Phosphorus Dendrimers as Carriers of siRNA—Characterisation of Dendriplexes
Abstract
:1. Introduction
| Ksv [L/mol] | V [L/mol] | |
|---|---|---|
| pdG3 | 1.1 × 106 | 1.5 × 106 |
| pdG4 | 2.1 × 106 | 2.9 × 106 |
2. Results and Discussion
2.1. Ethidium Bromide Intercalation Assay

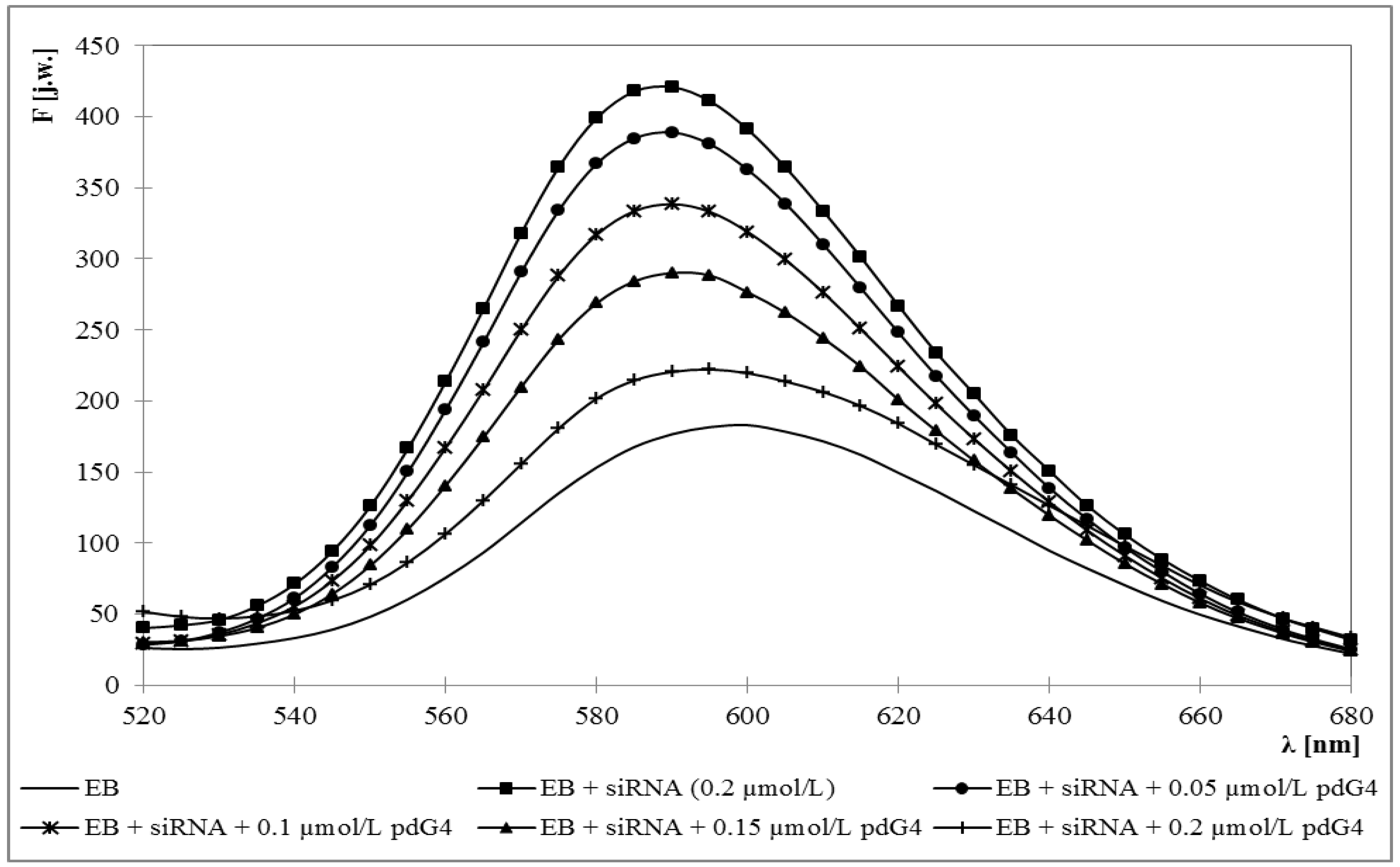
