Isotope Effects as Probes for Enzyme Catalyzed Hydrogen-Transfer Reactions
Abstract
:1. Introduction
2. Theory of KIEs
2.1. Semi-Classical Models of KIEs

 is the equilibrium constant between the TS and GS,
is the equilibrium constant between the TS and GS, 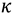 is the transmission coefficient (which can be smaller than unity due to friction [19] or recrossing [20], or be larger than unity due to tunneling as discussed below), T is the absolute temperature, h is Planck’s constant and kB is Boltzmann’s constant. The equilibrium constant,
is the transmission coefficient (which can be smaller than unity due to friction [19] or recrossing [20], or be larger than unity due to tunneling as discussed below), T is the absolute temperature, h is Planck’s constant and kB is Boltzmann’s constant. The equilibrium constant,  can be expressed in terms of partition functions as follows:
can be expressed in terms of partition functions as follows: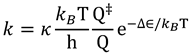
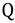 and
and 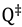 are the total partition functions of the GS and TS respectively and
are the total partition functions of the GS and TS respectively and 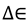 is the energy difference between the TS and GS. A KIE is the ratio of rate constants for a reaction involving a light isotope (
is the energy difference between the TS and GS. A KIE is the ratio of rate constants for a reaction involving a light isotope ( 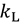 ) and a heavy isotope (
) and a heavy isotope ( 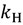 ). Taking this ratio using the TST rate constants of Equation (2) yields the Bigeleisen equation [21,22]:
). Taking this ratio using the TST rate constants of Equation (2) yields the Bigeleisen equation [21,22]: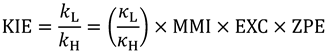
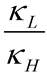 is the ratio of transmission coefficients, which semi-classically (no tunneling) is close to unity. MMI, the “Mass Moment of Inertia” term, refers to the isotope effect on translation and rotation. In the vast majority of reactions, isotope effects on translation and rotation are very small because isotopic substitution does not significantly perturb the system’s overall mass or moment of inertia, so the MMI term is usually smaller than one but close to unity [23]. EXC refers to the isotopic variations on excited vibrational levels. This term is bit larger than one but close to unity, since excited vibrational states have very small populations even at relatively high temperatures. The product of these two terms (MMI and EXC) is very close to unity and is usually negligible for hydrogen KIEs, but may make a more significant contribution to heavy atom KIEs because the relative contributions from other effects is smaller. ZPE is the contribution arising from the isotopic difference in vibrational zero-point energies, and is the primary contributor to KIEs in semi-classical models. Heavier isotopes have lower vibrational ZPEs at both the GS and TS, requiring different amounts of thermal activation to reach the TS. Thus, Equation (3) can be approximated as follows:
is the ratio of transmission coefficients, which semi-classically (no tunneling) is close to unity. MMI, the “Mass Moment of Inertia” term, refers to the isotope effect on translation and rotation. In the vast majority of reactions, isotope effects on translation and rotation are very small because isotopic substitution does not significantly perturb the system’s overall mass or moment of inertia, so the MMI term is usually smaller than one but close to unity [23]. EXC refers to the isotopic variations on excited vibrational levels. This term is bit larger than one but close to unity, since excited vibrational states have very small populations even at relatively high temperatures. The product of these two terms (MMI and EXC) is very close to unity and is usually negligible for hydrogen KIEs, but may make a more significant contribution to heavy atom KIEs because the relative contributions from other effects is smaller. ZPE is the contribution arising from the isotopic difference in vibrational zero-point energies, and is the primary contributor to KIEs in semi-classical models. Heavier isotopes have lower vibrational ZPEs at both the GS and TS, requiring different amounts of thermal activation to reach the TS. Thus, Equation (3) can be approximated as follows: 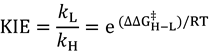
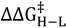 is the difference in the free energy of activation between isotopologues.
is the difference in the free energy of activation between isotopologues.2.2. Primary Kinetic Isotope Effects
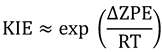
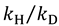 and
and 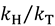 , respectively [23].
, respectively [23].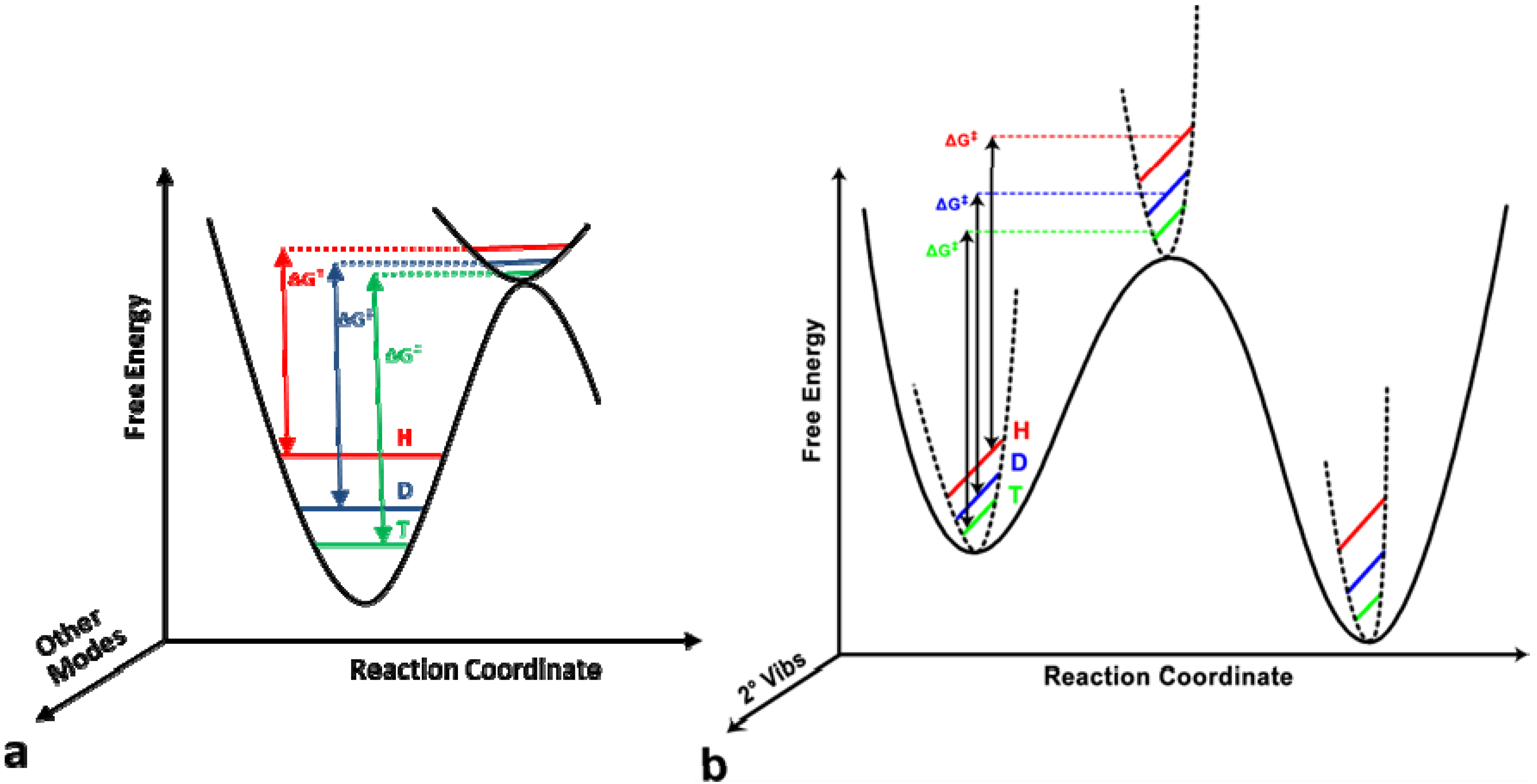
2.3. Secondary KIEs
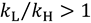 ; and “inverse” when heavier isotopes react faster than lighter isotopes
; and “inverse” when heavier isotopes react faster than lighter isotopes 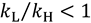 . An inverse KIE indicates that the ZPE of the isotopically sensitive modes increases in going from reactants to TS.
