Anionic Calixarene-Capped Silver Nanoparticles Show Species-Dependent Binding to Serum Albumins
Abstract
:1. Introduction
2. Results and Discussion
| BSA | HSA | PSA | SSA | |
|---|---|---|---|---|
| Molar Mass | 69,323.44 | 66,472.21 | 69,692.17 | 69,188.28 |
| Iso Electric Point | 5,82 | 5,67 | 6,08 | 5,8 |
| Amino Acid number | 607 | 585 | 607 | 607 |
| Lysine Residues | 60 | 59 | 58 | 61 |
| Arginine Residues | 26 | 24 | 29 | 25 |
| Histidine Residues | 17 | 16 | 19 | 18 |
| Aspartic Acid Residues | 40 | 36 | 36 | 44 |
| Glutamic Acid Residues | 59 | 62 | 61 | 56 |
| Homology | 100% | 76% | 80% | 92% |
| Structure (reference) | [10] | [29] | ND * | ND * |
| Accession Number PDB | PDB: 4F5S | PDB ID: 1AO6 | ||
| Accession Number NCBI | NP_851335.1 | PMID: 10388840 | NP_001005208.1 | NP_001009376.1 |

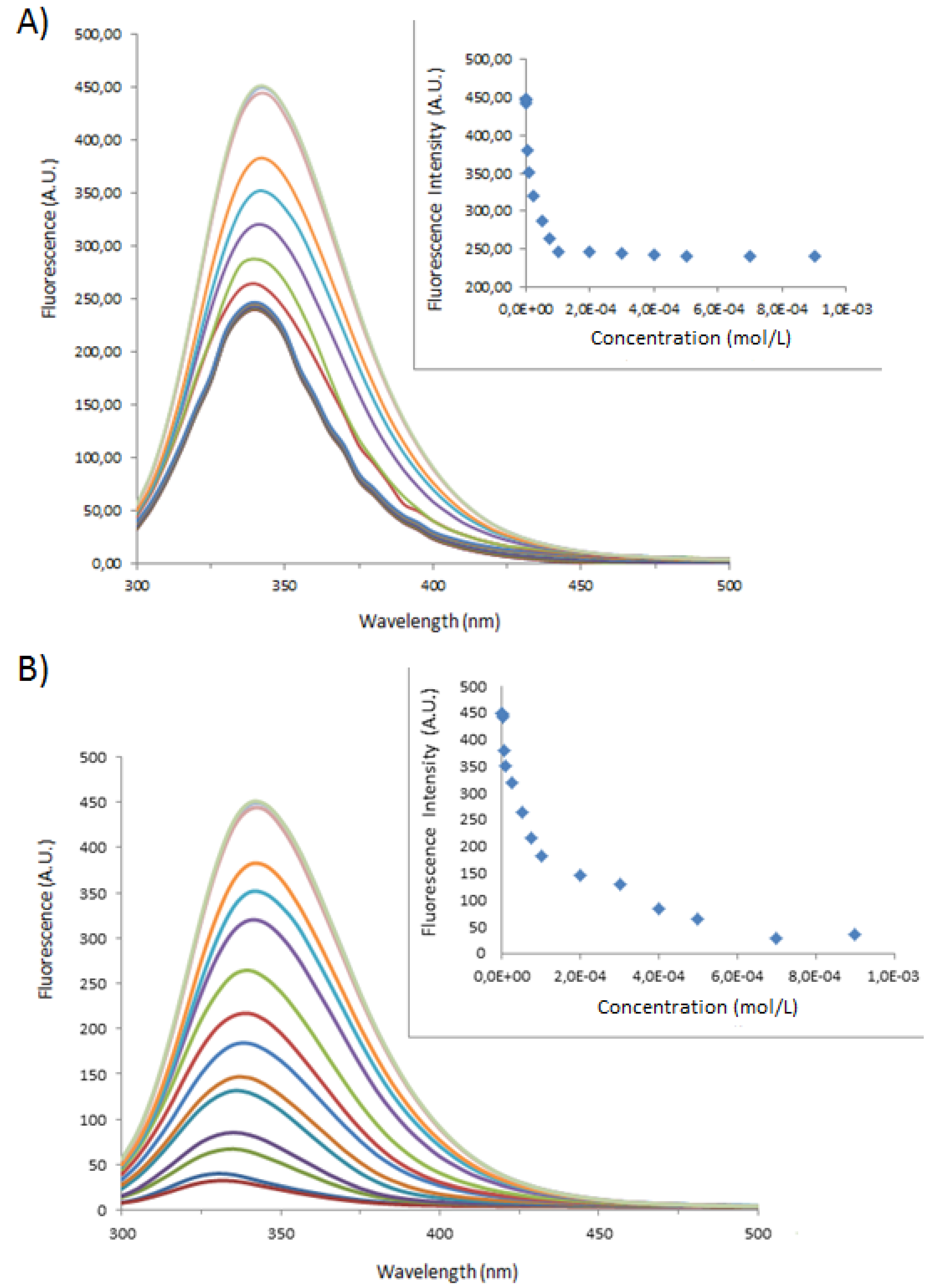
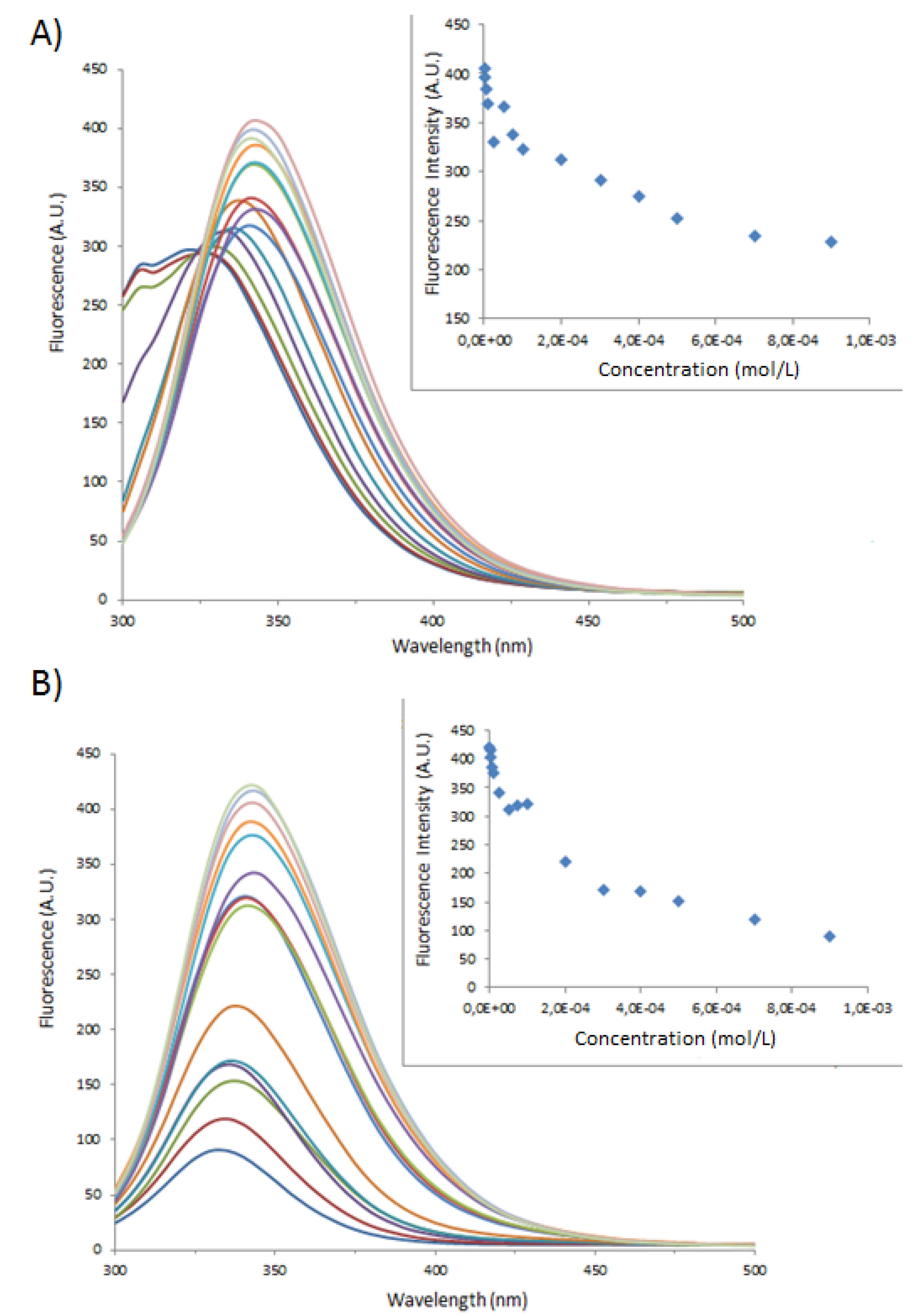
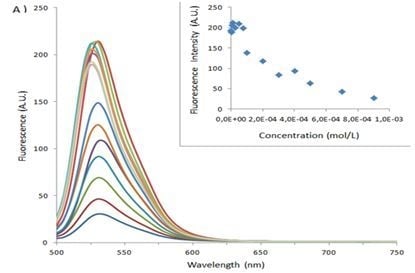
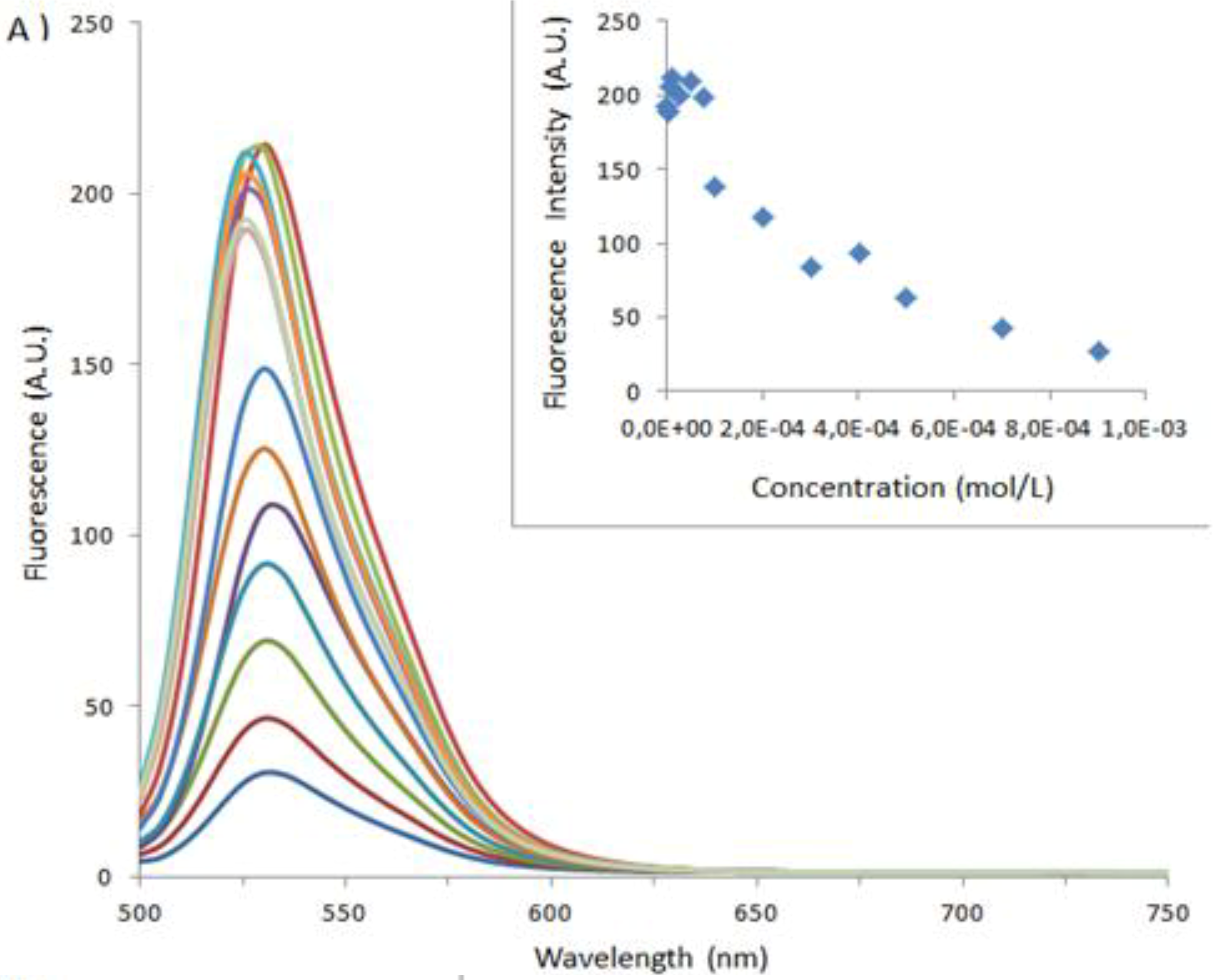
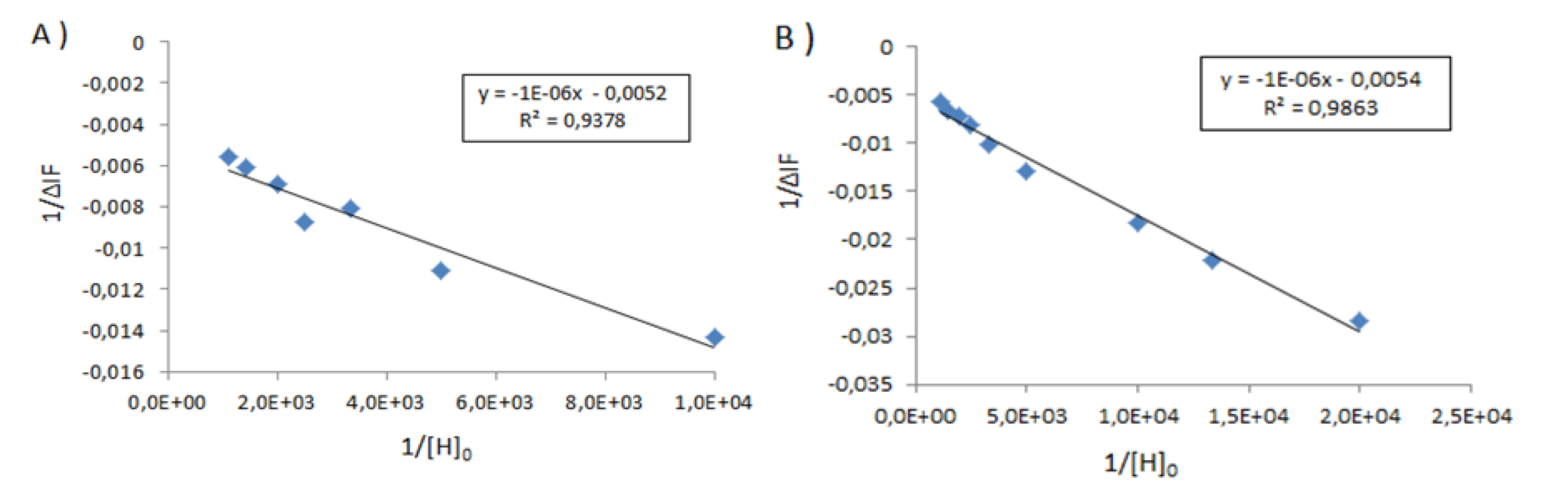
| Sample | Type of Bovine Albumin | Stoichiometry determined according to the method of Benesi [30] | Kass determined according to the method of Benesi [30] (M−1) | Kass apparent (M−1) |
|---|---|---|---|---|
| 1 | BSA | 1 : 1 | 71,666 | 100,000 |
| 1_Ag_NP | BSA | 1 : 1 | 24,000 | 16,600 |
| 1_Ag_NP | BSA-FITC | 1 : 1 | 5,200 | 4,540 |
| 2 | BSA | 1 : 1 | 10,000 | 5,500 |
