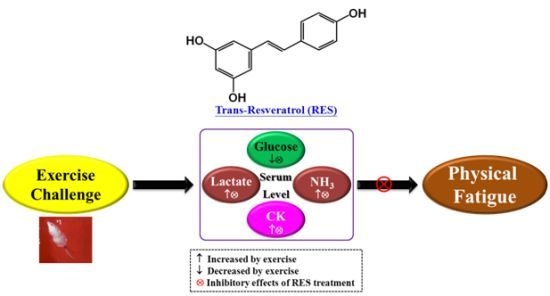
Resveratrol Protects against Physical Fatigue and Improves Exercise Performance in Mice
Abstract
:1. Introduction
2. Results and Discussion
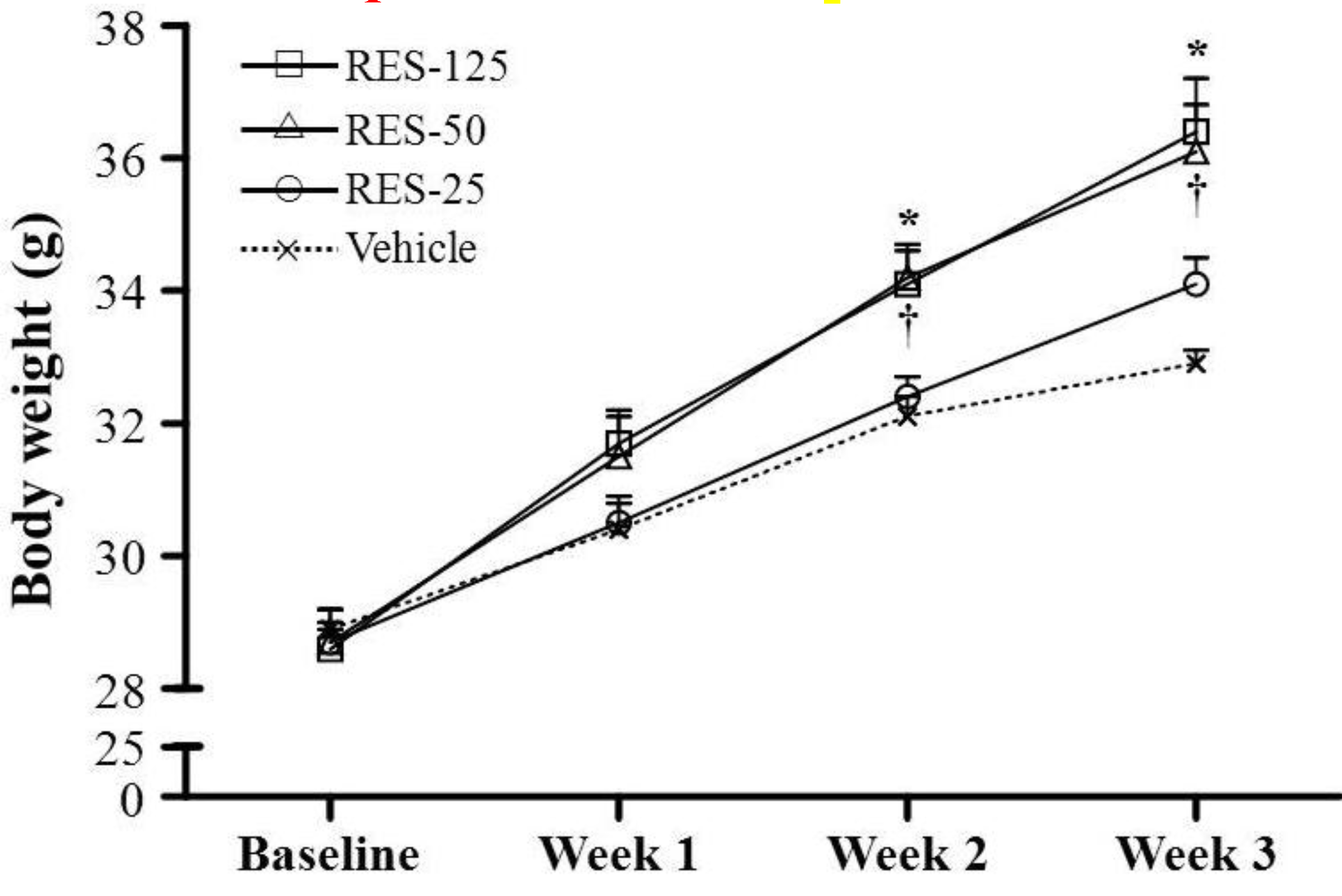
2.1. Body Weight (BW), Skeletal Muscle Mass, and Other Metabolism-Related Organ Weights
| Characteristic | Vehicle | RES-25 | RES-50 | RES-125 | Trend analysis |
|---|---|---|---|---|---|
| Initial BW (g) | 28.9 ± 0.3 | 28.7 ± 0.5 | 28.7 ± 0.2 | 28.6 ± 0.4 | 0.4956 |
| Final BW (g) | 32.9 ± 0.2 a | 34.1 ± 0.4 a | 36.1 ± 0.7 b | 36.4 ± 0.8 b | <0.0001 |
| Food intake (g/day) | 5.16 ± 0.14 a | 5.94 ± 0.12 a | 7.21 ± 0.30 b | 8.18 ± 0.50 c | <0.0001 |
| Food efficiency ratio | 0.79 ± 0.02 a | 0.91 ± 0.02 ab | 1.03 ± 0.05 b | 0.97 ± 0.07 b | 0.0007 |
| Liver (g) | 1.82 ± 0.05 a | 1.84 ± 0.06 a | 1.94 ± 0.07 ab | 2.05 ± 0.05 b | 0.0037 |
| Muscle (g) | 0.33 ± 0.01 a | 0.34 ± 0.01 a | 0.38 ± 0.01 b | 0.36 ± 0.01 ab | 0.0305 |
| Kidney (g) | 0.52 ± 0.01 a | 0.59 ± 0.02 ab | 0.62 ± 0.03 b | 0.65 ± 0.01 b | <0.0001 |
| EFP (g) | 0.54 ± 0.02 ab | 0.51 ± 0.02 a | 0.62 ± 0.04 ab | 0.69 ± 0.07 b | 0.0482 |
| Relative liver weight (%) | 5.57 ± 0.16 | 5.49 ± 0.12 | 5.37 ± 0.11 | 5.64 ± 0.18 | 0.8797 |
| Relative muscle weight (%) | 1.01 ± 0.03 | 1.01 ± 0.03 | 1.06 ± 0.03 | 0.99 ± 0.03 | 0.9878 |
| Relative kidney weight (%) | 1.59 ± 0.04 a | 1.76 ± 0.06 ab | 1.70 ± 0.06 ab | 1.79 ± 0.05 b | 0.0887 |
| Relative EFP weight (%) | 1.65 ± 0.08 | 1.51 ± 0.08 | 1.71 ± 0.11 | 1.87 ± 0.18 | 0.3194 |