. An inverse KIE indicates that the ZPE of the isotopically sensitive modes increases in going from reactants to TS.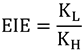
2.4. Intrinsic KIEs
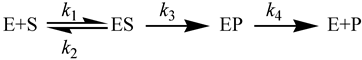
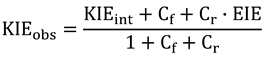
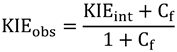
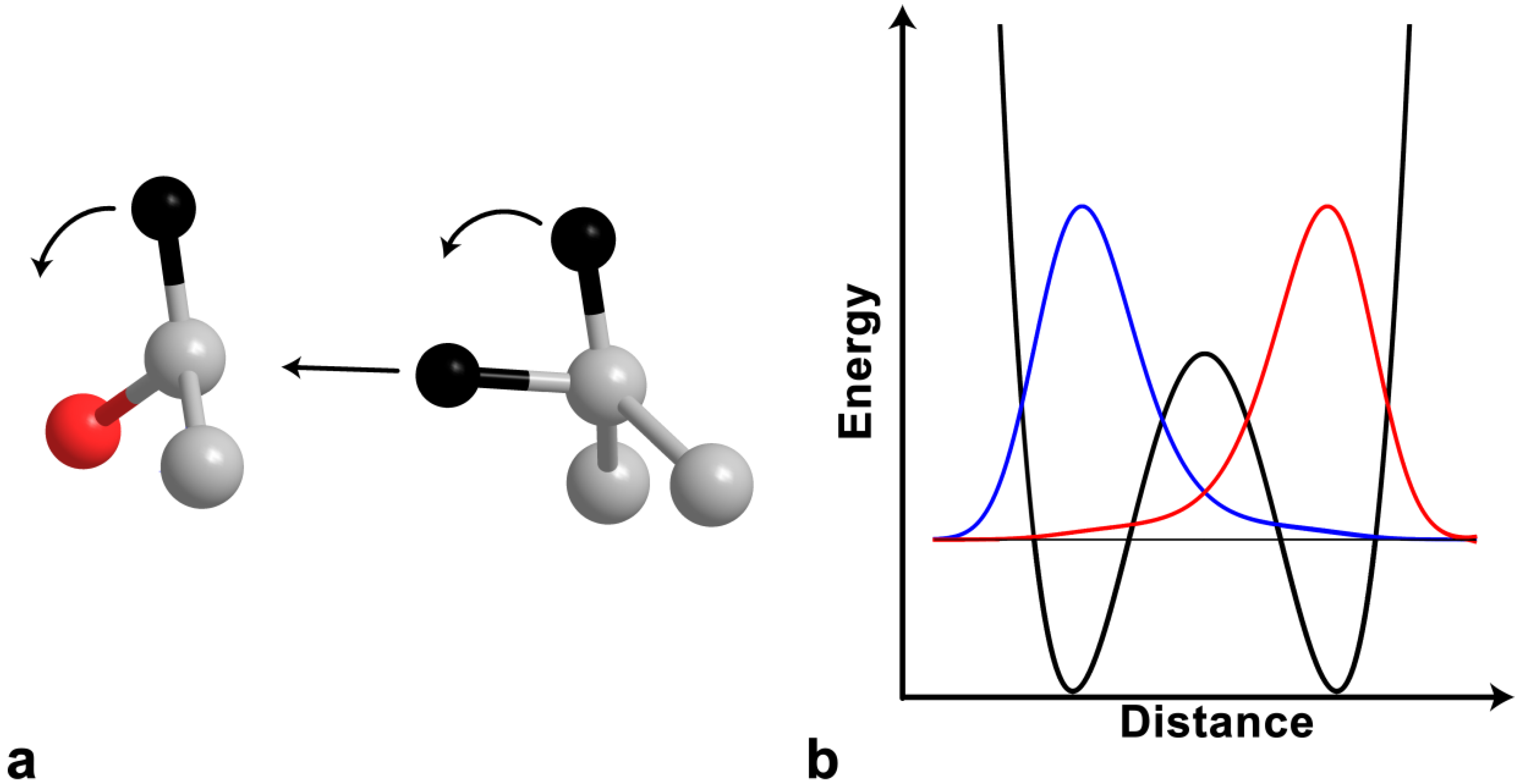
2.5. Swain-Schaad Relationships
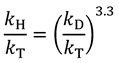
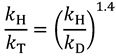
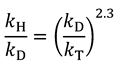
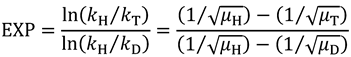

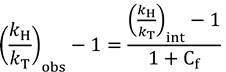
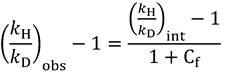
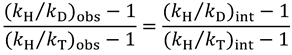

 and
and 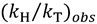 , one can assess
, one can assess 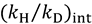 . A recent study showed the usefulness of all three combinations of hydrogen isotopes in assessing intrinsic KIEs for an enzyme affected by kinetic complexity [33]. The limitations and assumption associated with this method are discussed in detail in ref. [33] and many cited therein.