| 2_Ag_NP | BSA | 1 : 1 | 3,500 | 5,500 |
| 2_Ag_NP | BSA-FITC | 1 : 1 | 5,400 | 3,300 |
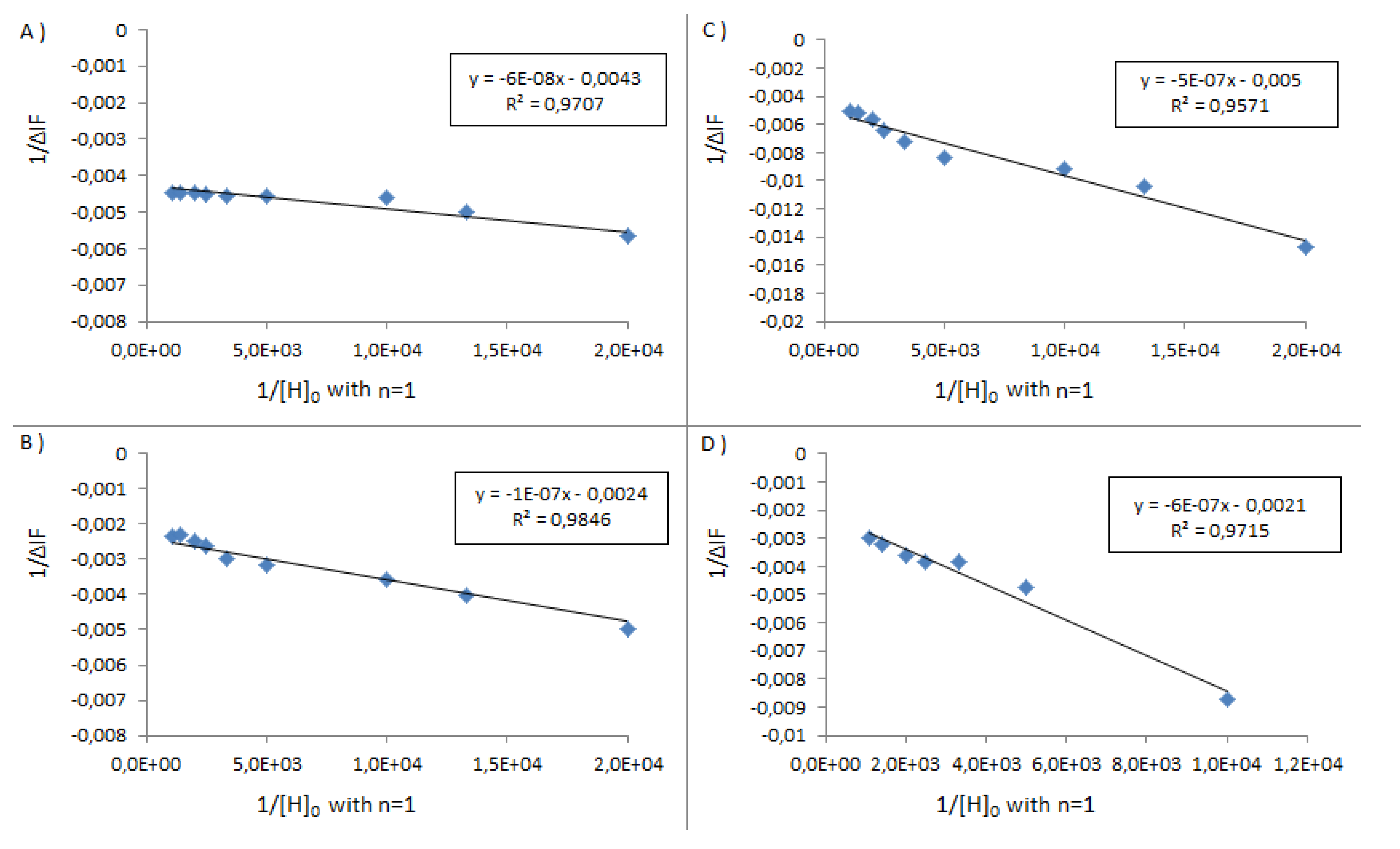
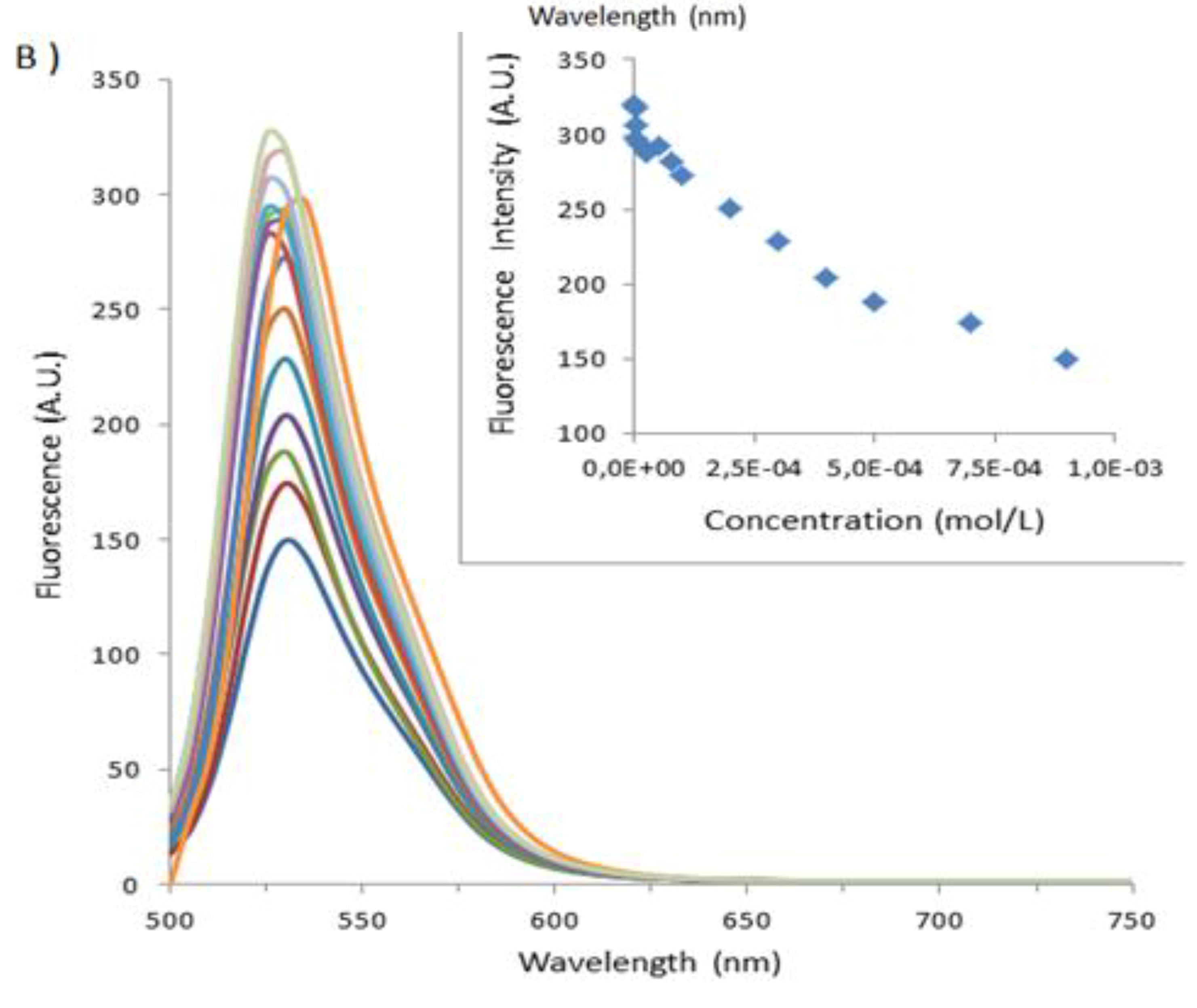
| BSA | HSA | PSA | SSA | |
|---|---|---|---|---|
| Denaturation Temperature (°C) | 50–80 | 50 | 68 | 60 |
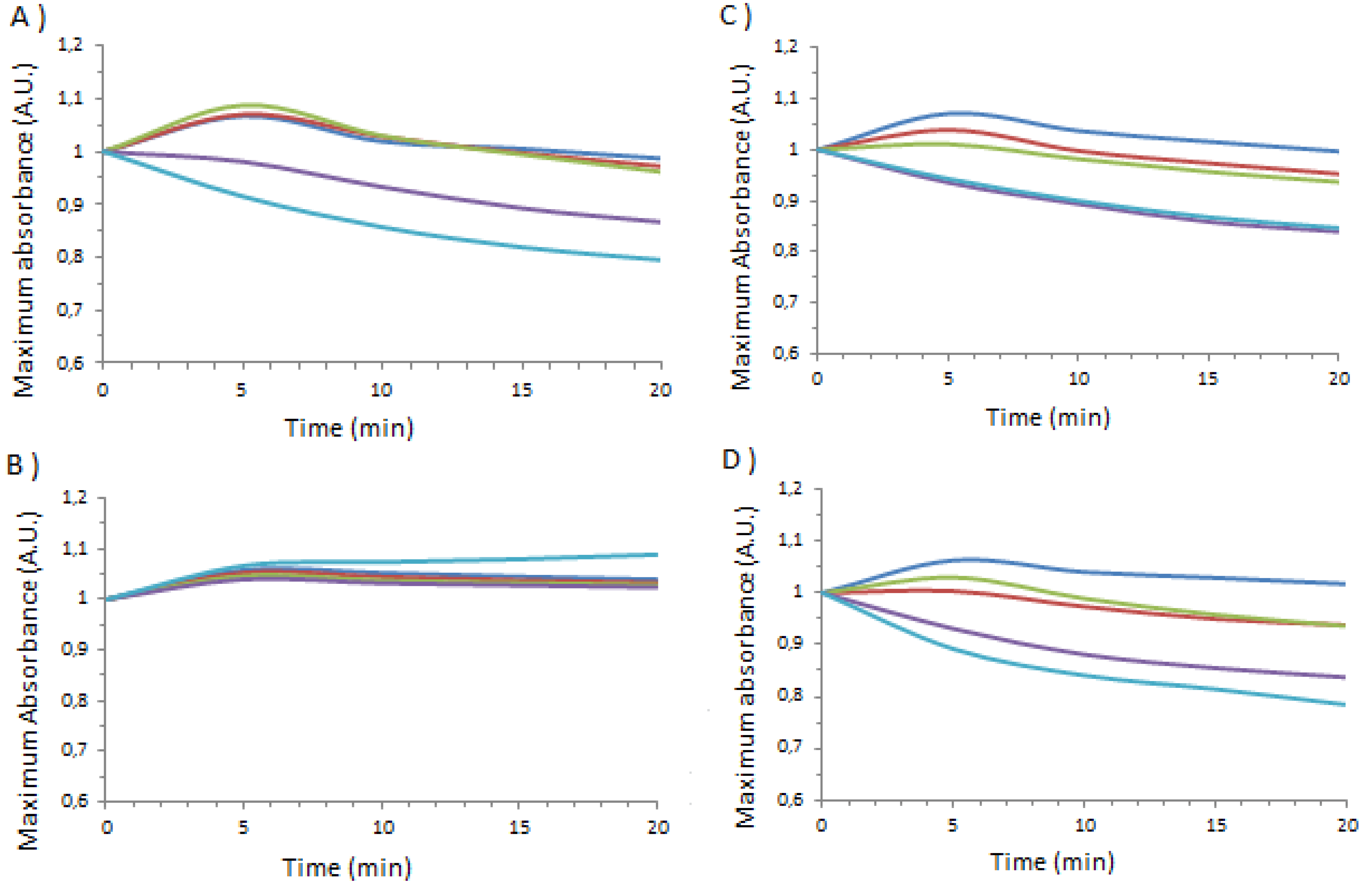
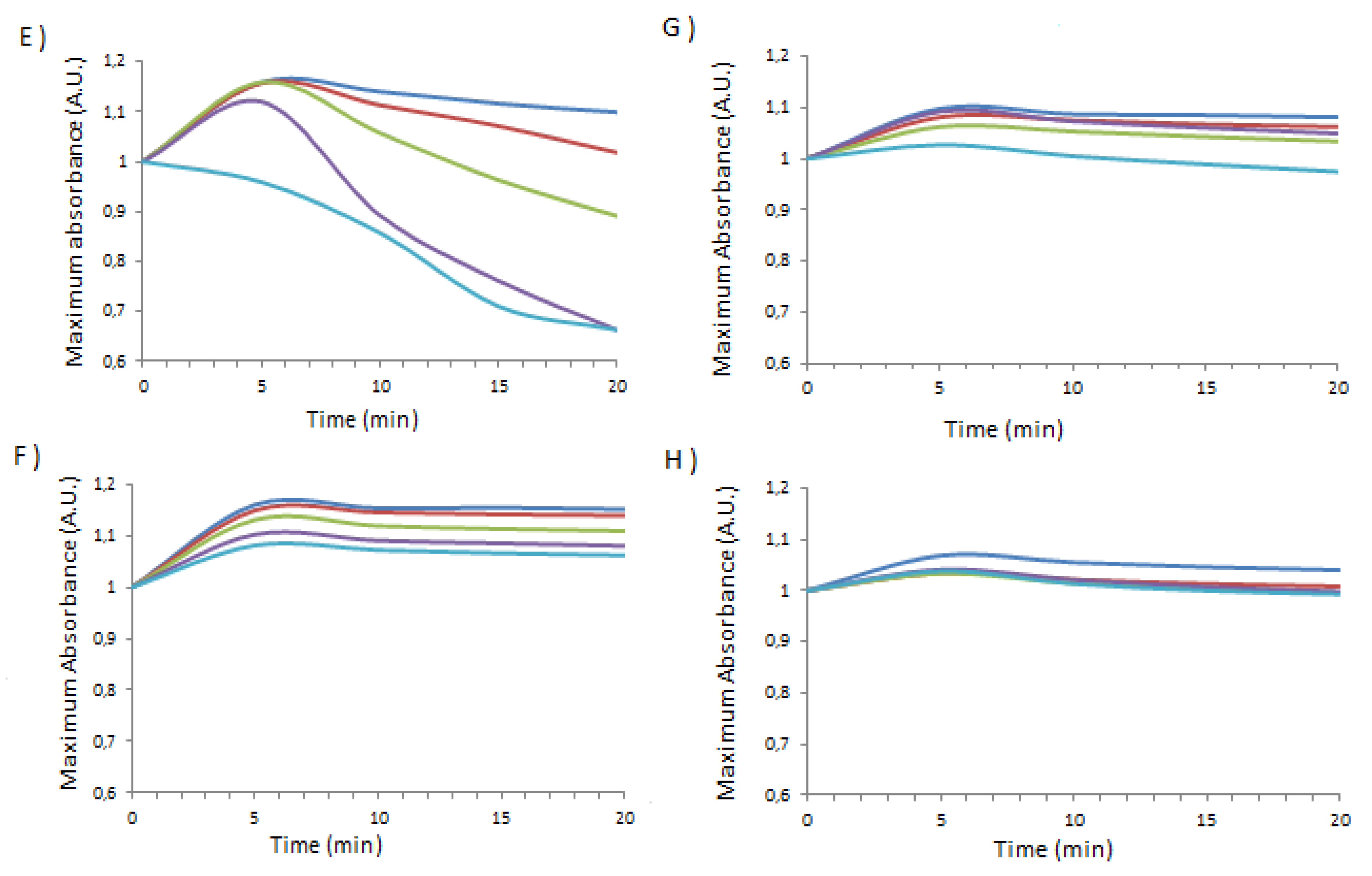
| Temperature applied (°C) | 1_Ag_NP / BSA | 1_Ag_NP / HSA | 1_Ag_NP / PSA | 1_Ag_NP / SSA | 2_Ag_NP / BSA | 2_Ag_NP / HSA | 2_Ag_NP / PSA | 2_Ag_NP / SSA |
|---|---|---|---|---|---|---|---|---|
| 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 30 | 0.3 | −0.5 | −3 | −5.62 | −0.17 | −0.95 | −1.55 | −3.18 |
| 40 | 1.93 | −1.1 | −5.6 | −3.13 | 0 | −2.5 | −3.28 | −3.28 |
| 50 | −8.08 | −1.71 | −12.61 | −12.46 | −3.36 | −5 | −0.55 | −2.53 |
| 60 | −14.26 | 0.8 | −11.95 | −16.16 | −17.26 | −6.81 | −6.47 | −2.81 |
| Temperature applied (°C) | 1_Ag_NP / BSA | 1_Ag_NP / HSA | 1_Ag_NP / PSA | 1_Ag_NP / SSA | 2_Ag_NP / BSA | 2_Ag_NP / HSA | 2_Ag_NP / PSA | 2_Ag_NP / SSA |
|---|---|---|---|---|---|---|---|---|
| 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 30 | −0.78 | −0.7 | −4.14 | −7.74 | −4.03 | −1.13 | −1.55 | −3.15 |
| 40 | −1.04 | −1.21 | −5.81 | −6.93 | −13.71 | −3.55 | −3.28 | −3.92 |
| 50 | −11.17 | −1.64 | −15.46 | −16.95 | −31.81 | −5.98 | −0.55 | −3.73 |
| 60 | −18.52 | 3.3 | −14.57 | −20.87 | −36.38 | −7.63 | −6.47 | −4.4 |
