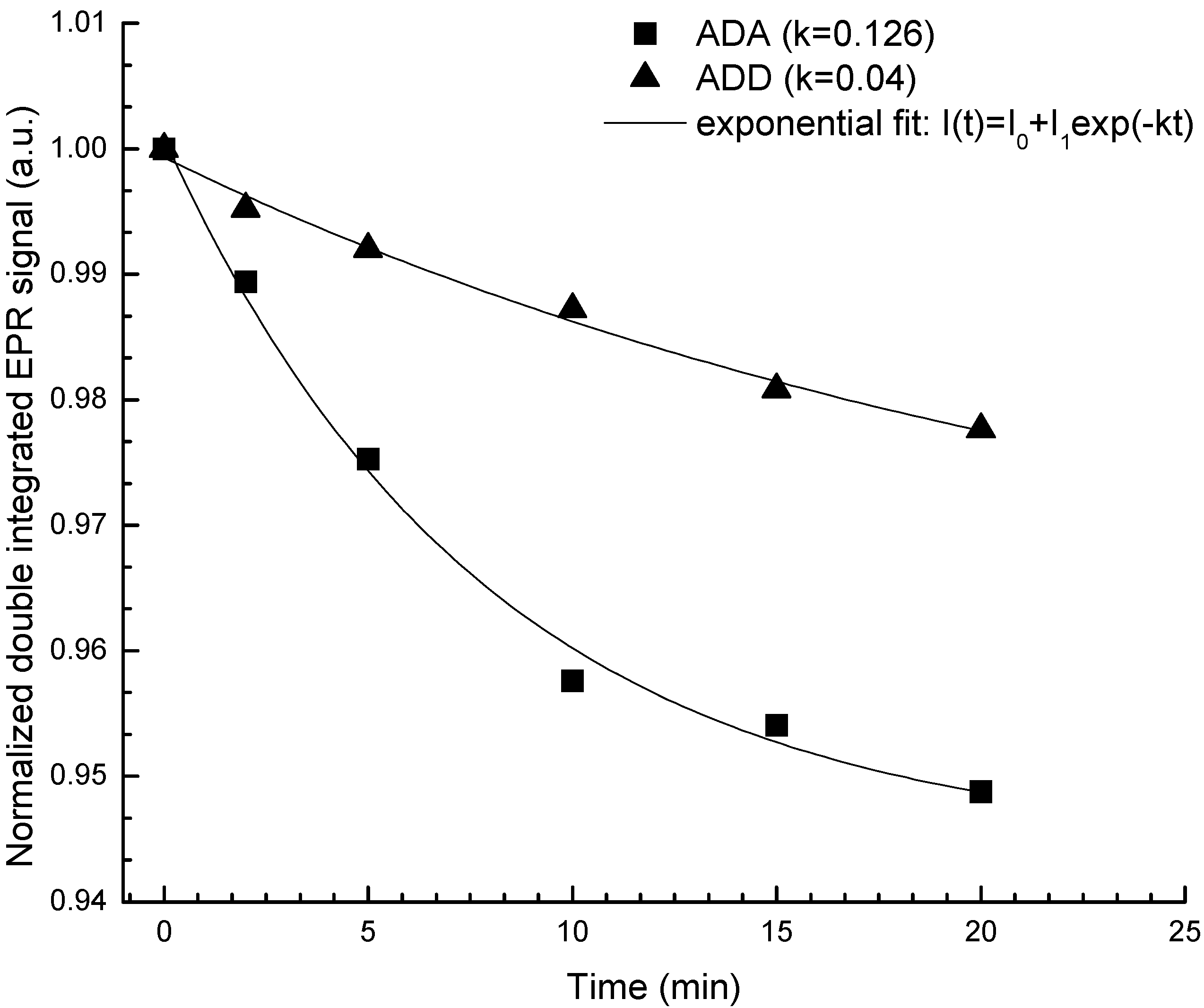
2.4. Antibacterial Activity
| Samples | Inhibition zone in diameter (mm) | |||
|---|---|---|---|---|
| Staphylococcus aureus | Listeria monocytogenes | Escherichia coli | Salmonella typhymurium | |
| A. distans subsp. distans | 13.0 ± 0.05 | 17.0 ± 0.1 | 10.0 ± 0.05 | 7.0 ± 0.00 |
| A. distans subsp. alpina | 12.0 ± 0.07 | 12.0 ± 0.00 | 8.0 ± 0.05 | 6.0 ± 0.02 |
| Gentamicin | 19 ± 0.05 | 18 ± 0.1 | 22 ± 0.00 | 18 ± 0.05 |
3. Experimental
3.1. Plant Material and Extraction Procedure
3.2. Chemicals
3.3. HPLC/MS Analysis
3.3.1. Apparatus and Chromatographic Conditions
3.3.2. Identification and Quantification of Polyphenols
3.4. Determination of Total Polyphenols and Flavonoids Content
3.5. Antioxidant Activity Test
3.5.1. DPPH• Radical Scavenging Assay
3.5.2. Trolox Equivalent Antioxidant Capacity (TEAC) Assay
3.5.3. Hemoglobin/Ascorbate Peroxidase Activity Inhibition (HAPX) Assay
3.5.4. EPR (Electron Paramagnetic Resonance) Spectroscopy Method
3.6. Antibacterial Activity Test
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ciocârlan, V. Illustrated Flora of Romania. Pteridophyta et Spermatophyta; Ceres Publishing House: Bucharest, Romania, 2009; pp. 794–799. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea; Cambridge University Press: Cambridge, UK, 1976; Volune 4, pp. 162–163. [Google Scholar]
- Tamas, M.; Popovici, M.; Oniga, I.; Oprean, I.; Coldea, G. Infraspecific chemical taxa of Achillea distans Waldst. et Kit. from the Rodnei Mountains (Eastern Carpathians) -Romania. Planta Med. 2009, 75, 932. [Google Scholar]
- Popovici, M.; Vlase, L.; Oniga, I.; Tamas, M. HPLC analyses on polyphenolic compounds from Achillea species. Farmacia 2007, 3, 353–357. [Google Scholar]
- Popovici, M.; Pârvu, A.E.; Oniga, I.; Toiu, A.; Tamas, M.; Benedec, D. Effects of two Achillea species tinctures on experimental acute inflammation. Farmacia 2008, 1, 15–23. [Google Scholar]
- Todorova, M.N.; Mikhova, B.; Trendafilova, A.; Vitkova, A.; Duddeck, H. Terpenoids from Achillea distans Waldst. & Kit. ex Willed. Biochem. Syst. Ecol. 2007, 12, 852–858. [Google Scholar]
- Konakchiev, A.; Todorova, M.; Mikhova, B.; Vitkova, A.; Najdenski, H. Composition and antimicrobial activity of Achillea distans essential oil. Nat. Prod. Commun. 2011, 6, 905–906. [Google Scholar]
- Lazarevic, J.; Radulović, N.; Zlatković, B.; Palić, R. Composition of Achillea distans Willd. subsp. distans root essential oil. Nat. Prod. Res. 2010, 8, 718–731. [Google Scholar]