. A recent study showed the usefulness of all three combinations of hydrogen isotopes in assessing intrinsic KIEs for an enzyme affected by kinetic complexity [33]. The limitations and assumption associated with this method are discussed in detail in ref. [33] and many cited therein.2.6. Breakdown of the Semi-Classical Model: Quantum Tunneling
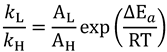
2.7. Models of Tunneling
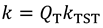
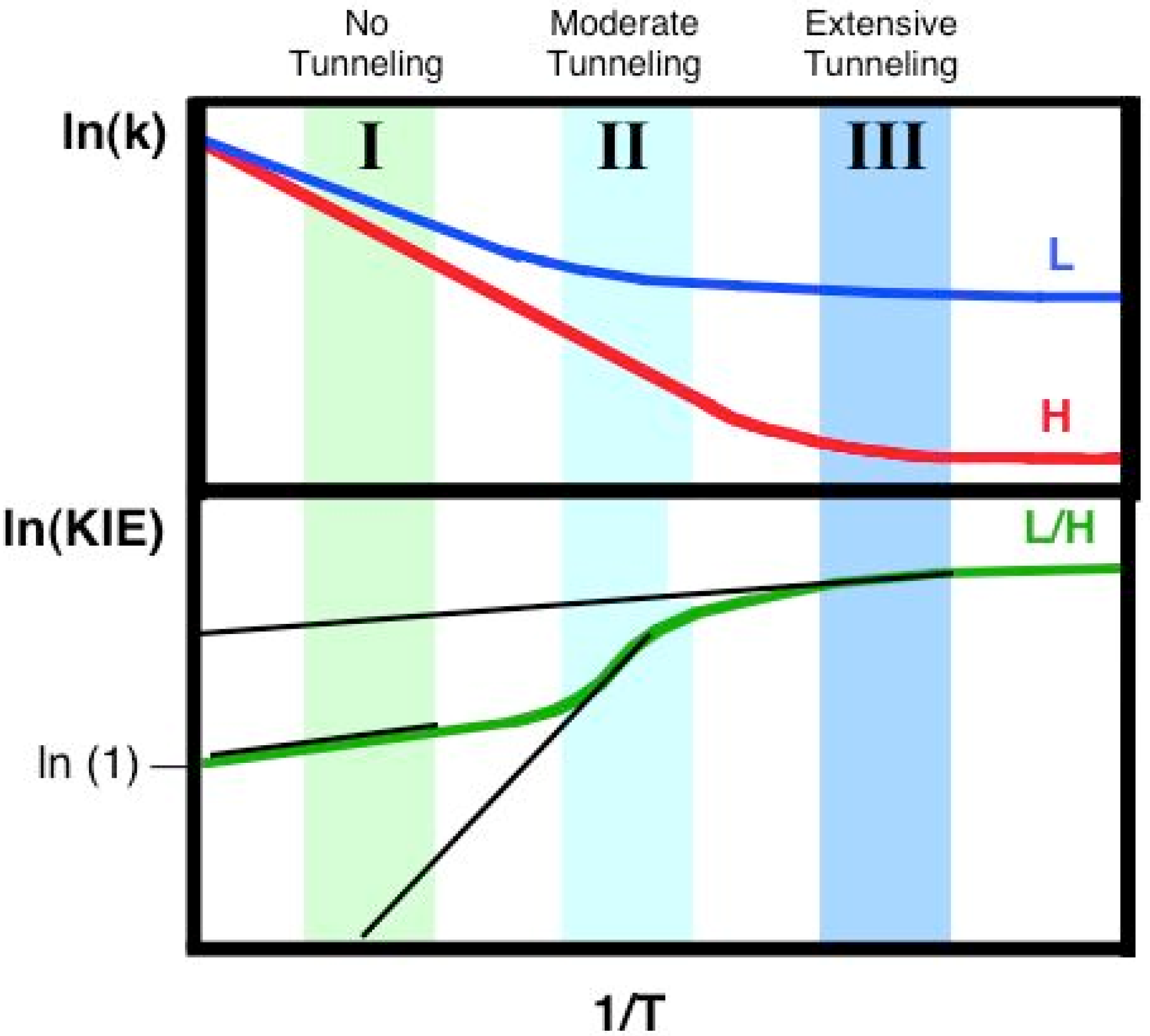
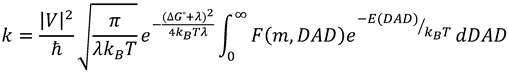

3. Alcohol Dehydrogenase
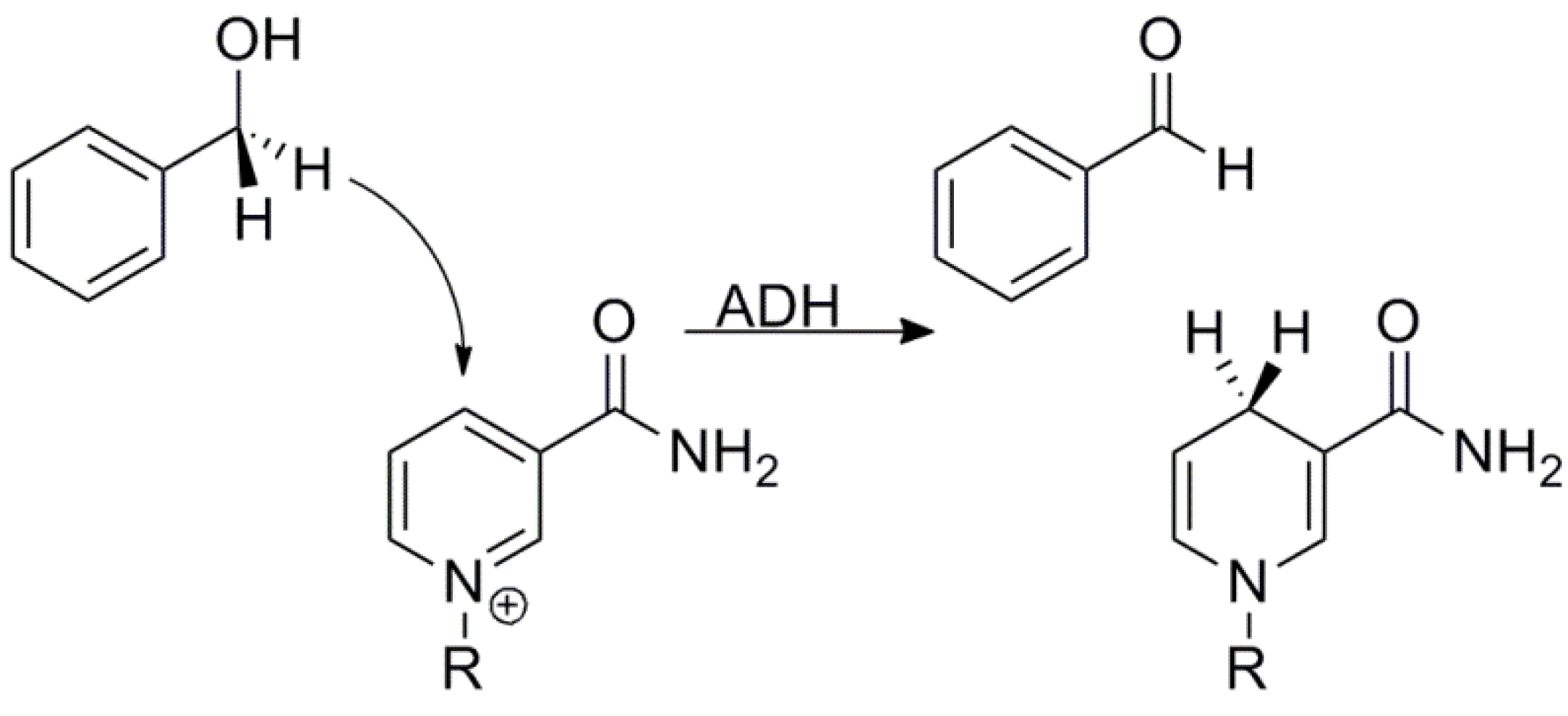

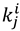 indicates the rate with isotope i at the 1° position and isotope j at the 2° position. Semi-classical models typically predict that mSSE = SSE = 3.3, although some calculations have suggested that even without tunneling the SSE can be somewhat larger than that [27,67]. No semi-classical models, however, can explain the experimental value in yADH, which was over 10, clearly supporting the theory of tunneling and coupled motion [34].
