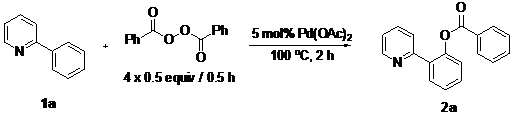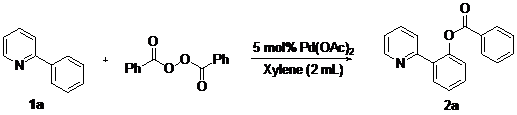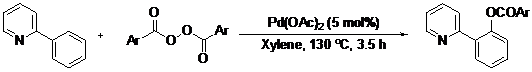Palladium(II)-Catalyzed othro-C–H-Benzoxylation of 2-Arylpyridines by Oxidative Coupling with Aryl Acylperoxides
Abstract
:1. Introduction
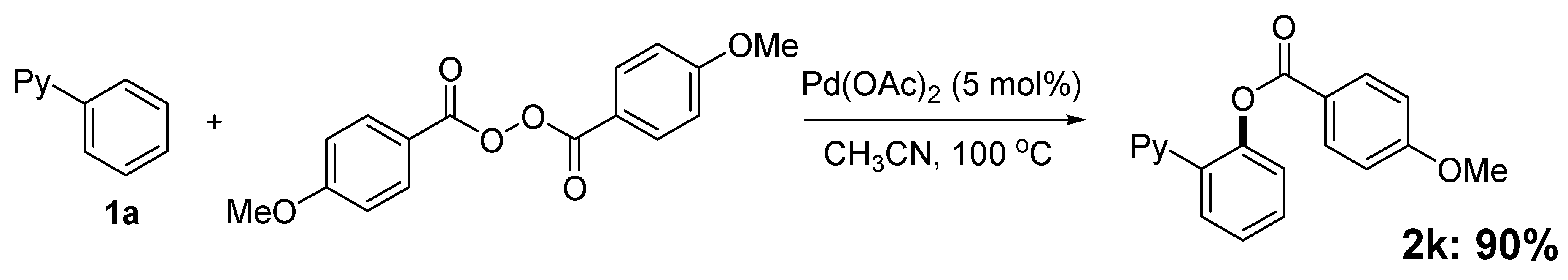
2. Results and Discussion
| Entry | Solvent (2 mL) | GC Yield (%) |
|---|---|---|
| 1 | CH3CN | 7 |
| 2 | dioxane | 10 |
| 3 | THF | 29 |
| 4 | DMF | 33 |
| 5 | DMA | 3 |
| 6 | DCE | 11 |
| 7 | toluene | 47 |
| 8 | xylene | 49 |
| Entry | Addition method | Temp. (°C) | Time (h) | GC Yield (%) |
|---|---|---|---|---|
| 1 | 4 × 0.5 equiv./0.5 h | 100 | 2 | 49 |
| 2 | 4 × 0.5 equiv./0.5 h | 120 | 2 | 41 |
| 3 | 4 × 0.5 equiv./0.5 h | 130 | 2 | 59 |
| 4 | 4 × 0.5 equiv./1 h | 130 | 4 | 53 |
| 5 | 4 × 0.5 equiv./0.5 h | 150 | 2 | 52 |
| 6 | 7 × 0.5 equiv./0.5 h | 130 | 3.5 | 64 |
| 7 | 8 × 0.5 equiv./0.5 h | 130 | 4 | 60 |
| 8 * | 7 × 0.5 equiv./0.5 h | 130 | 3.5 | 70 |
| 9 *,# | 7 × 0.5 equiv./0.5 h | 130 | 3.5 | 76 |
| Entry | Substrate | Product | Isolated Yield (%) | ||
|---|---|---|---|---|---|
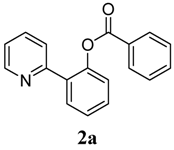
| 1 | 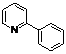 1a 1a | 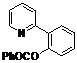 2a 2a | 71 | ||