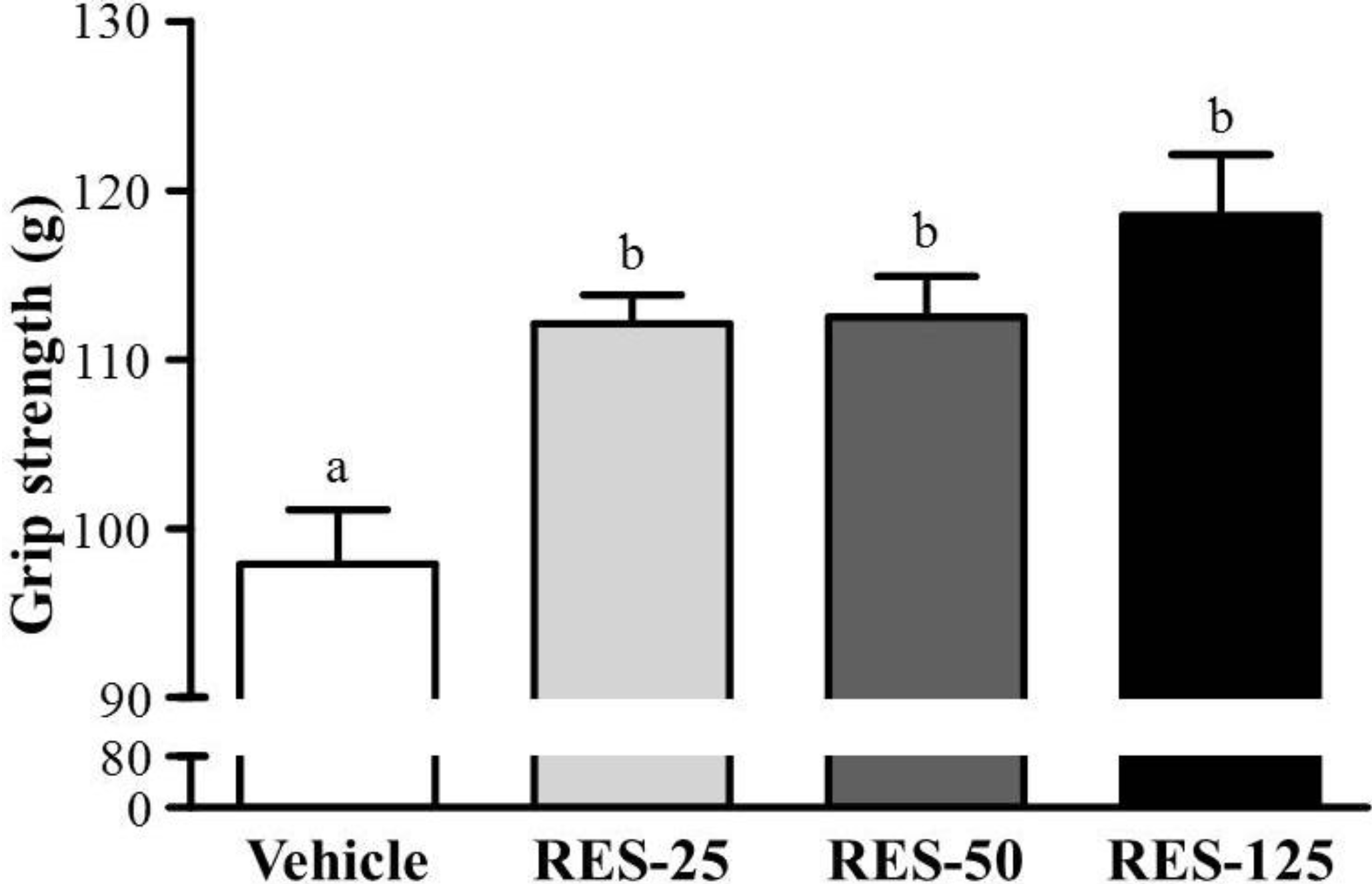
2.2. Effect of RES Supplementation on Forelimb Grip Strength
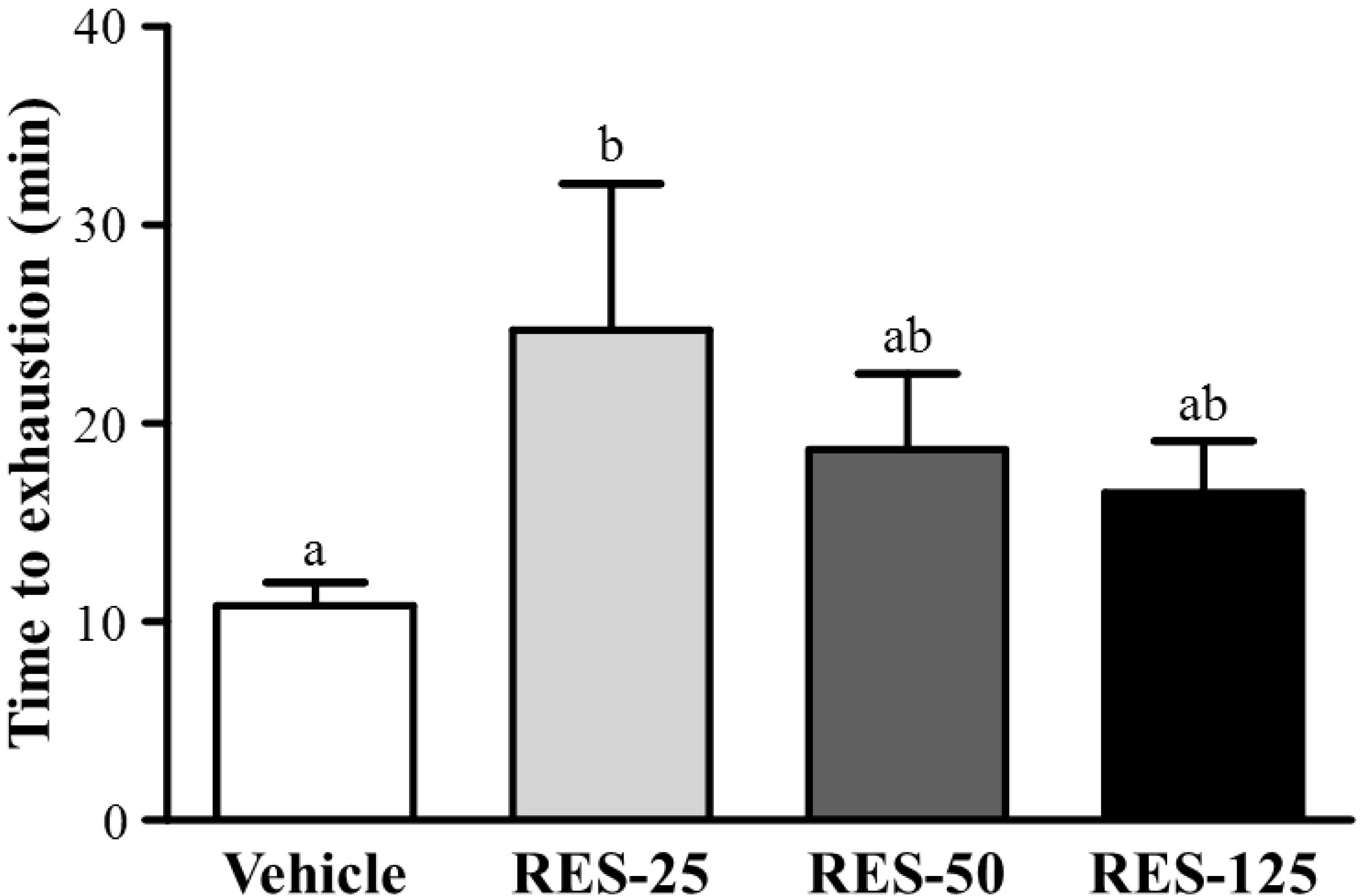
2.3. Effect of RES Supplementation on Exercise Performance in a Weight-loaded Swimming Test
2.4. Effect of RES Supplementation on Serum Lactate, Ammonia, Glucose, and CK Levels after Acute Exercise Challenge
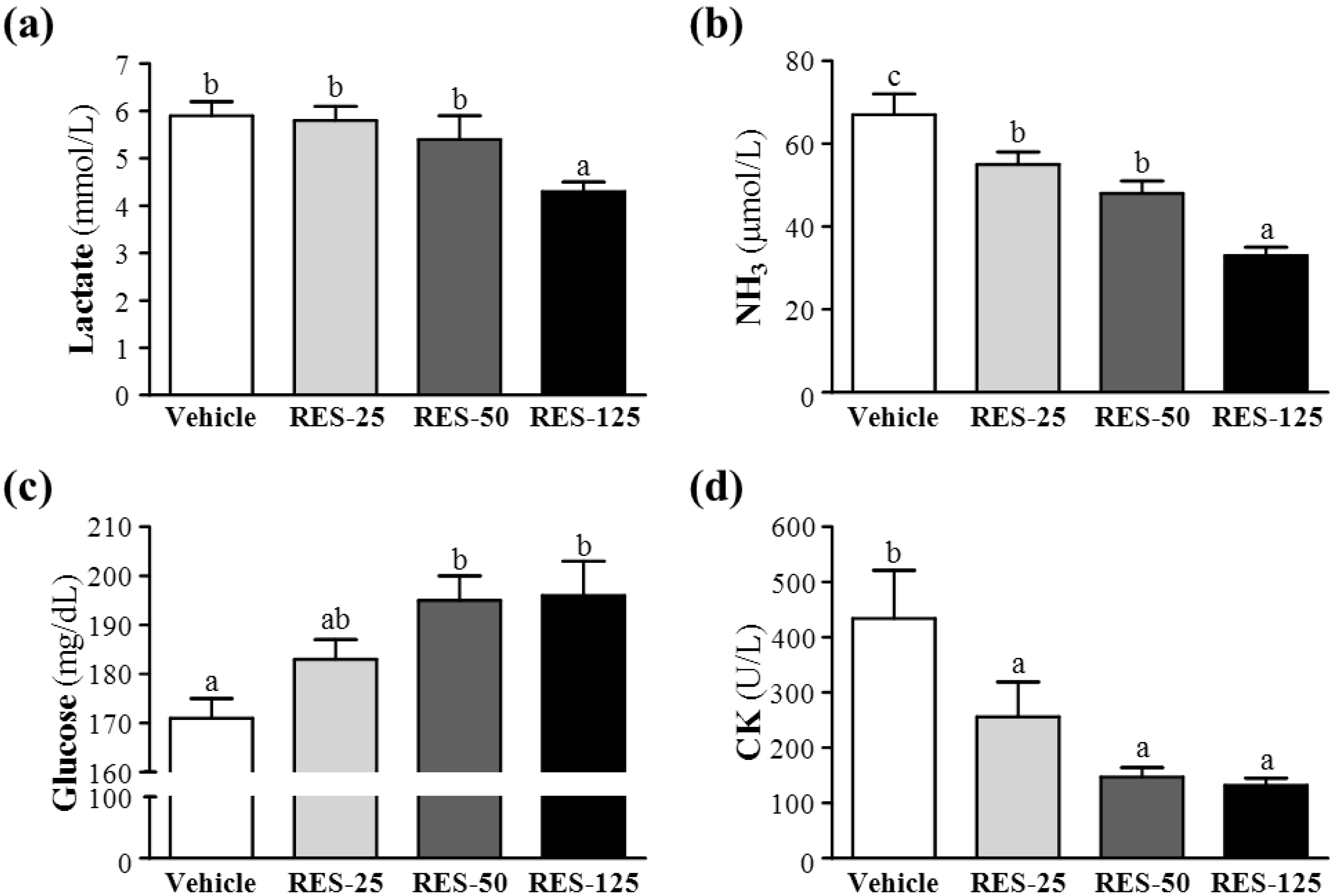
2.5. Effect of RES Supplementation on Hepatic Glycogen Level
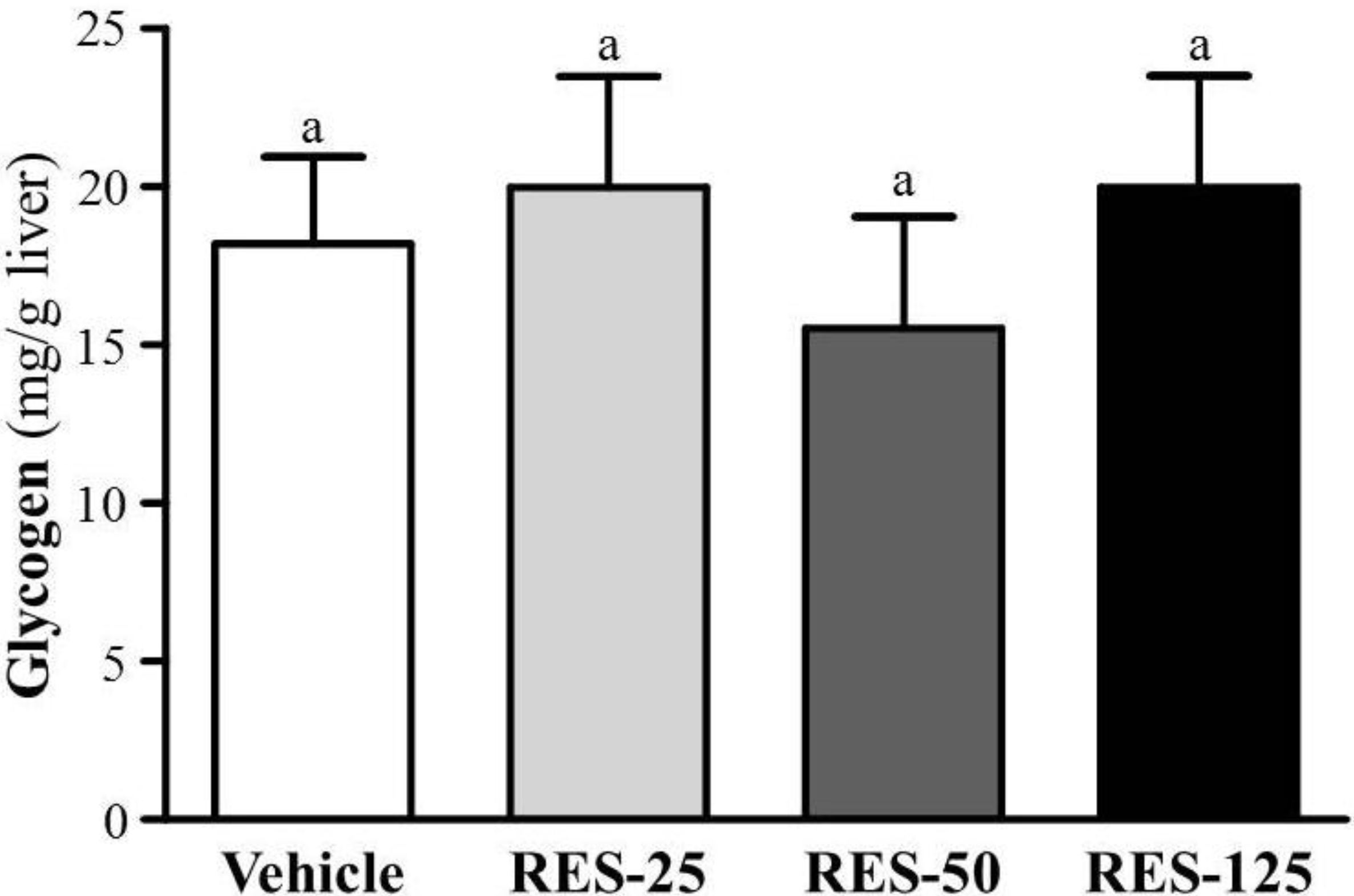
2.6. Effect of RES Supplementation on Biochemical Analyses at the End of the Experiment
| Parameter | Vehicle | RES-25 | RES-50 | RES-125 | Trend analysis |
|---|---|---|---|---|---|
| AST (U/L) | 64 ± 4 b | 61 ± 4 b | 62 ± 3 b | 49 ± 1 a | 0.0036 |
| ALT (U/L) | 32 ± 2 | 33 ± 2 | 38 ± 3 | 31 ± 1 | 0.9216 |
| ALP (U/L) | 287 ± 28 | 315 ± 11 | 284 ± 15 | 292 ± 10 | 0.7657 |
| LDH (U/L) | 416 ± 27 c | 388 ± 39 bc | 326 ± 29 ab | 256 ± 5 a | <0.0001 |
| Albumin (g/dL) | 3.3 ± 0.0 | 3.3 ± 0.0 | 3.3 ± 0.1 | 3.2 ± 0.0 | 0.2861 |
| TBIL (μg/dL) | 95 ± 7 | 84 ± 5 | 96 ± 5 | 99 ± 6 | 0.6861 |
| TP (g/dL) | 5.6 ± 0.1 | 5.6 ± 0.1 | 5.5 ± 0.1 | 5.5 ± 0.0 | 0.1361 |
| BUN (mg/dL) | 21.0 ± 0.6 | 19.3 ± 0.5 | 20.9 ± 0.8 | 20.0 ± 0.5 | 0.7720 |
| Creatinine (mg/dL) | 0.28 ± 0.01 b | 0.28 ± 0.01 ab | 0.27 ± 0.01 ab | 0.26 ± 0.01 a | 0.0087 |
| UA (mg/dL) | 1.94 ± 0.14 c | 1.66 ± 0.11 bc | 1.20 ± 0.22 ab | 0.80 ± 0.07 a | <0.0001 |
| TC (mg/dL) | 148 ± 4 | 144 ± 4 | 145 ± 5 | 156 ± 4 | 0.2133 |