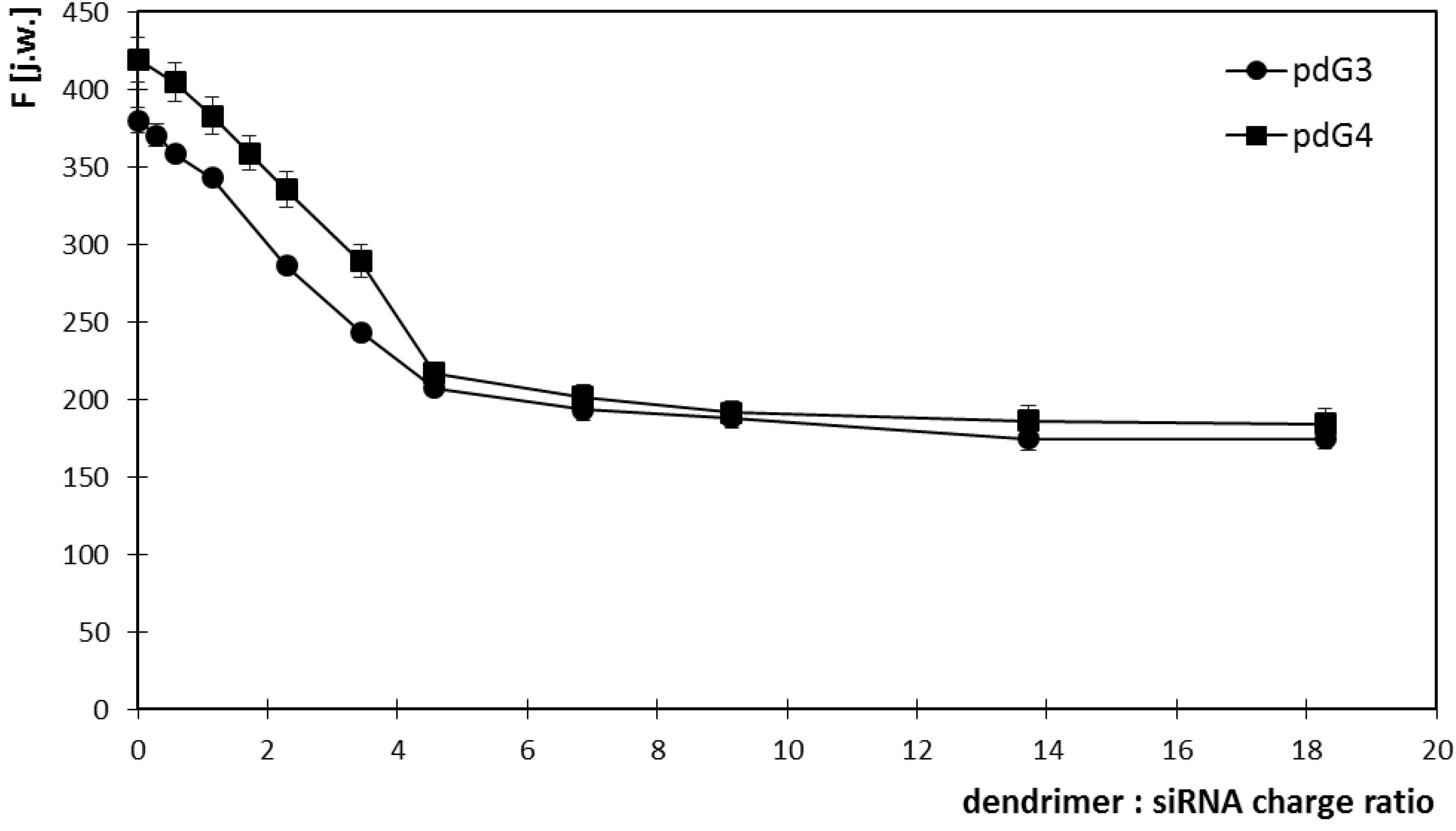


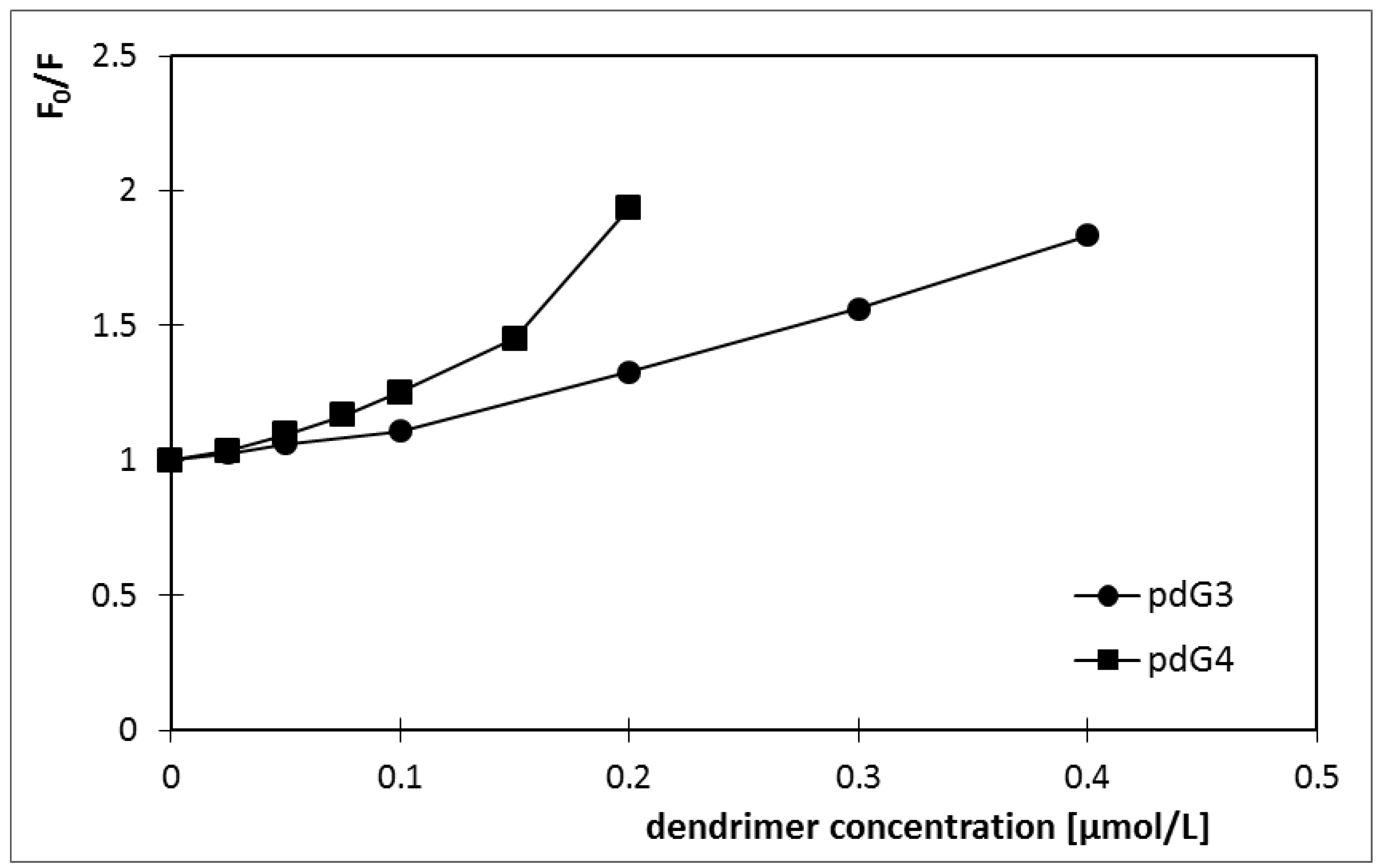
2.2. Zeta Potential

2.3. Hydrodynamic Diameter