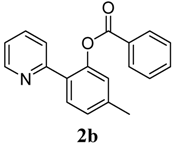
| 2 | 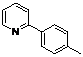 1b 1b | 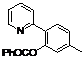 2b 2b | 70 | ||
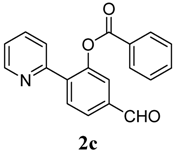
| 3 | 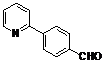 1c 1c | 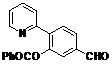 2c 2c | 44 | ||
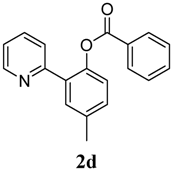
| 4 | 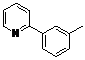 1d 1d | 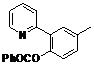 2d 2d | 87 | ||
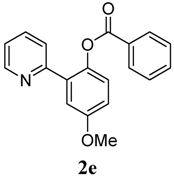
| 5 | 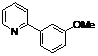 1e 1e | 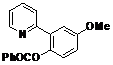 2e 2e | 83 | ||
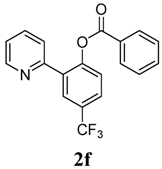
| 6 | 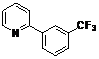 1f 1f | 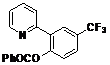 2f 2f | 54 | ||
| 7 | 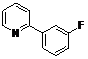 1g 1g | 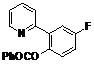 2g 2g | 62 | ||
| 8 | 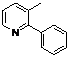 1h 1h | 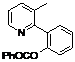 2h 2h | 87 | ||
| 9 | 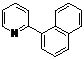 1i 1i | 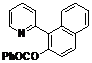 2i 2i | 57 | ||