| TG (mg/dL) | 103 ± 10 b | 83 ± 7 ab | 65 ± 6 a | 66 ± 3 a | <0.0001 |
| Glucose (mg/dL) | 191 ± 6 | 189 ± 4 | 178 ± 4 | 195 ± 4 | 0.8109 |
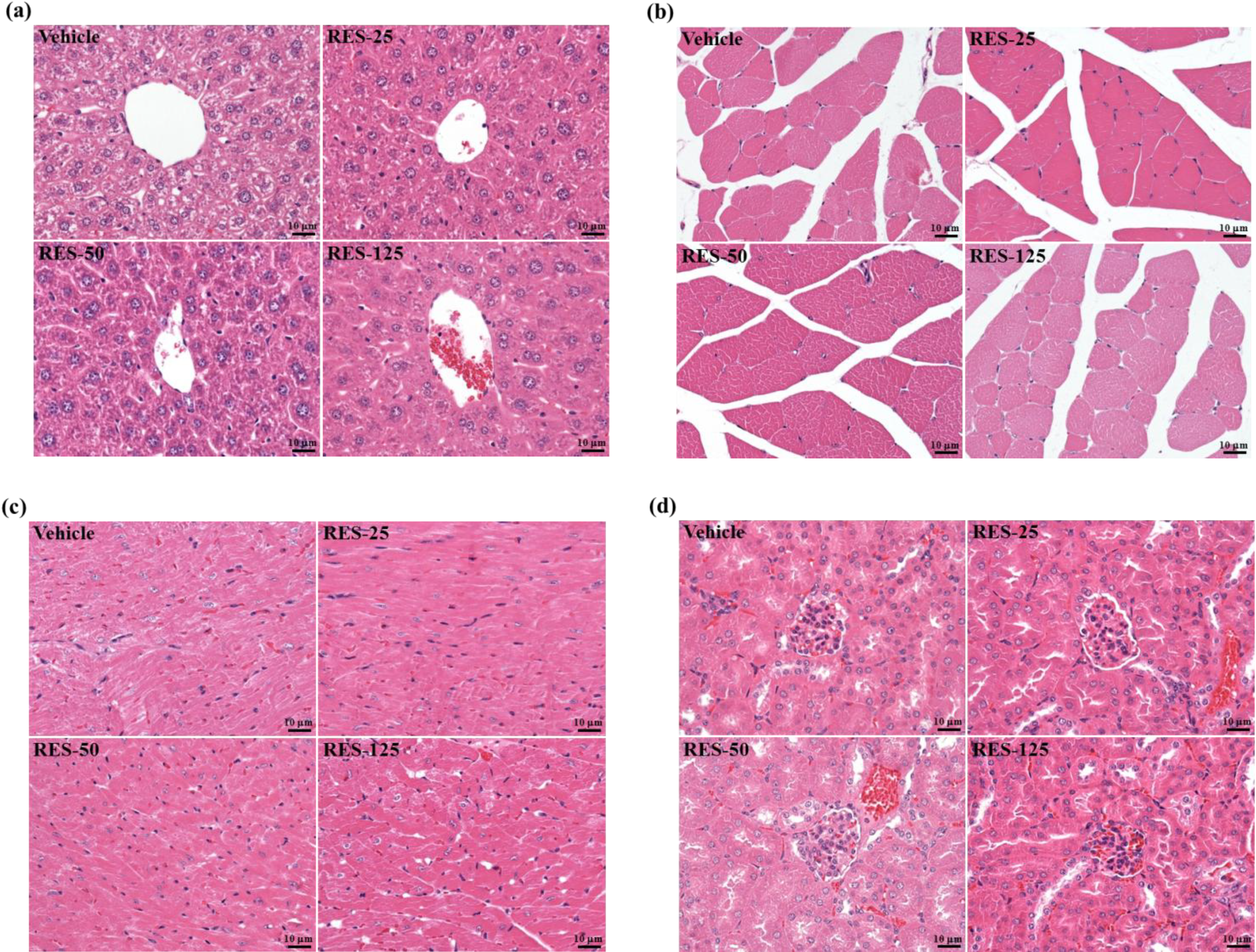
3. Experimental
3.1. Materials, Animals, and Experiment Design
3.2. Forelimb Grip Strength
3.3. Swimming Exercise Performance Test
3.4. Determination of Blood Biochemical Variables
3.5. Tissue Glycogen Determination
3.6. Histological Staining of Tissues
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Delmas, D.; Lancon, A.; Colin, D.; Jannin, B.; Latruffe, N. Resveratrol as a chemopreventive agent: A promising molecule for fighting cancer. Curr. Drug Targets 2006, 7, 423–442. [Google Scholar] [CrossRef]
- Bavaresco, L. Role of viticultural factors on stilbene concentrations of grapes and wine. Drugs Exp. Clin. Res. 2003, 29, 181–187. [Google Scholar]
- Joe, A.K.; Liu, H.; Suzui, M.; Vural, M.E.; Xiao, D.; Weinstein, I.B. Resveratrol induces growth inhibition, S-phase arrest, apoptosis, and changes in biomarker expression in several human cancer cell lines. Clin. Cancer Res. 2002, 8, 893–903. [Google Scholar]
- Regev-Shoshani, G.; Shoseyov, O.; Bilkis, I.; Kerem, Z. Glycosylation of resveratrol protects it from enzymic oxidation. Biochem. J. 2003, 374, 157–163. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption buy very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1482. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Soleas, G.J.; Yan, J.; Goldberg, D.M. Ultrasensitive assay for three polyphenols (catechin, quercetin and resveratrol) and their conjugates in biological fluids utilizing gas chromatography with mass selective detection. J. Chromatogr. B Biomed. Sci. Appl. 2001, 757, 161–172. [Google Scholar] [CrossRef]
- Zhao, H.; Niu, Q.; Li, X.; Liu, T.; Xu, Y.; Han, H.; Wang, W.; Fan, N.; Tian, Q.; Zhang, H.; Wang, Z. Long-term resveratrol consumption protects ovariectomized rats chronically treated with D-galactose from developing memory decline without effects on the uterus. Brain Res. 2012, 1467, 67–80. [Google Scholar] [CrossRef]
- Busch, F.; Mobasheri, A.; Shayan, P.; Lueders, C.; Stahlmann, R.; Shakibaei, M. Resveratrol modulates interleukin-1β-induced phosphatidylinositol 3-kinase and nuclear factor κB signaling pathways in human tenocytes. J. Biol. Chem. 2012, 287, 38050–38063. [Google Scholar] [CrossRef]
- Castillo-Pichardo, L.; Dharmawardhane, S.F. Grape polyphenols inhibit Akt/mammalian target of rapamycin signaling and potentiate the effects of gefitinib in breast cancer. Nutr. Cancer 2012, 64, 1058–1069. [Google Scholar] [CrossRef]
- Ramar, M.; Manikandan, B.; Raman, T.; Priyadarsini, A.; Palanisamy, S.; Velayudam, M.; Munusamy, A.; Marimuthu Prabhu, N.; Vaseeharan, B. Protective effect of ferulic acid and resveratrol against alloxan-induced diabetes in mice. Eur. J. Pharmacol. 2012, 690, 226–235. [Google Scholar] [CrossRef]