indicates the rate with isotope i at the 1° position and isotope j at the 2° position. Semi-classical models typically predict that mSSE = SSE = 3.3, although some calculations have suggested that even without tunneling the SSE can be somewhat larger than that [27,67]. No semi-classical models, however, can explain the experimental value in yADH, which was over 10, clearly supporting the theory of tunneling and coupled motion [34].4. Thymidylate Synthase
4.1. Hydride and Proton Transfers
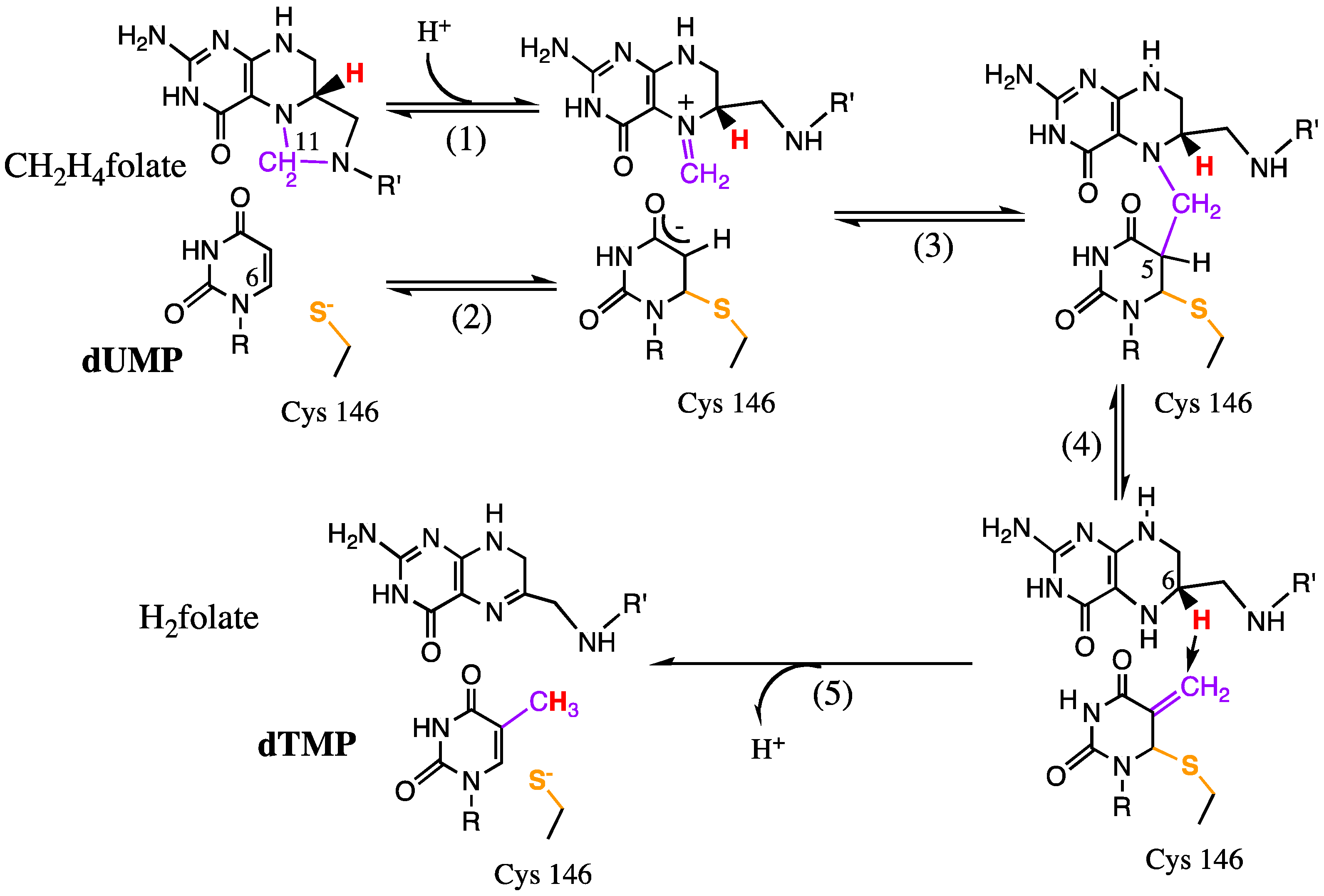
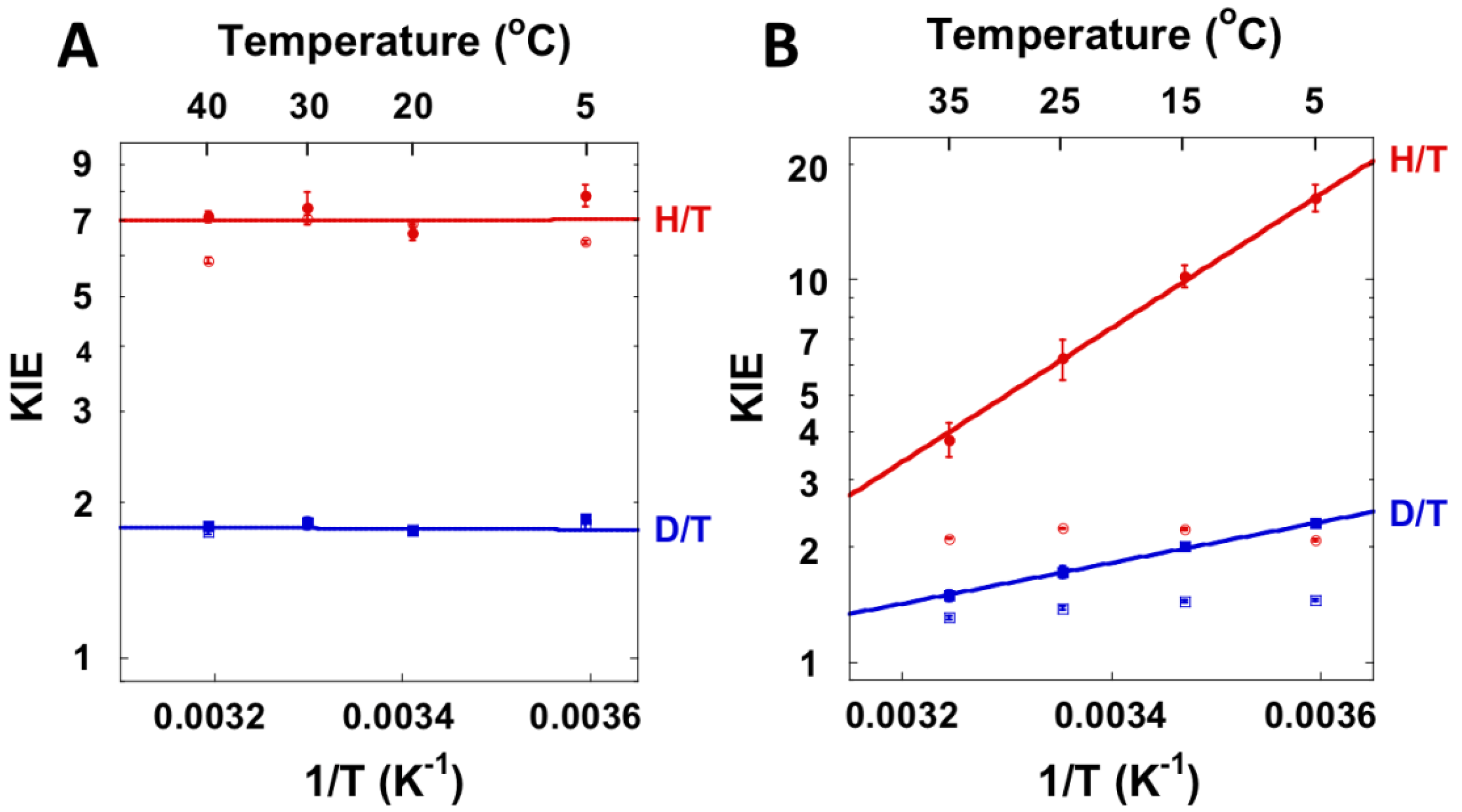
4.2. Mutagenesis Studies
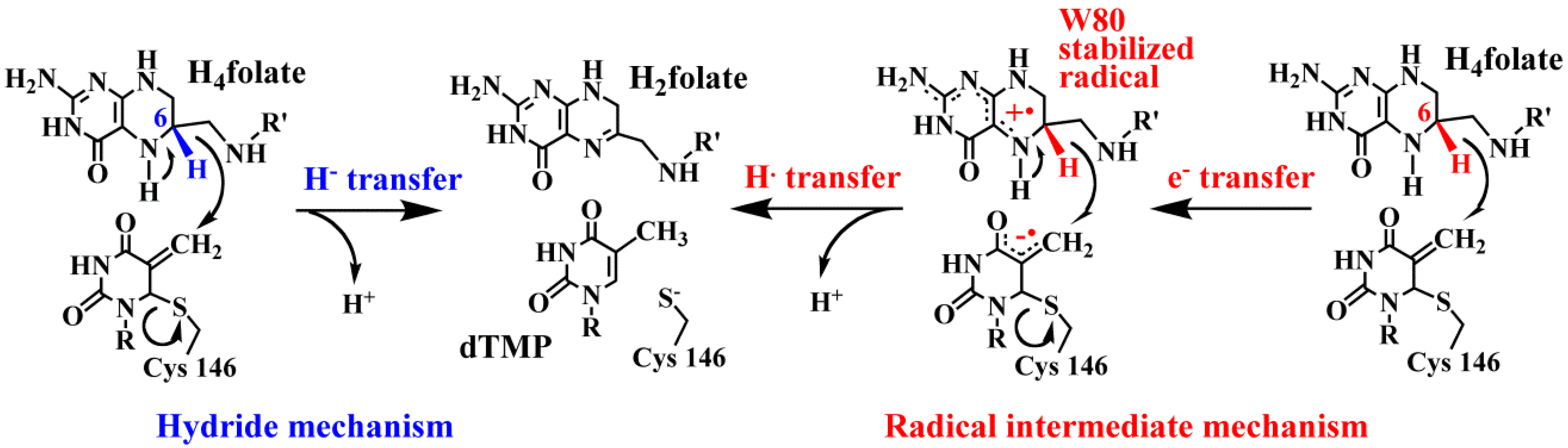
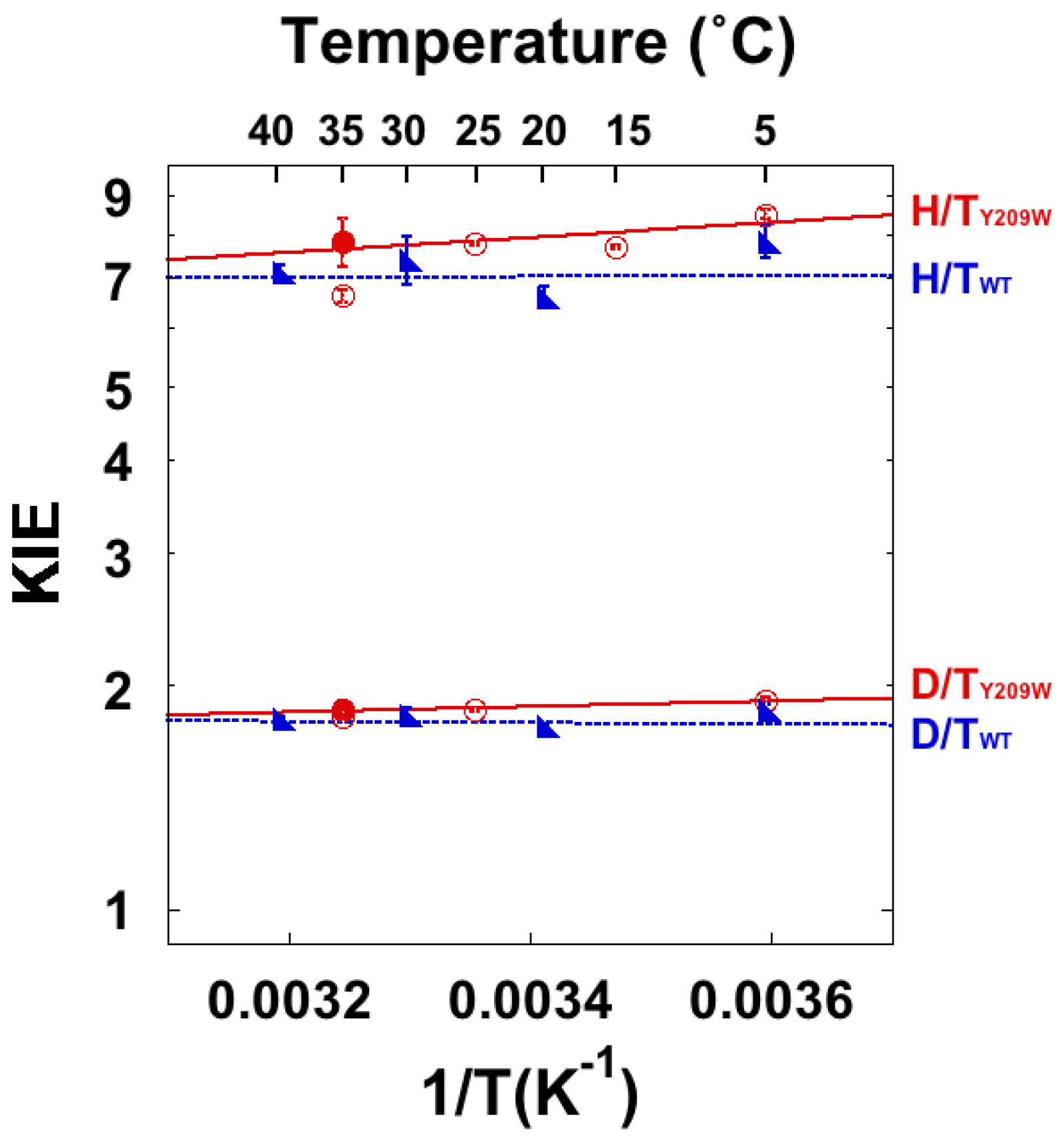
4.3. High-Level Simulations
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Schramm, V.L. Enzymatic transition states, transition-state analogs, dynamics, thermodynamics, and lifetimes. Annu. Rev. Biochem. 2011, 80, 703–732. [Google Scholar] [CrossRef]
- Truhlar, D.G. Tunneling in enzymatic and nonenzymatic hydrogen transfer reactions. J. Phys. Org. Chem. 2010, 23, 660–676. [Google Scholar] [CrossRef]
- Sen, A.; Kohen, A. Enzymatic tunneling and kinetic isotope effects: Chemistry at the crossroads. J. Phys. Org. Chem. 2010, 23, 613–619. [Google Scholar] [CrossRef]
- Warshel, A.; Sharma, P.K.; Kato, M.; Xiang, Y.; Liu, H.B.; Olsson, M.H.M. Electrostatic basis for enzyme catalysis. Chem. Rev. 2006, 106, 3210–3235. [Google Scholar] [CrossRef]
- Hammes-Schiffer, S. Hydrogen tunneling and protein motion in enzyme reactions. Acc. Chem. Res. 2006, 39, 93–100. [Google Scholar] [CrossRef]
- Schwartz, S.D. Vibrationally enhanced tunneling from the temperature dependence of KIE. In Isotope Effects in Chemistry and Biology; Kohen, A., Limbach, H.H., Eds.; Taylor & Francis,CRC Press: Boca Raton, FL, USA, 2006; pp. 475–498. [Google Scholar]
- Nagel, Z.D.; Klinman, J.P. Update 1 of: Tunneling and dynamics in enzymatic hydride transfer. Chem. Rev. 2010, 110, PR41–PR67. [Google Scholar] [CrossRef]
- Wang, Z.; Roston, D.; Kohen, A. Experimental and theoretical studies of enzyme-catalyzed hydrogen-transfer reactions. In Structural and Mechanistic Enzymology: Bringing Together Experiments and Computing; Christov, C., Karabencheva-Christova, T., Eds.; Elsevier Academic Press Inc: San Diego, CA,USA, 2012; pp. 155–180. [Google Scholar]
- Nagel, Z.D.; Klinman, J.P. A 21st century revisionist’s view at a turning point in enzymology. Nat. Chem. Biol. 2009, 5, 543–550. [Google Scholar] [CrossRef]
- Kamerlin, S.C.L.; Warshel, A. At the dawn of the 21st century: Is dynamics the missing link for understanding enzyme catalysis? Proteins 2010, 78, 1339–1375. [Google Scholar]
- Hay, S.; Scrutton, N.S. Good vibrations in enzyme-catalysed reactions. Nat. Chem. 2012, 4, 161–168. [Google Scholar] [CrossRef]
- Glowacki, D.R.; Harvey, J.N.; Mulholland, A.J. Taking ockham's razor to enzyme dynamics and catalysis. Nat. Chem. 2012, 4, 169–176. [Google Scholar] [CrossRef]
- Editorial. Of polemics and progress. Nat. Chem. 2012, 4, 141–141. [CrossRef]
- Boekelheide, N.; Salomon-Ferrer, R.; Miller, T.F. Dynamics and dissipation in enzyme catalysis. Proc. Natl. Acad. Sci. USA 2011, 108, 16159–16163. [Google Scholar] [CrossRef]
- Bhabha, G.; Lee, J.; Ekiert, D.C.; Gam, J.; Wilson, I.A.; Dyson, H.J.; Benkovic, S.J.; Wright, P.E. A dynamic knockout reveals that conformational fluctuations influence the chemical step of enzyme catalysis. Science 2011, 332, 234–238. [Google Scholar]
- Adamczyk, A.J.; Cao, J.; Kamerlin, S.C.L.; Warshel, A. Catalysis by dihydrofolate reductase and other enzymes arises from electrostatic preorganization, not conformational motions. Proc. Natl. Acad. Sci. USA 2011, 108, 14115–14120. [Google Scholar]
- Dametto, M.; Antoniou, D.; Schwartz, S.D. Barrier crossing in dihydrofolate reductase does not involve a rate-promoting vibration. Mol. Phys. 2012, 110, 531–536. [Google Scholar] [CrossRef]
- Kelly, K.K.; Hirschi, J.S.; Singleton, D.A. Newtonian kinetic isotope effects. Observation, prediction, and origin of heavy-atom dynamic isotope effects. J. Am. Chem. Soc. 2009, 131, 8382–8383. [Google Scholar]