2.4. Circular Dichroism
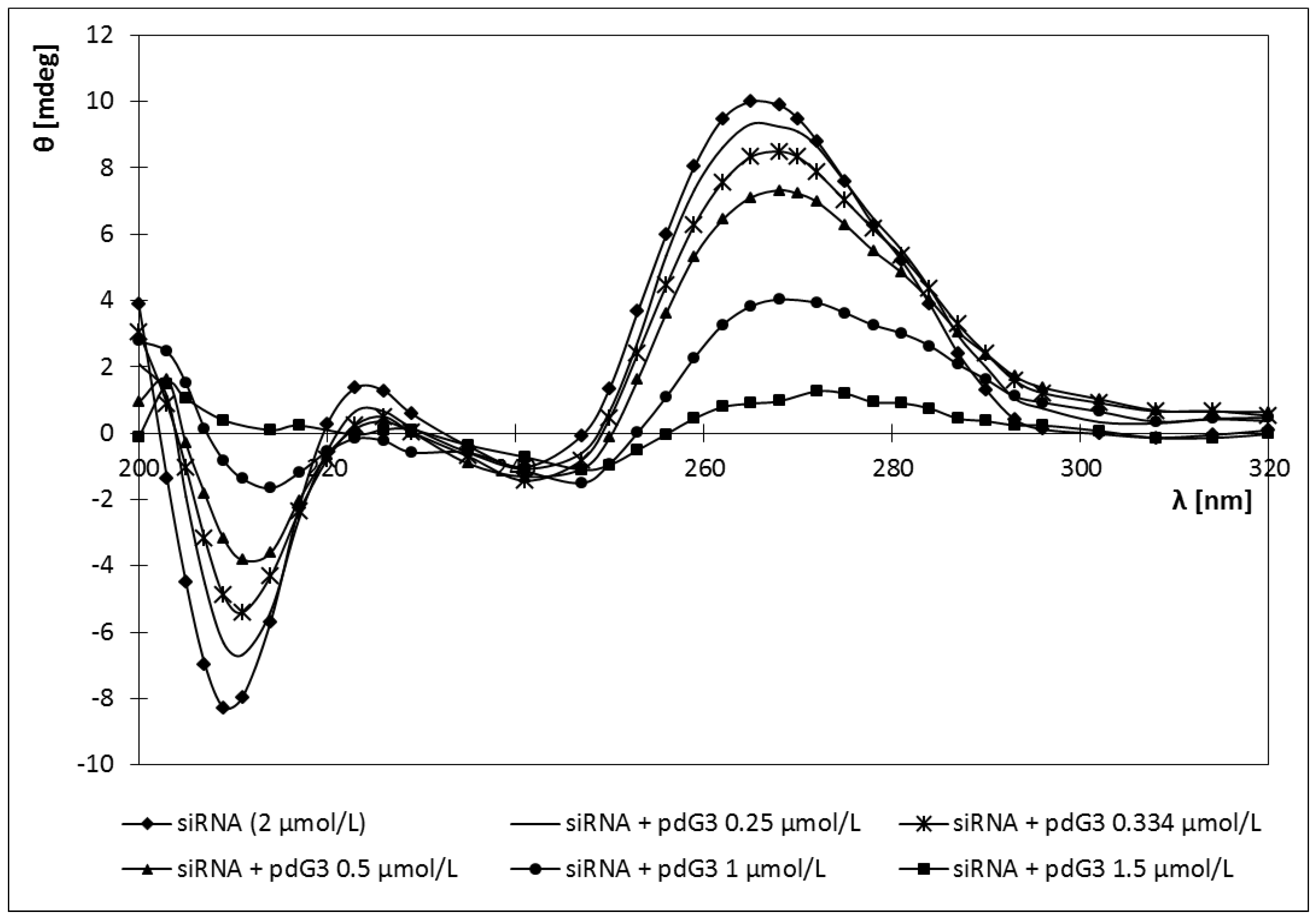
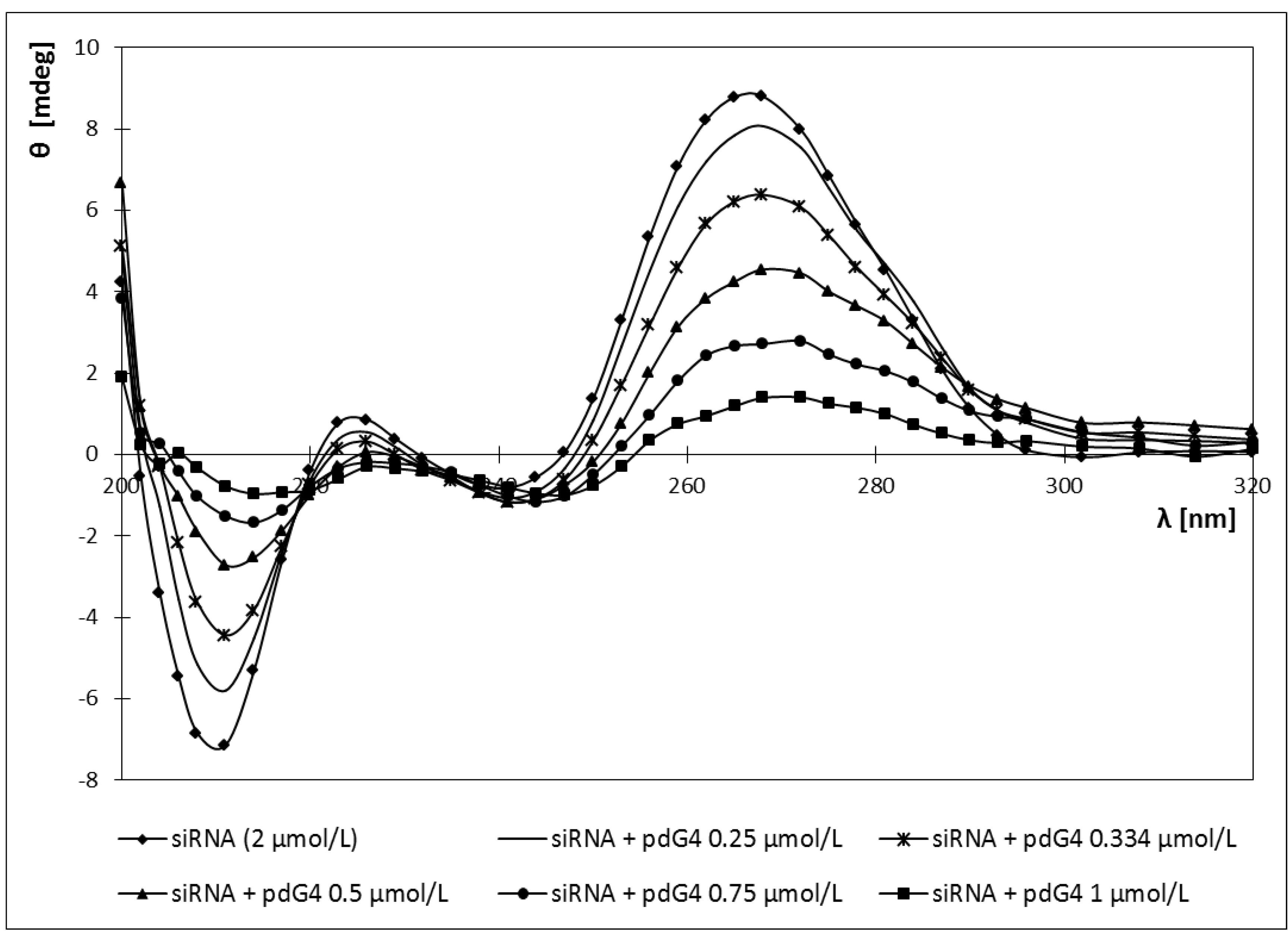