- Ichikawa, T.; Hayashi, R.; Suzuki, K.; Imanishi, S.; Kambara, K.; Okazawa, S.; Inomata, M.; Yamada, T.; Yamazaki, Y.; Koshimizu, Y.; et al. The Sirt1 activator SRT1720 suppresses inflammation in an ova-induced mouse model of asthma. Respirology 2013, 18, 332–339. [Google Scholar] [CrossRef]
- Falchi, M.; Bertelli, A.; Galazzo, R.; Viganò, P.; Dib, B. Central antalgic activity of resveratrol. Arch. Ital. Biol. 2010, 148, 389–396. [Google Scholar]
- Mehta, R.K.; Agnew, M.J. Influence of mental workload on muscle endurance, fatigue, and recovery during intermittent static work. Eur. J. Appl. Physiol. 2012, 112, 2891–2902. [Google Scholar] [CrossRef]
- Fitts, R.H. Cellular mechanisms of muscle fatigue. Physiol. Rev. 1994, 74, 49–94. [Google Scholar] [CrossRef]
- Coombes, J.S.; Rowell, B.; Dodd, S.L.; Demirel, H.A.; Naito, H.; Powers, S.K. Effects of vitamin E deficiency on fatigue and muscle contractile properties. Eur. J. Appl. Physiol. 2002, 87, 272–277. [Google Scholar] [CrossRef]
- You, L.; Zhao, M.; Regenstein, J.M.; Ren, J. In vitro antioxidant activity and in vivo anti-fatigue effect of loach (Misgurnus anguillicaudatus) peptides prepared by papain digestion. Food Chem. 2011, 124, 188–194. [Google Scholar] [CrossRef]
- Schwer, B.; Verdin, E. Conserved metabolic regulatory functions of sirtuins. Cell Metab. 2008, 7, 104–112. [Google Scholar] [CrossRef]
- Huang, C.C.; Hsu, M.C.; Huang, W.C.; Yang, H.R.; Hou, C.C. Triterpenoid-rich extract from Antrodia camphorata improves physical fatigue and exercise performance in mice. Evid. Based Complement. Alternat. Med. 2012, 2012. [Google Scholar] [CrossRef]