- Dai, J.; Mumper, J.R. Plant phenolics: Extraction, analysis and their antioxidant an anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Kundaković, T.; Stanojković, T.; Juranić, Z.; Kovacević, N. Cytotoxic and antioxidant activity of Achillea alexandri-regis. Pharmazie 2005, 4, 319–320. [Google Scholar]
- Özer, H.; Sökmen, M.; Bariş, Ö.; Şahin, F.; Özkan, H.; Güllüce, M.; Kiliç, H.; Özbek, T. Biological activities of the essential oil and methanol extract of Achillea biebersteinii Afan (Asteraceae). Turk. J. Biol. 2006, 30, 65–73. [Google Scholar]
- Vitalini, S.; Grande, S.; Visioli, F.; Agradi, E.; Fico, G.; Tome, F. Antioxidant activity of wild plants collected in Valsesia, an alpine region of Northern Italy. Phytother. Res. 2006, 7, 576–580. [Google Scholar]
- Nickavar, B.; Kamalinejad, M.; Haj-Yahya, M.; Shafaghi, B. Comparison of the free radical scavenging activity of six Iranian Achillea species. Pharm. Biol. 2006, 44, 208–212. [Google Scholar] [CrossRef]
- Ardestani, A.; Yazdanparast, R. Antioxidant and free radical scavenging potential of Achillea santolina extracts. Food Chem. 2007, 104, 21–29. [Google Scholar] [CrossRef]
- Koleckar, V.; Opletal, L.; Brojerova, E.; Rehakova, Z.; Cervenka, F.; Kubikova, K.; Kuca, K.; Jun, D.; Polasek, M.; Kunes, J.; et al. Evaluation of natural antioxidants of Leuzea carthamoides as a result of a screening study of 88 plant extracts from the European Asteraceae and Cichoriaceae. J. Enzyme Inhib. Med. Chem. 2008, 2, 218–224. [Google Scholar]
- Eghdami, A.; Sadeghi, F. Determination of total phenolic and flavonoids contents in methanolic and aqueous extract of Achillea millefolium. Org. Chem. J. 2010, 2, 81–84. [Google Scholar]
- Vitalini, S.; Beretta, G.; Iriti, M.; Orsenigo, S.; Basilico, N.; Dall'Acqua, S.; Iorizzi, M.; Fico, G. Phenolic compounds from Achillea millefolium L. and their bioactivity. Acta Biochim. Pol. 2011, 2, 203–209. [Google Scholar]
- Wojdyło, A.; Oszmianski, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Saeidnia, S.; Gohari, A.; Mokhber-Dezfuli, N.; Kiuchi, F. A review on phytochemistry and medicinal properties of the genus Achillea. Daru. 2011, 3, 173–186. [Google Scholar]
- Tuberoso, C.I.; Montoro, P.; Piacente, S.; Corona, G.; Deiana, M.; Dessì, M.A.; Pizza, C.; Cabras, P. Flavonoid characterization and antioxidant activity of hydroalcoholic extracts from Achillea ligustica All. J. Pharm. Biomed. Anal. 2009, 3, 440–448. [Google Scholar]