2.5. Discussion
3. Experimental
3.1. Materials
| Dendrimer | MW (Da) | Structure | Number of cationic end groups |
|---|---|---|---|
| pdG3 | 16280 |  | 48 |
| pdG4 | 33702 |  | 96 |
| Sense | Antisense | |
|---|---|---|
| Sequence (5'→3') | CGCAUGUUCCGGGACAAAAtt | UUUUGUCCCGGAACAUGCGgt |
| Length (number of base pairs) | 21 | 21 |
| G/C content | 48% | 48% |
| Molar weight | 6800 | 6600 |
| Total molar weight | 13,400 | |
3.2. Ethidium Bromide Intercalation Assay
3.3. Laser Doppler Electrophoresis (LDE)
3.4. Dynamic Light Scattering (DLS).
3.5. Circular Dichroism (CD).
4. Conclusions
Acknowledgments
References
- Verma, M.; Somia, N. Gene therapy—promises, problems and prospects. Nature 1997, 389, 239–242. [Google Scholar] [CrossRef]
- Huang, L.; Viroonchatapan, E. Introduction. In Non-viral Vector for Gene Therapy; Huang, L., Wagner, M., Eds.; Academic Press: New York, NY, USA, 1999; pp. 3–22. [Google Scholar]
- Hannon, G.J. RNA interference. Nature 2002, 418, 244–251. [Google Scholar] [CrossRef]
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001, 411, 494–498. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A new class of polymers: Starburst-dendritic macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Klajnert, B.; Bryszewska, M. Dendrimers: Properties and applications. Acta Biochim. Pol. 2001, 48, 199–208. [Google Scholar]
- Galliot, C.; Larré, C.; Caminade, A.M.; Majoral, J.P. Regioselective stepwise growth of dendrimer units in the internal voids of a main dendrimer. Science 1997, 277, 1981–1984. [Google Scholar] [CrossRef]
- Merino, S.; Brauge, L.; Caminade, A.M.; Majoral, J.P.; Taton, D.; Gnanou, Y. Synthesis and characterization of linear, Hyperbranched, and dendrimer-like polymers constituted of the same repeating unit. Chemistry 2001, 7, 3095–3105. [Google Scholar] [CrossRef]
- Caminade, A.M.; Majoral, J.P. Water-soluble phosphorus-containing dendrimers. Prog. Polym. Sci. 2005, 30, 491–505. [Google Scholar] [CrossRef]
- Solassol, J.; Crozet, C.; Perrier, V.; Leclaire, J.; Béranger, F.; Caminade, A.M.; Meunier, B.; Dormont, D.; Majoral, J.P.; Lehmann, S. Cationic phosphorus-containing dendrimers reduce prion replication both in cell culture and in mice infected with scrapie. J. Gen. Virol. 2004, 85, 1791–1799. [Google Scholar] [CrossRef]
- Loup, C.; Zanta, M.A.; Caminade, A.M.; Majoral, J.P.; Meunier, B. Preparation of water-soluble cationic phosphorus-containing dendrimers as DNA transfecting agents. Chem. Eur. J. 1999, 5, 3644–3650. [Google Scholar] [CrossRef]
- Maksimenko, A.V.; Mandrouguine, V.; Gottikh, M.B.; Bertrand, J.R.; Majoral, J.P.; Malvy, C. Optimisation of dendrimer-mediated gene transfer by anionic oligomers. J. Gene. Med. 2003, 5, 61–71. [Google Scholar]
- Ionov, M.; Wróbel, D.; Gardikis, K.; Hatziantoniou, S.; Demetzos, C.; Majoral, J.P.; Klajnert, B.; Bryszewska, M. Effect of phosphorus dendrimers on DMPC lipid membranes. Chem. Phys. Lipids 2011, 165, 408–413. [Google Scholar]
- Wrobel, D.; Ionov, M.; Gardikis, K.; Demetzos, C.; Majoral, J.P.; Palecz, B.; Klajnert, B.; Bryszewska, M. Interactions of phosphorus-containing dendrimers with liposomes. Biochim. Biophys. Acta 2010, 1811, 221–226. [Google Scholar]
- Griffe, L.; Poupot, M.; Marchand, P.; Maraval, A.; Turrin, C.O.; Rolland, O.; Métivier, P.; Bacquet, G.; Fournié, J.J.; Caminade, A.M.; et al. Multiplication of human natural killer cells by nanosized phosphonate-capped dendrimers. Angew. Chem. Int. Ed. Engl. 2007, 46, 2523–2526. [Google Scholar] [CrossRef]