- Wang, S.Y.; Huang, W.C.; Liu, C.C.; Wang, M.F.; Ho, C.S.; Huang, W.P.; Hou, C.C.; Chuang, H.L.; Huang, C.C. Pumpkin (Cucurbita moschata) fruit extract improves physical fatigue and exercise performance in mice. Molecules 2012, 17, 11864–11876. [Google Scholar] [CrossRef]
- Belluardo, N.; Westerblad, H.; Mudó, G.; Casabona, A.; Bruton, J.; Caniglia, G.; Pastoris, O.; Grassi, F.; Ibáñez, C.F. Neuromuscular junction disassembly and muscle fatigue in mice lacking neurotrophin-4. Mol. Cell. Neurosci. 2001, 18, 56–67. [Google Scholar]
- Dal-Pan, A.; Pifferi, F.; Marchal, J.; Picq, J.L.; Aujard, F. RESTRIKAL Consortium. Cognitive performances are selectively enhanced during chronic caloric restriction or resveratrol supplementation in a primate. PLoS One 2011, 6, e16581. [Google Scholar]
- Brancaccio, P.; Maffulli, N.; Limongelli, F.M. Creatine kinase monitoring in sport medicine. Br. Med. Bull. 2007, 81–82, 209–230. [Google Scholar]
- Cairns, S.P. Lactic acid and exercise performance: Culprit or friend? Sports Med. 2006, 36, 279–291. [Google Scholar] [CrossRef]
- Carvalho-Peixoto, J.; Alves, R.C.; Cameron, L.C. Glutamine and carbohydrate supplements reduce ammonemia increase during endurance field exercise. Appl. Physiol. Nutr. Metab. 2007, 32, 1186–1190. [Google Scholar] [CrossRef]
- Suh, S.H.; Paik, I.Y.; Jacobs, K. Regulation of blood glucose homeostasis during prolonged exercise. Mol. Cells 2007, 23, 272–279. [Google Scholar]