- Kramers, H.A. Brownian motion in a field of force and the diffusion model of chemical reactions. Physica 1940, 7, 284–304. [Google Scholar]
- Pu, J.; Gao, J.; Truhlar, D.G. Multidimensional tunneling, recrossing, and the transmission coefficient for enzymatic reaction. Chem. Rev. 2006, 106, 3140–3169. [Google Scholar] [CrossRef]
- Bigeleisen, J.; Mayer, M.G. Calculation of equilibrium constants for isotopic exchange reactions. J. Chem. Phys. 1947, 15, 261–267. [Google Scholar] [CrossRef]
- Bigeleisen, J. The relative reaction velocities of isotopic molecules. J. Chem. Phys. 1949, 17, 675–678. [Google Scholar]
- Melander, L.; Saunders, W.H. Reaction Rates of Isotopic Molecules, 4th ed; Wiley: New York, NY, USA, 1987. [Google Scholar]
- Westheimer, F.H. The magnitude of the primary kinetic isotope effect for compounds of hydrogen and deuterium. Chem. Rev. 1961, 61, 265–273. [Google Scholar]
- Streitwieser, A.; Jagow, R.H.; Fahey, R.C.; Suzuki, S. Kinetic isotope effects in the acetolyses of deuterated cyclopentyl tosylates. J. Am. Chem. Soc. 1958, 80, 2326–2332. [Google Scholar]
- Cook, P.F.; Oppenheimer, N.J.; Cleland, W.W. Secondary deuterium and n-15 isotope effects in enzyme-catalyzed reactions - chemical mechanism of liver alcohol-dehydrogenase. Biochemistry 1981, 20, 1817–1825. [Google Scholar]
- Kohen, A.; Jensen, J.H. Boundary conditions for the swain-schaad relationship as a criterion for hydrogen tunneling. J. Am. Chem. Soc. 2002, 124, 3858–3864. [Google Scholar]
- Kurz, L.C.; Frieden, C. Anomalous equilibrium and kinetic alpha-deuterium secondary isotope effects accompanying hydride transfer from reduced nicotinamide adenine-dinucleotide. J. Am. Chem. Soc. 1980, 102, 4198–4203. [Google Scholar]
- Hermes, J.D.; Morrical, S.W.; O'Leary, M.H.; Cleland, W.W. Variation of transition-state structure as a function of the nucleotide in reactions catalyzed by dehydrogenases. 2. Formate dehydrogenase. Biochemistry 1984, 23, 5479–5488. [Google Scholar]
- Cook, P.F.; Cleland, W.W. Enzyme Kinetics and Mechanism; Taylor & Francis Group: New York, NY, USA, 2007. [Google Scholar]
- Northrop, D.B. Intrinsic isotope effects in enzyme catalyzed reactions. In Enzyme Mechanism from Isotope Effects; Cook, P.F., Ed.; CRC Press: Boca Raton, FL, USA, 1991; pp. 181–202. [Google Scholar]
- Swain, C.G.; Stivers, E.C.; Reuwer, J.F.; Schaad, L.J. Use of hydrogen isotope effects to identify the attacking nucleophile in the enolization of ketones catalyzed by acetic acid. J. Am. Chem. Soc. 1958, 80, 5885–5893. [Google Scholar]
- Sen, A.; Yahashiri, A.; Kohen, A. Triple isotopic labeling and kinetic isotope effects: Exposing h-transfer steps in enzymatic systems. Biochemistry 2011, 50, 6462–6468. [Google Scholar]
- Cha, Y.; Murray, C.J.; Klinman, J.P. Hydrogen tunneling in enzyme reactions. Science 1989, 243, 1325–1330. [Google Scholar]
- Bahnson, B.J.; Park, D.H.; Kim, K.; Plapp, B.V.; Klinman, J.P. Unmasking of hydrogen tunneling in the horse liver alcohol-dehydrogenase reaction by site-directed mutagenesis. Biochemistry 1993, 32, 5503–5507. [Google Scholar]
- Kohen, A.; Cannio, R.; Bartolucci, S.; Klinman, J.P. Enzyme dynamics and hydrogen tunneling in a thermophilic alcohol dehydrogenase. Nature 1999, 399, 496–499. [Google Scholar]
- Knapp, M.J.; Rickert, K.; Klinman, J.P. Temperature-dependent isotope effects in soybean lipoxygenase-1: Correlating hydrogen tunneling with protein dynamics. J. Am. Chem. Soc. 2002, 124, 3865–3874. [Google Scholar]
- Sikorski, R.S.; Wang, L.; Markham, K.A.; Rajagopalan, P.T.; Benkovic, S.J.; Kohen, A. Tunneling and coupled motion in the escherichia coli dihydrofolate reductase catalysis. J. Am. Chem. Soc. 2004, 126, 4778–4779. [Google Scholar]
- Hay, S.; Sutcliffe, M.J.; Scrutton, N.S. Probing coupled motions in enzymatic hydrogen tunnelling reactions: Beyond temperature-dependence studies of kinetic isotope effects. In Quantum Tunnelling in Enzyme-Catalysed Reactions; Allemann, R.K., Scrutton, N.S., Eds.; Royal Society of Chemistry: Cambridge, UK, 2009; pp. 199–218. [Google Scholar]