- Poupot, M.; Griffe, L.; Marchand, P.; Maraval, A.; Rolland, O.; Martinet, L.; L'Faqihi-Olive, F.E.; Turrin, C.O.; Caminade, A.M.; Fournié, J.J.; et al. Design of phosphorylated dendritic architectures to promote human monocyte activation. FASEB J. 2006, 20, 2339–2351. [Google Scholar] [CrossRef]
- Hayder, M.; Poupot, M.; Baron, M.; Nigon, D.; Turrin, C.O.; Caminade, A.M.; Majoral, J.P.; Eisenberg, R.A.; Fournie, J.J.; Cantagrel, A.; et al. A phosphorus-based dendrimer targets inflammation and osteoclastogenesis in experimental arthritis. Sci. Transl. Med. 2011, 3, 81ra35. [Google Scholar] [CrossRef]
- Rolland, O.; Griffe, L.; Poupot, M.; Maraval, A.; Ouali, A.; Coppel, Y.; Fournié, J.J.; Bacquet, G.; Turrin, C.O.; Caminade, A.M.; et al. Tailored control and optimisation of the number of phosphonic acid termini on phosphorus-containing dendrimers for the ex vivo activation of human monocytes. Chemistry 2008, 14, 4836–4850. [Google Scholar]
- Shcharbin, D.; Dzmitruk, V.; Shakhbazau, A.; Goncharova, N.; Seviaryn, I.; Kosmacheva, S.; Potapnev, M.; Pedziwiatr-Werbicka, E.; Bryszewska, M.; Talabaev, M.; et al. Fourth generation phosphorus-containing dendrimers: Prospective drug and gene delivery carrier. Pharmaceutics 2011, 3, 458–473. [Google Scholar] [CrossRef]
- Eftink, M.R. Fluorescence quenching reactions: Probing biological macromolecular structures. In Biophysical and Biochemical Aspects of Fluorescence Spectroscopy; Dewey, T.G., Ed.; Plenum: New York, NY, USA, 1991; pp. 1–41. [Google Scholar]
- Akhtar, S.; Hughes, M.D.; Khan, A.; Bibby, M.; Hussain, M.; Nawaz, Q.; Double, J.; Sayyed, P. The delivery of antisense therapeutics. Adv. Drug Deliver. Rev. 2000, 44, 3–21. [Google Scholar] [CrossRef]
- Akhtar, S.; Juliano, R.L. Cellular and intracellular fate of antisense oligonucleotides. Trends Cell Biol. 1992, 2, 139–144. [Google Scholar] [CrossRef]
- Hussain, M.; Shchepinov, M.; Sohail, M.; Benter, I.F.; Hollins, A.J.; Southern, E.M.; Akhtar, S. A novel anionic dendrimer for improved cellular delivery of antisense oligonucleotides. J. Control. Release 2004, 99, 139–155. [Google Scholar] [CrossRef]
- Bumcrot, D.; Manoharan, M.; Koteliansky, V.; Sah, D.W. RNAi therapeutics: A potential new class of pharmaceutical drugs. Nat. Chem. Biol. 2006, 2, 711–719. [Google Scholar] [CrossRef]
- Kathlen, F.; Zon, G.; Rati, A.; Zhou, Q.; Yo, W. Targeting Nanoimmunoliposome Complex for Short Interfering RNA Delivery. Hum. Gene Ther. 2006, 17, 117–124. [Google Scholar] [CrossRef]
- Lam, J.K.W.; Liang, W.; Chan, H.K. Pulmonary delivery of therapeutic siRNA. Adv. Drug Deliver. Rev. 2011, 64, 1–15. [Google Scholar]
- Juliano, R.; Alam, M.R.; Dixit, V.; Kang, H. Mechanisms and strategies for effective delivery of antisense and siRNA oligonucleotides. Nucleic Acids Res. 2008, 36, 4158–4171. [Google Scholar] [CrossRef]
- Aigner, A. Nonviral in vivo delivery of therapeutic small interfering RNAs. Curr. Opin. Mol. Ther. 2007, 9, 345–352. [Google Scholar]
- D’Emanuele, A.; Attwood, D. Dendrimer-drug interactions. Adv. Drug Deliver. Rev. 2005, 57, 2147–2162. [Google Scholar] [CrossRef]
- Quillardet, P.; Hofnung, M. Ethidium bromide and safety—Readers suggest alternative solutions. Trends Genet. 1988, 4, 89–93. [Google Scholar] [CrossRef]