3. Experimental
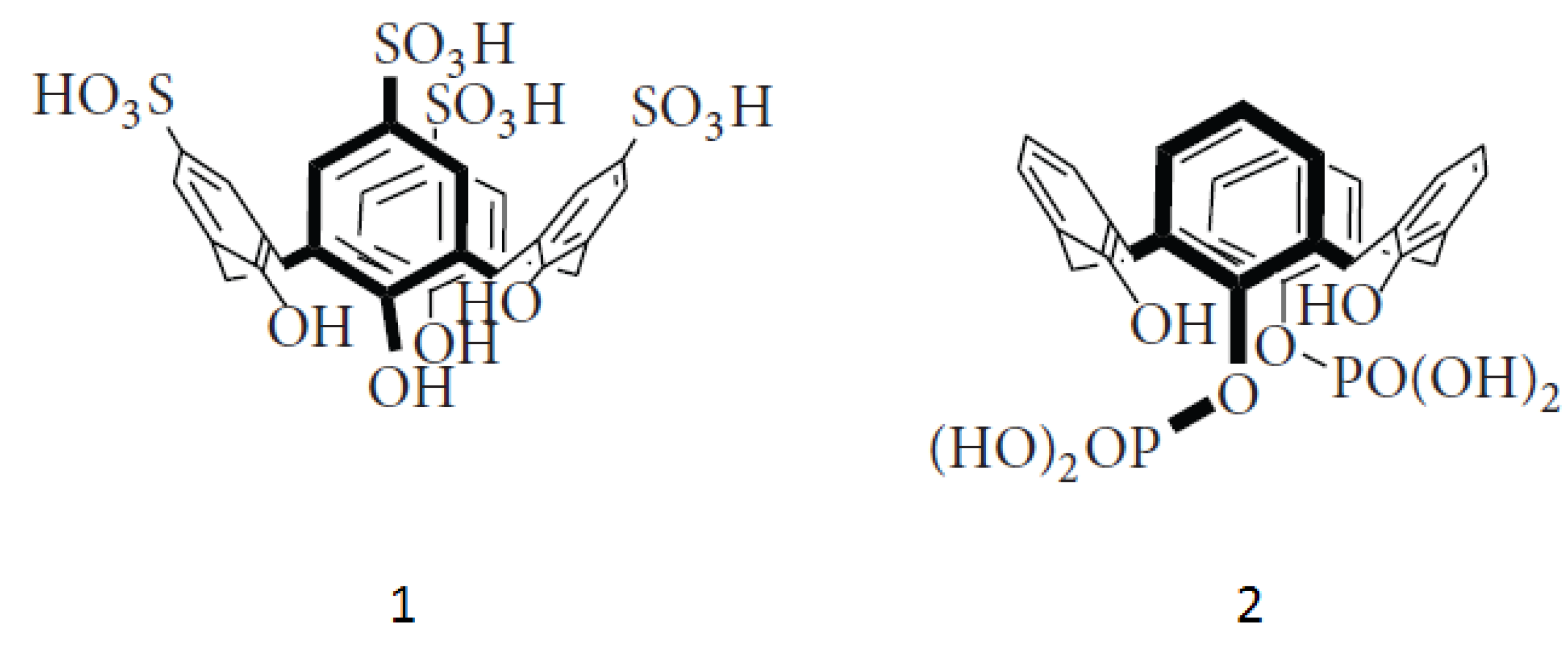
3.1. Synthesis
3.2. Nanoparticle Preparation and Characterization
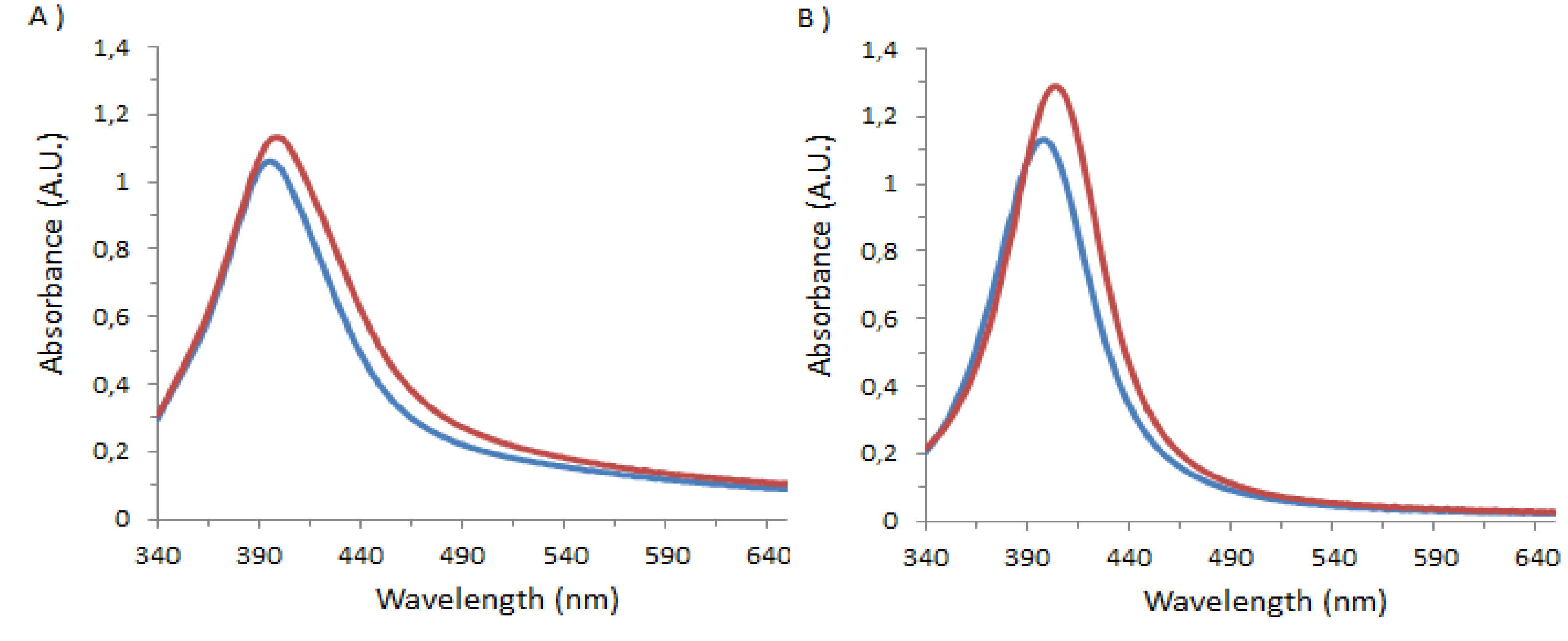
3.3. Fluorimetry Experiments
3.4. Thermal Complexation Titrations
4. Conclusion
Conflicts of Interest
References
- FSA statement on horse meat investigation. Food Standard Agency, 8 February 2013.
- Shen, S.; Lee, M. Yum’s China chicken antibiotics within limits: Shanghai government. Reuters Agency, 24 December 2012. [Google Scholar]
- Dunmore, C.; Croft, A. Horsemeat scandal set to spur tougher EU food tests. Reuters Agency, 13 February 2013. [Google Scholar]
- How the horsemeat scandal unfolded—Timeline. The Guardian. Press Association (London: Guardian News and Media), 15 February 2013.
- Reilly, A. CEO Statement to the Joint Oireachtas Committee on Agriculture, Food and the Marine. Food Safety Authority of Ireland, 5 February 2013. [Google Scholar]
- Supplier of halal meat containing pork DNA is named. BBC Press Association, 3 February 2013.
- FSAI Survey Finds Horse DNA in Some Beef Burger Products. Food Safety Authority of Ireland, 15 January 2013.
- Winter, A.K.; Thomsen, P.D. A comparison of DNA-hybridization, immunodiffusion, countercurrent immunoelectrophoresis and isoelectric focusing for detecting the admixture of pork to beef. Meat Sci. 1990, 27, 75–85. [Google Scholar] [CrossRef]