- Stern, M.J.; Weston, R.E. Phenomenological manifestations of quantum-mechanical tunneling .2. Effect on arrhenius pre-exponential factors for primary hydrogen kinetic isotope-effects. J. Chem. Phys. 1974, 60, 2808–2814. [Google Scholar] [CrossRef]
- Bell, R.P. Tunnel-effect correction for parabolic potential barriers. Trans. Faraday Soc. 1959, 55, 1–4. [Google Scholar]
- Kohen, A.; Klinman, J.P. Hydrogen tunneling in biology. Chem. Biol. (Oxford, UK) 1999, 6, 191–198. [Google Scholar]
- Bell, R.P. The Tunnel Effect in Chemistry; Chapman & Hall: London,UK, 1980. [Google Scholar]
- Nagel, Z.D.; Klinman, J.P. Tunneling and dynamics in enzymatic hydride transfer. Chem. Rev. 2006, 106, 3095–3118. [Google Scholar]
- Pu, J.; Ma, S.; Gao, J.; Truhlar, D.G. Small temperature dependence of the kinetic isotope effect for the hydride transfer reaction catalyzed by escherichia coli dihydrofolate reductase. J. Phys. Chem. B 2005, 109, 8551–8556. [Google Scholar]
- Kanaan, N.; Ferrer, S.; Martin, S.; Garcia-Viloca, M.; Kohen, A.; Moliner, V. Temperature dependence of the kinetic isotope effects in thymidylate synthase. A theoretical study. J. Am. Chem. Soc. 2011, 133, 6692–6702. [Google Scholar]
- Liu, H.; Warshel, A. Origin of the temperature dependence of isotope effects in enzymatic reactions: The case of dihydrofolate reductase. J. Phys. Chem. B 2007, 111, 7852–7861. [Google Scholar] [CrossRef]
- Major, D.T.; Heroux, A.; Orville, A.M.; Valley, M.P.; Fitzpatrick, P.F.; Gao, J. Differential quantum tunneling contributions in nitroalkane oxidase catalyzed and the uncatalyzed proton transfer reaction. Proc. Natl. Acad. Sci. USA 2009, 106, 20734–20739. [Google Scholar]
- Fan, Y.; Cembran, A.; Ma, S.; Gao, J. Connecting protein conformational dynamics with catalytic function as illustrated in dihydrofolate reductase. Biochemistry 2013, 52, 2036–2049. [Google Scholar]
- Marcus, R.A.; Sutin, N. Electron transfers in chemistry and biology. Biochim. Biophys. Acta 1985, 811, 265–322. [Google Scholar]
- Kuznetsov, A.M.; Ulstrup, J. Proton and hydrogen atom tunnelling in hydrolytic and redox enzyme catalysis. Can. J. Chem. 1999, 77, 1085–1096. [Google Scholar]
- Kohen, A. Isotope Effects in Chemistry and Biology; Kohen, A., Limbach, H.-H., Eds.; Taylor & Francis: Boca Raton, FL, USA, 2006; pp. 743–764. [Google Scholar]
- Marcus, R.A. H and other transfers in enzymes and in solution: Theory and computations, a unified view. 2. Applications to experiment and computations. J. Phys. Chem. B 2007, 111, 6643–6654. [Google Scholar] [CrossRef]
- Roston, D.; Cheatum, C.M.; Kohen, A. Hydrogen donor-acceptor fluctuations from kinetic isotope effects: A phenomenological model. Biochemistry 2012, 51, 6860–6870. [Google Scholar]
- Knapp, M.J.; Klinman, J.P. Environmentally coupled hydrogen tunneling - linking catalysis to dynamics. Eur. J. Biochem. 2002, 269, 3113–3121. [Google Scholar] [CrossRef]
- Pudney, C.R.; Johannissen, L.O.; Sutcliffe, M.J.; Hay, S.; Scrutton, N.S. Direct analysis of donor acceptor distance and relationship to isotope effects and the force constant for barrier compression in enzymatic h-tunneling reactions. J. Am. Chem. Soc. 2010, 132, 11329–11335. [Google Scholar]
- Klinman, J.P. Linking protein structure and dynamics to catalysis: The role of hydrogen tunnelling. Philos. Trans. R. Soc. B-Biol. Sci. 2006, 361, 1323–1331. [Google Scholar] [CrossRef]
- Nagel, Z.D.; Meadows, C.W.; Dong, M.; Bahnson, B.J.; Klinman, J.P. Active site hydrophobic residues impact hydrogen tunneling differently in a thermophilic alcohol dehydrogenase at optimal versus nonoptimal temperatures. Biochemistry 2012, 51, 4147–4156. [Google Scholar]
- Kohen, A.; Roston, D.; Stojković, V.; Wang, Z. Kinetic isotope effects in enzymes. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons, Ltd: Chichester, UK, 2011; pp. 77–99. [Google Scholar]
- Klinman, J.P. Mechanism of enzyme-catalyzed reduced nicotinamide adenine dinucleotide-dependent reductions - substituent and isotope-effects in yeast alcohol-dehydrogenase reaction. J. Biol. Chem. 1972, 247, 7977–7987. [Google Scholar]
- Klinman, J.P. Isotope-effects and structure-reactivity correlations in yeast alcohol-dehydrogenase reaction - study of enzyme-catalyzed oxidation of aromatic alcohols. Biochemistry 1976, 15, 2018–2026. [Google Scholar] [CrossRef]