- Fujii, N.; Jessen, N.; Goodyear, L.J. AMP-activated protein kinase and the regulation of glucose transport. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E867–E877. [Google Scholar] [CrossRef]
- Manabe, Y.; Miyatake, S.; Takagi, M.; Nakamura, M.; Okeda, A.; Nakano, T.; Hirshman, M.F.; Goodyear, L.J.; Fujii, N.L. Characterization of an acute muscle contraction model using cultured C2C12 myotubes. PLoS One 2012, 7, e52592. [Google Scholar] [CrossRef]
- Sahlin, K.; Tonkonogi, M.; Söderlund, K. Energy supply and muscle fatigue in humans. Acta Physiol. Scand. 1998, 162, 261–266. [Google Scholar] [CrossRef]
- Young, A.J.; Castellani, J.W. Exertion-induced fatigue and thermoregulation in the cold. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 128, 769–776. [Google Scholar] [CrossRef]
- Rodgers, J.T.; Lerin, C.; Haas, W.; Gygi, S.P.; Spiegelman, B.M.; Puigserver, P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 2005, 434, 113–118. [Google Scholar] [CrossRef]
- Frescas, D.; Valenti, L.; Accili, D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J. Biol. Chem. 2005, 280, 20589–20595. [Google Scholar] [CrossRef]
- Sehirli, O.; Tozan, A.; Omurtag, G.Z.; Cetinel, S.; Contuk, G.; Gedik, N.; Sener, G. Protective effect of resveratrol against naphthalene-induced oxidative stress in mice. Ecotoxicol. Environ. Saf. 2008, 71, 301–308. [Google Scholar] [CrossRef]