- Curry, S.; Brick, P.; Franks, N.P. Fatty Acid Binding to Human Serum Albumin: New Insights from Crystallographic Studies. Biochim. Biophys. Acta 1999, 1441, 131–140. [Google Scholar] [CrossRef]
- Bujacz, A. Structures of bovine, equine and leporine serum albumin. Acta Crystallogr. Sect. D Biol. Crystallogr. 2012, D68, 1278–1289. [Google Scholar] [CrossRef]
- Perret, F.; Coleman, A.W. Biochemistry of the anionic calix[n]arenes. Chem. Commun. 2011, 47, 7303–7319. [Google Scholar] [CrossRef]
- Da Silva, E.; Ficheux, D.; Coleman, A.W. Anti-thrombotic activity of water-soluble calix[n] arenes. J. Inclusion Phenom. Macrocyclic Chem. 2005, 52, 201–206. [Google Scholar] [CrossRef]
- Matar-Merheb, R.; Rhimi, M.; Leydier, A.; Huche, F.; Galian, C.; Desuzinges-Mandon, E.; Ficheux, D.; Flot, D.; Aghajari, N.; Kahn, R.; et al. Structuring Detergents for Extracting and Stabilizing Functional Membrane Proteins. PLoS One 2011, 6, e18036. [Google Scholar] [CrossRef]
- Atwood, J.L.; Bridges, R.J.; Juneja, R.K.; Singh, A.K. Calixarene chloride channel blockers. U.S. Patent 5489612, 1996. [Google Scholar]
- Beshara, C.S.; Jones, C.E.; Daze, K.D.; Lilgert, B.J.; Hof, F. A Simple Calixarene Recognizes Post-translationally Methylated Lysine. ChemBioChem 2010, 11, 63–66. [Google Scholar]