- Bozin, B.; Mimica-Dukic, N.; Bogavac, M.; Suvajdzic, L.; Simin, N.; Samojlik, I.; Couladis, M. Chemical composition, antioxidant and antibacterial properties of Achillea collina Becker ex Heimerl s.l. and A.pannonica Scheele essential oils. Molecules 2008, 13, 2058–2068. [Google Scholar]
- Giorgi, A.; Madeo, M.; Speranza, G.; Cocucci, M. Influence of environmental factors on composition of phenolic antioxidants of Achillea collina Becker ex Rchb. Nat. Prod. Res. 2010, 16, 1546–1559. [Google Scholar]
- Turkoglu, I.; Turkoglu, S.; Celik, S.; Kahyaoglu, M. Antioxidant and antimicrobial activities of Turkish endemic Achillea species. Afr. J. Microbiol. Res. 2010, 4, 2034–2042. [Google Scholar]
- Meda, R.N.T.; Vlase, L.; Lamien-Meda, A.; Lamien, C.E.; Muntean, D.; Tiperciuc, B.; Oniga, I.; Nacoulma, O.G. Identification and quantification of phenolic compounds from Balanites aegyptiaca (L) Del (Balanitaceae) galls and leaves by HPLC-MS. Nat. Prod. Res. 2011, 25, 93–99. [Google Scholar] [CrossRef]
- Vlase, L.; Marcel Parvu, M.; Parvu, E.A.; Toiu, A. Chemical Constituents of Three Allium Species from Romania. Molecules 2013, 18, 114–127. [Google Scholar]
- Benedec, D.; Vlase, L.; Hanganu, D.; Oniga, I. Antioxidant potential and polyphenolic content of Romanian Ocimum basilicum. Dig. J. Nanomater. Bios. 2012, 7, 1263–1270. [Google Scholar]
- Ghasemzadeh, A.; Ghasemzadeh, N. Flavonoids and phenolic acids: Role and biochemical activity in plants and human. J. Med. Plants Res. 2011, 31, 6697–6703. [Google Scholar]
- Reeves, D.S.; White, L.O. Principles of Methods of Assaying Antibiotics in Pharmaceutical Microbiology, 3rd ed.; Blackwel: Oxford, UK, 1983; pp. 140–162. [Google Scholar]
- Zbakh, H.; Chiheb, H.; Bouziane, H.; Motilva Sánchez, V.; Riadi, H. Antibacterial activity of benthic marine algae extracts from the Mediterranean coast of Morocco. JMBFS 2012, 1, 219–228. [Google Scholar]
- Antonini, E.; Brunori, M. Hemoglobin and Myoglobin in Their Reaction with Ligands; North-Holland Pub. Co.: Amsterdam, The Netherlands, 1971. [Google Scholar]
- Mot, A.C.; Damian, G.; Sarbu, C.; Silaghi-Dumitrescu, R. Redox reactivity in propolis: Direct detection of free radicals in basic medium and interaction with hemoglobin. Redox Rep. 2009, 14, 267–274. [Google Scholar] [CrossRef]
- Council of Europe, European Pharmacopoeia, 5th ed.; Council of Europe: Strasbourg Cedex, France, 2005; Volume 1, p. 221.