- Tsuda, H.; Kawada, N.; Kaimori, J.Y.; Kitamura, H.; Moriyama, T.; Rakugi, H.; Takahara, S.; Isaka, Y. Febuxostat suppressed renal ischemia-reperfusion injury via reduced oxidative stress. Biochem. Biophys. Res. Commun. 2012, 427, 266–272. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Szkudelska, K.; Szkudelski, T. Resveratrol, obesity and diabetes. Eur. J. Pharmacol. 2010, 635, 1–8. [Google Scholar] [CrossRef]
- Zhang, X.H.; Huang, B.; Choi, S.K.; Seo, J.S. Anti-obesity effect of resveratrol-amplified grape skin extracts on 3T3-L1 adipocytes differentiation. Nutr. Res. Pract. 2012, 6, 286–293. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, R.-E.; Huang, W.-C.; Liao, C.-C.; Chang, Y.-K.; Kan, N.-W.; Huang, C.-C. Resveratrol Protects against Physical Fatigue and Improves Exercise Performance in Mice. Molecules 2013, 18, 4689-4702. https://doi.org/10.3390/molecules18044689
Wu R-E, Huang W-C, Liao C-C, Chang Y-K, Kan N-W, Huang C-C. Resveratrol Protects against Physical Fatigue and Improves Exercise Performance in Mice. Molecules. 2013; 18(4):4689-4702. https://doi.org/10.3390/molecules18044689
Chicago/Turabian StyleWu, Ruei-Er, Wen-Ching Huang, Chen-Chung Liao, Yu-Kai Chang, Nai-Wen Kan, and Chi-Chang Huang. 2013. "Resveratrol Protects against Physical Fatigue and Improves Exercise Performance in Mice" Molecules 18, no. 4: 4689-4702. https://doi.org/10.3390/molecules18044689
APA StyleWu, R.-E., Huang, W.-C., Liao, C.-C., Chang, Y.-K., Kan, N.-W., & Huang, C.-C. (2013). Resveratrol Protects against Physical Fatigue and Improves Exercise Performance in Mice. Molecules, 18(4), 4689-4702. https://doi.org/10.3390/molecules18044689