- Coleman, A.W.; Perret, F.; Cecillon, S.; Moussa, A.; Martin, A.; Dupin, M.; Perron, H. Enhanced detection of the pathogenic prion protein by its supramolecular association with para-sulfonato-calix[n]arene derivatives. New J. Chem. 2007, 31, 711–717. [Google Scholar] [CrossRef]
- Cecillon, S.; Coleman, A.W.; Eveno-Nobile, A.; Perron, H.; Rodrigue, M. Method for detecting aggregate-forming circulating protein forms and agent for capturing formed aggregates. U.S. Patent 8158441, 2006. [Google Scholar]
- Memmi, L.; Lazar, A.; Brioude, A.; Ball, V.; Coleman, A.W. Protein–calixarene interactions: Complexation of Bovine Serum Albumin by sulfonatocalix[n]arenes. Chem. Commun. 2001, 23, 2474–2475. [Google Scholar]
- Da Silva, E.; Rousseau, C.F.; Zanella-Cleon, I.; Becchi, M.; Coleman, A.W. Mass Spectrometric Determination of Association Constants of Bovine Serum Albumin (BSA) with para-Sulphonato-Calix[n]arene Derivatives. J. Inclusion Phenom. Macrocyclic Chem. 2006, 54, 53–59. [Google Scholar] [CrossRef]
- Xiong, D.; Chen, M.; Li, H. Synthesis of para-sulfonatocalix[4]arene-modified silver nanoparticles as colorimetric histidine probes. Chem. Commun. 2008, 7, 880–882. [Google Scholar] [CrossRef]
- Tauran, Y.; Grosso, M.; Brioude, A.; Kassab, R.; Coleman, A.W. Colourimetric and spectroscopic discrimination between nucleotides and nucleosides using para-sulphonato-calix[4]arene capped silver nanoparticles. Chem. Commun. 2011, 47, 10013–10015. [Google Scholar] [CrossRef]