- Tamokou, J.D.D.; Chouna, J.R.; Fischer-Fodor, E.; Chereches, G.; Barbos, O.; Damian, G.; Benedec, D.; Duma, M.; Nkeng Efouet, P.A.; Wabo, H.K.; et al. Anticancer and antimicrobial activities of some antioxidant-rich cameroonian medicinal plants. PLoS One 2013, 2, e55880. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method. Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total phenol analyses: Automation and Comparison with Manual Methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Romanian Pharmacopoeia Commission National Medicines Agency, Romanian Pharmacopoeia, Xth ed.; Medical Publishing House Bucharest: Bucharest, Romania, 1993; p. 335.
- Adiguzel, A.; Ozer, H.; Sokmen, M.; Gulluce, M.; Sokmen, A.; Kilic, H.; Sahin, F.; Baris, O. Antimicrobial and antioxidant activity of the essential oil and methanol extract of Nepeta cataria. Pol. J. Microbiol. 2009, 58, 69–76. [Google Scholar] [CrossRef]
- Nimmi, O.S.; George, P. Evaluation of the antioxidant potential of a newly developed polyherbal formulation for antiobesity. Int. J. Pharm. Pharm. Sci. 2012, 4, 505–510. [Google Scholar]
- Simirgiotis, M.J. Antioxidant Capacity and HPLC-DAD-MS Profiling of Chilean Peumo (Cryptocarya alba) Fruits and Comparison with German Peumo (Crataegus monogyna) from Southern Chile. Molecules 2013, 18, 2061–2080. [Google Scholar] [CrossRef]
- Zadra, M.; Piana, M.; Faccim de Brum, T.; Boligon, A.A.; Borba de Freitas, R.; Machado, M.M.; Stefanello, S.T.; Soares, F.A.A.; Athayde, M.L. antioxidant activity and phytochemical composition of the leaves of Solanum guaraniticum A. St.-Hil. Molecules 2012, 17, 12560–12574. [Google Scholar] [CrossRef]
- Obón, J.M.; Castellar, M.R.; Cascales, J.A.; Fernández-López, J.A. Assessment of the TEAC method for determining the antioxidant capacity of synthetic red food colorants. Food Res. Int. 2005, 38, 843–845. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Cooper, C.E.; Silaghi-Dumitrescu, R.; Rukengwa, M.; Alayash, A.I.; Buehler, P.W. Peroxidase activity of hemoglobin towards ascorbate and urate: A synergistic protective strategy against toxicity of hemoglobin-based oxygen carriers (HBOC). Biochim. Biophys. Acta 2008, 1784, 1415–1420. [Google Scholar]
- Espinoza, M.; Olea-Azar, C.; Speisky, H.; Rodríguez, J. Determination of reactions between free radicals and selected Chilean wines and transition metals by ESR and UV–Vis technique. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 71, 1638–1643. [Google Scholar] [CrossRef]
- Sample Availability: Samples are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Benedec, D.; Vlase, L.; Oniga, I.; Mot, A.C.; Damian, G.; Hanganu, D.; Duma, M.; Silaghi-Dumitrescu, R. Polyphenolic Composition, Antioxidant and Antibacterial Activities for Two Romanian Subspecies of Achillea distans Waldst. et Kit. ex Willd. Molecules 2013, 18, 8725-8739. https://doi.org/10.3390/molecules18088725
Benedec D, Vlase L, Oniga I, Mot AC, Damian G, Hanganu D, Duma M, Silaghi-Dumitrescu R. Polyphenolic Composition, Antioxidant and Antibacterial Activities for Two Romanian Subspecies of Achillea distans Waldst. et Kit. ex Willd. Molecules. 2013; 18(8):8725-8739. https://doi.org/10.3390/molecules18088725
Chicago/Turabian StyleBenedec, Daniela, Laurian Vlase, Ilioara Oniga, Augustin C. Mot, Grigore Damian, Daniela Hanganu, Mihaela Duma, and Radu Silaghi-Dumitrescu. 2013. "Polyphenolic Composition, Antioxidant and Antibacterial Activities for Two Romanian Subspecies of Achillea distans Waldst. et Kit. ex Willd." Molecules 18, no. 8: 8725-8739. https://doi.org/10.3390/molecules18088725
APA StyleBenedec, D., Vlase, L., Oniga, I., Mot, A. C., Damian, G., Hanganu, D., Duma, M., & Silaghi-Dumitrescu, R. (2013). Polyphenolic Composition, Antioxidant and Antibacterial Activities for Two Romanian Subspecies of Achillea distans Waldst. et Kit. ex Willd. Molecules, 18(8), 8725-8739. https://doi.org/10.3390/molecules18088725