- Welsh, K.M.; Creighton, D.J.; Klinman, J.P. Transition-state structure in the yeast alcohol-dehydrogenase reaction - the magnitude of solvent and alpha-secondary hydrogen isotope effects. Biochemistry 1980, 19, 2005–2016. [Google Scholar]
- Cook, P.F.; Blanchard, J.S.; Cleland, W.W. Primary and secondary deuterium isotope effects on equilibrium-constants for enzyme-catalyzed reactions. Biochemistry 1980, 19, 4853–4858. [Google Scholar]
- Huskey, W.P.; Schowen, R.L. Reaction-coordinate tunneling in hydride-transfer reactions. J. Am. Chem. Soc. 1983, 105, 5704–5706. [Google Scholar] [CrossRef]
- Bigeleisen, J. Second-order sum rule for the vibrations of isotopic molecules and the second rule of the mean. J. Chem. Phys. 1958, 28, 694–699. [Google Scholar] [CrossRef]
- Saunders, W.H. Calculations of isotope effects in elimination reactions - new experimental criteria for tunneling in slow proton transfers. J. Am. Chem. Soc. 1985, 107, 164–169. [Google Scholar] [CrossRef]
- Hirschi, J.; Singleton, D.A. The normal range for secondary swain-schaad exponents without tunneling or kinetic complexity. J. Am. Chem. Soc. 2005, 127, 3294–3295. [Google Scholar] [CrossRef]
- Bahnson, B.J.; Colby, T.D.; Chin, J.K.; Goldstein, B.M.; Klinman, J.P. A link between protein structure and enzyme catalyzed hydrogen tunneling. Proc. Natl. Acad. Sci. USA 1997, 94, 12797–12802. [Google Scholar]
- Stojkovic, V.; Perissinotti, L.L.; Lee, J.; Benkovic, S.J.; Kohen, A. The effect of active-site isoleucine to alanine mutation on the dhfr catalyzed hydride-transfer. Chem. Commun. (London) 2010, 46, 8974–8976. [Google Scholar]
- Stojkovic, V.; Perissinotti, L.L.; Willmer, D.; Benkovic, S.J.; Kohen, A. Effects of the donor-acceptor distance and dynamics on hydride tunneling in the dihydrofolate reductase catalyzed reaction. J. Am. Chem. Soc. 2012, 134, 1738–1745. [Google Scholar]
- Roston, D.; Kohen, A. Elusive transition state of alcohol dehydrogenase unveiled. Proc. Natl. Acad. Sci. USA 2010, 107, 9572–9577. [Google Scholar] [CrossRef]
- Karsten, W.E.; Hwang, C.C.; Cook, P.F. Alpha-secondary tritium kinetic isotope effects indicate hydrogen tunneling and coupled motion occur in the oxidation of l-malate by nad-malic enzyme. Biochemistry 1999, 38, 4398–4402. [Google Scholar] [CrossRef]
- Pudney, C.R.; Hay, S.; Sutcliffe, M.J.; Scrutton, N.S. Alpha-secondary isotope effects as probes of "Tunneling-ready" Configurations in enzymatic h-tunneling: Insight from environmentally coupled tunneling models. J. Am. Chem. Soc. 2006, 128, 14053–14058. [Google Scholar] [CrossRef]
- Alston, W.C.; Kanska, M.; Murray, C.J. Secondary wt and d/t isotope effects in enzymatic enolization reactions. Coupled motion and tunneling in the triosephosphate isomerase reaction. Biochemistry 1996, 35, 12873–12881. [Google Scholar] [CrossRef]
- Carreras, C.W.; Santi, D.V. The catalytic mechanism and structure of thymidylate synthase. Annu. Rev. Biochem. 1995, 64, 721–762. [Google Scholar] [CrossRef]
- Sergeeva, O.A.; Khambatta, H.G.; Cathers, B.E.; Sergeeva, M.V. Kinetic properties of human thymidylate synthase, an anticancer drug target. Biochem. Biophys. Res. Commun. 2003, 307, 297–300. [Google Scholar] [CrossRef]
- Stroud, R.M.; Finer-Moore, J.S. Conformational dynamics along an enzymatic reaction pathway: Thymidylate synthase, “The movie”. Biochemistry 2003, 42, 239–247. [Google Scholar] [CrossRef]
- Finer-Moore, J.S.; Santi, D.V.; Stroud, R.M. Lessons and conclusions from dissecting the mechanism of a bisubstrate enzyme: Thymidylate synthase mutagenesis, function, and structur. Biochemistry 2003, 42, 248–256. [Google Scholar] [CrossRef]
- Bruice, T.W.; Santi, D.V. Isotope effects in reactions catalyzed by thymidylate synthase. In Enzyme Mechanism from Isotope Effects; Cook, P.F., Ed.; CRC Press: Boca Raton, FL, USA, 1991; pp. 457–479. [Google Scholar]
- Spencer, H.T.; Villafranca, J.E.; Appleman, J.R. Kinetic scheme for thymidylate synthase from escherichia coli: Determination from measurements of ligand binding, primary and secondary isotope effects and pre-steady-state catalysis. Biochemistry 1997, 36, 4212–4222. [Google Scholar]