- Perret, F.; Tauran, Y.; Suwinska, K.; Kim, B.J.; Chassain-Nely, C.; Boulet, M.; Coleman, A.W. Molecular recognition and transport of active pharmaceutical ingredients on anionic calix[4]arene-capped silver nanoparticles. J. Chem. 2013, 2013. [Google Scholar] [CrossRef]
- Coleman, A.W.; Jebors, S.; Cecillon, S.; Perret, P.; Garin, D.; Marti-Battle, D.; Moulin, M. Toxicity and biodistribution of para-sulfonato-calix[4]arene in mice. New J. Chem. 2008, 32, 780–782. [Google Scholar] [CrossRef]
- Da Silva, E.; Coleman, A.W. Synthesis and complexation properties towards amino acids of mono-substituted p-sulphonato-calix-[n]-arenes. Tetrahedron 2003, 59, 7357–7364. [Google Scholar] [CrossRef]
- Selkti, M.; Tomas, A.; Coleman, A.W.; Douteau-Guevel, N.; Nicolis, I.; Villain, F.; de Rango, C. The first example of a substrate spanning the calix[4]arene bilayer: the solid state complex of p-sulfonatocalix[4]arene with L-lysine. Chem. Commun. 2000, 161–162. [Google Scholar]
- Adina, L.; Da Silva, E.; Navaza, A.; Barbey, C.; Coleman, A.W. A new packing motif for para-sulfonatocalix[4]arene: the solid state structure of the para-sulfonatocalix[4]arene D-arginine complex. Chem. Comm. 2004, 2162–2163. [Google Scholar]
- Perret, F.; Morel-Desrosiers, N.; Ficheux, D.; Coleman, A.W. An ESI/MS study of the formation of ternary 25,27-bis(dihydroxy-phosphoryloxy) calix[4]arene- metal ion-aminoacid complexes. J. Supramol. Chem. 2004, 2, 533–536. [Google Scholar]