- Hashizume, H.; Imahori, M. Circular dichroism and the conformation of natural and synthetic polynucleotides. J. Biochem. 1967, 61, 738–749. [Google Scholar]
- Tritton, T.R.; Crothers, D.M. Physical characterization of a ribosomal nucleoprotein complex. Biochemistry 1976, 15, 4377–4385. [Google Scholar] [CrossRef]
- Chiu, Y.L.; Rana, T.M. RNAi in human cells: Basic structural and functional features of small interfering RNA. Mol. Cell 2002, 10, 549–561. [Google Scholar] [CrossRef]
- Plank, C.; Mechtler, K.; Szoka, F.C.; Wagner, E. Activation of the complement system by synthetic DNA complexes: A potential barrier to intravenous gene delivery. Hum. Gene Ther. 1996, 7, 1437–1446. [Google Scholar] [CrossRef]
- Pouton, C.W.; Seymour, L.W. Key issues in non-viral gene delivery. Adv. Drug Deliver. Rev. 1998, 34, 3–19. [Google Scholar] [CrossRef]
- Jensen, L.B.; Pavan, G.M.; Kasimova, M.R.; Rutherford, S.; Danani, A.; Nielsen, H.M.; Foged, C. Elucidating the molecular mechanism of PAMAM-siRNA dendriplex self-assembly: Effect of dendrimer charge density. Int. J. Pharm. 2011, 416, 410–418. [Google Scholar] [CrossRef]
- Fang, M.; Cheng, Y.; Zhang, J.; Wu, Q.; Hu, J.; Zhao, L.; Xu, T. New insights into interactions between dendrimers and surfactants. 4. Fast-exchange/slow-exchange transitions in the structure of dendrimer-surfactant aggregates. J. Phys. Chem. B 2010, 114, 6048–6055. [Google Scholar] [CrossRef]
- Oh, Y.K.; Park, T.G. siRNA delivery systems for cancer treatment. Adv. Drug Deliver. Rev. 2009, 61, 850–862. [Google Scholar] [CrossRef]
- Tsai, C.C.; Jain, S.C.; Sobel, H.M. Visualization of drug–nucleic acid interactions at atomic resolution. I. Structure of an ethidium/dinucleoside monophosphate crystalline complex, ethidium:5-iodouridylyl (3'–5') adenosine. J. Mol. Biol. 1977, 114, 301–315. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, Q.; Nagasawa, H.; Okayasu, R.; Liber, H.; Bedford, J. Silencing expression of the catalytic subunit of DNA-dependent protein kinase by small interfering RNA sensitizes human cells for radiation-induced chromosome damage, cell killing, and mutation. Cancer Res. 2002, 62, 6400–6404. [Google Scholar]
- Sample Availability: Samples of the compounds (phosphorus dendrimers G3 and G4) are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ferenc, M.; Pedziwiatr-Werbicka, E.; Nowak, K.E.; Klajnert, B.; Majoral, J.-P.; Bryszewska, M. Phosphorus Dendrimers as Carriers of siRNA—Characterisation of Dendriplexes. Molecules 2013, 18, 4451-4466. https://doi.org/10.3390/molecules18044451
Ferenc M, Pedziwiatr-Werbicka E, Nowak KE, Klajnert B, Majoral J-P, Bryszewska M. Phosphorus Dendrimers as Carriers of siRNA—Characterisation of Dendriplexes. Molecules. 2013; 18(4):4451-4466. https://doi.org/10.3390/molecules18044451
Chicago/Turabian StyleFerenc, Malgorzata, Elzbieta Pedziwiatr-Werbicka, Katarzyna E. Nowak, Barbara Klajnert, Jean-Pierre Majoral, and Maria Bryszewska. 2013. "Phosphorus Dendrimers as Carriers of siRNA—Characterisation of Dendriplexes" Molecules 18, no. 4: 4451-4466. https://doi.org/10.3390/molecules18044451
APA StyleFerenc, M., Pedziwiatr-Werbicka, E., Nowak, K. E., Klajnert, B., Majoral, J.-P., & Bryszewska, M. (2013). Phosphorus Dendrimers as Carriers of siRNA—Characterisation of Dendriplexes. Molecules, 18(4), 4451-4466. https://doi.org/10.3390/molecules18044451