- Agrawal, N.; Hong, B.; Mihai, C.; Kohen, A. Vibrationally enhanced hydrogen tunneling in the e. Coli thymidylate synthase catalyzed reaction. Biochemistry 2004, 43, 1998–2006. [Google Scholar] [CrossRef]
- Hong, B.; Maley, F.; Kohen, A. Role of y94 in proton and hydride transfers catalyzed by thymidylate synthase. Biochemistry 2007, 46, 14188–14197. [Google Scholar] [CrossRef]
- Wang, Z.; Kohen, A. Thymidylate synthase catalyzed h-transfers: Two chapters in one tale. J. Am. Chem. Soc. 2010, 132, 9820–9825. [Google Scholar] [CrossRef]
- Barrett, J.E.; Lucero, C.M.; Schultz, P.G. A model for hydride transfer in thymidylate synthase based on unnatural amino acid mutagenesis. J. Am. Chem. Soc. 1999, 121, 7965–7966. [Google Scholar]
- Fritz, T.A.; Liu, L.; Finer-Moore, J.S.; Stroud, R.M. Tryptophan 80 and leucine 143 are critical for the hydride transfer step of thymidylate synthase by controlling active site access. Biochemistry 2002, 41, 7021–7029. [Google Scholar]
- Hong, B.; Haddad, M.; Maley, F.; Jensen, J.H.; Kohen, A. Hydride transfer versus hydrogen radical transfer in thymidylate synthase. J. Am. Chem. Soc. 2006, 128, 5636–5637. [Google Scholar]
- Wang, Z.; Abeysinghe, T.; Finer-Moore, J.S.; Stroud, R.M.; Kohen, A. A remote mutation affects the hydride transfer by disrupting concerted protein motions in thymidylate synthase. J. Am. Chem. Soc. 2012, 134, 17722–17730. [Google Scholar]
- Newby, Z.; Lee, T.T.; Morse, R.J.; Liu, Y.; Liu, L.; Venkatraman, P.; Santi, D.V.; Finer-Moore, J.S.; Stroud, R.M. The role of protein dynamics in thymidylate synthase catalysis: Variants of conserved 2'-deoxyuridine 5'-monophosphate (dump)-binding tyr-261. Biochemistry 2006, 45, 7415–7428. [Google Scholar]
- Wang, L.; Goodey, N.M.; Benkovic, S.J.; Kohen, A. Coordinated effects of distal mutations on environmentally coupled tunneling in dihydrofolate reductase. Proc. Natl. Acad. Sci. USA 2006, 103, 15753–15758. [Google Scholar]
- Silva, R.G.; Murkin, A.S.; Schramm, V.L. Femtosecond dynamics coupled to chemical barrier crossing in a born-oppenheimer enzyme. Proc. Natl. Acad. Sci. USA 2011, 108, 18661–18665. [Google Scholar]
- Kanaan, N.; Marti, S.; Moliner, V.; Kohen, A. A quantum mechanics/molecular mechanics study of the catalytic mechanism of the thymidylate synthase. Biochemistry 2007, 46, 3704–3713. [Google Scholar]
- Kanaan, N.; Marti, S.; Moliner, V.; Kohen, A. Qm/mm study of thymidylate synthase: Enzymatic motions and the temperature dependence of the rate limiting step. J. Phys. Chem. A 2009, 113, 2176–2181. [Google Scholar]
- Wang, Z.; Ferrer, S.; Moliner, V.; Kohen, A. Qm/mm calculations suggest a novel intermediate following the proton abstraction catalyzed by thymidylate synthase. Biochemistry 2013, 52, 2348–2358. [Google Scholar]
- Siegel, J.B.; Zanghellini, A.; Lovick, H.M.; Kiss, G.; Lambert, A.R.; Clair, J.L.S.; Gallaher, J.L.; Hilvert, D.; Gelb, M.H.; Stoddard, B.L.; et al. Computational design of an enzyme catalyst for a stereoselective bimolecular diels-alder reaction. Science 2010, 329, 309–313. [Google Scholar]
- Baker, D. An exciting but challenging road ahead for computational enzyme design. Protein Sci. 2010, 19, 1817–1819. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Roston, D.; Islam, Z.; Kohen, A. Isotope Effects as Probes for Enzyme Catalyzed Hydrogen-Transfer Reactions. Molecules 2013, 18, 5543-5567. https://doi.org/10.3390/molecules18055543
Roston D, Islam Z, Kohen A. Isotope Effects as Probes for Enzyme Catalyzed Hydrogen-Transfer Reactions. Molecules. 2013; 18(5):5543-5567. https://doi.org/10.3390/molecules18055543
Chicago/Turabian StyleRoston, Daniel, Zahidul Islam, and Amnon Kohen. 2013. "Isotope Effects as Probes for Enzyme Catalyzed Hydrogen-Transfer Reactions" Molecules 18, no. 5: 5543-5567. https://doi.org/10.3390/molecules18055543
APA StyleRoston, D., Islam, Z., & Kohen, A. (2013). Isotope Effects as Probes for Enzyme Catalyzed Hydrogen-Transfer Reactions. Molecules, 18(5), 5543-5567. https://doi.org/10.3390/molecules18055543