- Lazar, A.N.; Danylyuk, O.; Suwinska, K.; Coleman, A.W. Hydrogen Bonding Effects in the structure of Calix[4]arene dihydroxyphosphonic acid and L-Lysine. J. Mol. Struct. 2006, 825, 20–25. [Google Scholar] [CrossRef]
- Sugio, S.; Kashima, A.; Mochizuki, S.; Noda, M.; Kobayashi, K. Crystal structure of human serum albumin at 2.5 Å resolution. Protein Eng. 1999, 12, 439–446. [Google Scholar] [CrossRef]
- Benesi, H.; Hildebrand, J. A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J. Am. Chem. Soc. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]
- Aoki, K.; Hiramatsu, K.; Kimura, K.; Kaneshina, S.; Nakamura, Y.; Sato, K. Heat Denaturation of Bovine Serum Albumin. I. Analysis by Acrylamide-gel Electrophoresis. Bull. Inst. Chem. Res. 1969, 47, 274–282. [Google Scholar]
- Wetzel, R.; Becker, M.; Behlke, J.; Billwitz, H.; Böhm, S.; Ebert, B.; Hamann, H.; Krumbiegel, J.; Lassmann, G. Temperature behaviour of human serum albumin. Eur. J. Biochem. 1980, 104, 469–478. [Google Scholar] [CrossRef]
- Saguer, E.; Alvarez, P.; Ismail, A.A. Heat-induced denaturation/aggregation of porcine plasma and its fractions studied by FTIR spectroscopy. Food Hydrocolloids 2012, 27, 208–219. [Google Scholar] [CrossRef]
- Ghazaei, C.; Ahmadi, M.; Hosseini, J.N. Optimization and comparative characterization of neuraminidase activities from Pseudomonas aeruginosa with Klebsiella pneumoniae,Hep-2 cell, sheep kidney and rat liver lysosome. Iran. J. Microbiol. 2010, 2, 33–40. [Google Scholar]
- Kalchenko, V.I.; Rudkevich, D.M.; Markovskii, L.N. Phosphorylation of 3,5,10,12,17,19,24,26-octahydroxy-1,8,14,22-tetramethyl[1–4]metacyclophane. Zh. Obshch. Khim. 1990, 60, 2813–2814. [Google Scholar]
- Sample Availability: Samples of the compounds 1 and 2 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tauran, Y.; Brioude, A.; Kim, B.; Perret, F.; Coleman, A.W. Anionic Calixarene-Capped Silver Nanoparticles Show Species-Dependent Binding to Serum Albumins. Molecules 2013, 18, 5993-6007. https://doi.org/10.3390/molecules18055993
Tauran Y, Brioude A, Kim B, Perret F, Coleman AW. Anionic Calixarene-Capped Silver Nanoparticles Show Species-Dependent Binding to Serum Albumins. Molecules. 2013; 18(5):5993-6007. https://doi.org/10.3390/molecules18055993
Chicago/Turabian StyleTauran, Yannick, Arnaud Brioude, Beomjoon Kim, Florent Perret, and Anthony W. Coleman. 2013. "Anionic Calixarene-Capped Silver Nanoparticles Show Species-Dependent Binding to Serum Albumins" Molecules 18, no. 5: 5993-6007. https://doi.org/10.3390/molecules18055993
APA StyleTauran, Y., Brioude, A., Kim, B., Perret, F., & Coleman, A. W. (2013). Anionic Calixarene-Capped Silver Nanoparticles Show Species-Dependent Binding to Serum Albumins. Molecules, 18(5), 5993-6007. https://doi.org/10.3390/molecules18055993