| 10 | 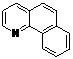 1j 1j | 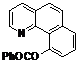 2j 2j | 85 | ||
| Entry | Ar | Isolated Yield (%) |
|---|---|---|
| 1 | 4-OMe-C6H5 | 54 |
| 2 | 3-OMe-C6H5 | 69 |
| 3 | 2-Cl-C6H5 | 43 |
| 4 | 3-Cl-C6H5 | 73 |
| 5 | 2-Me-C6H5 | 66 |
| 6 | 4-Me-C6H5 | 64 |
| 7 | 4-F-C6H5 | 55 |
| 8 | 4-Br-C6H5 | 30 |
| 9 | 4-CF3-C6H5 | 81 |
| 10 | 4-OEt-C6H5 | 27 |
| 11 | 4-tBu-C6H5 | 77 |
| 12 | 2,6-F2-C6H4 | 46 |
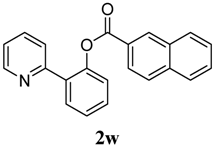
| 13 | 2-naphthalene | 44 |
3. Experimental
3.1. General
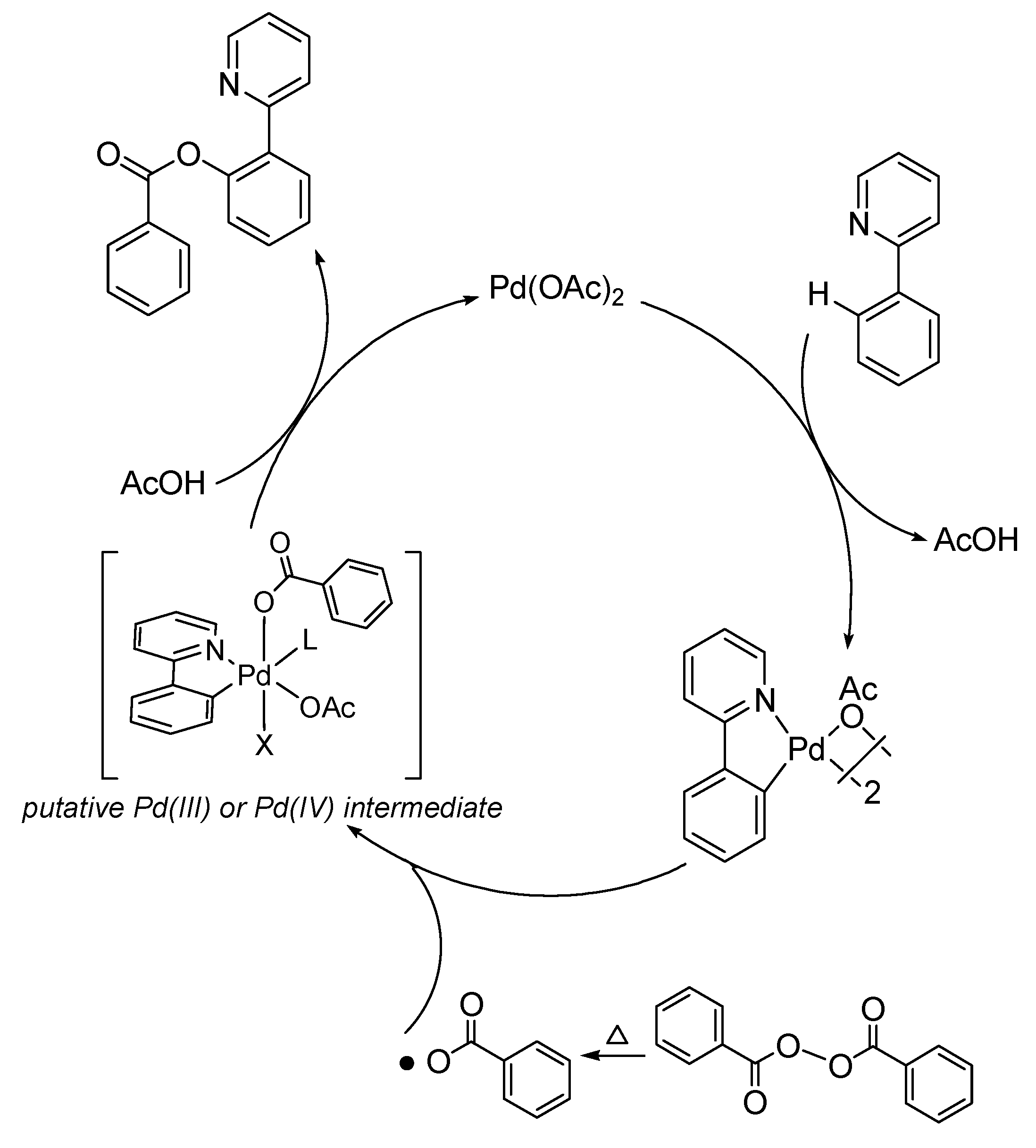
3.2. General Procedure for the Pd-Catalyzed Benzoxylation
3.3. Characterization of Products
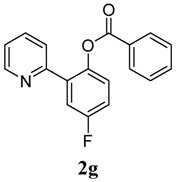
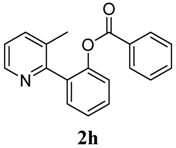
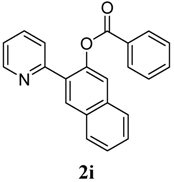
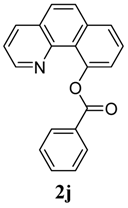
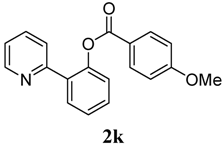
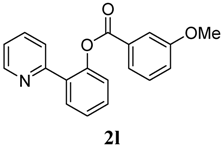
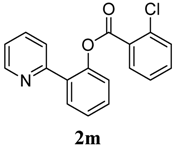
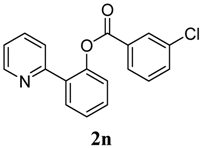
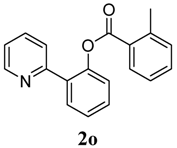
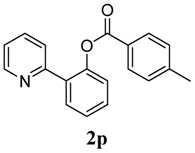
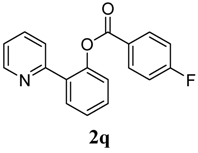
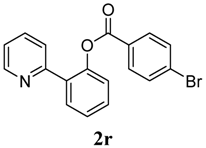
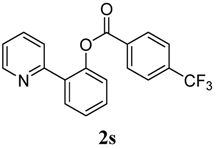
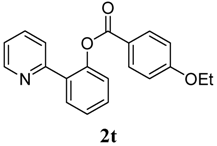
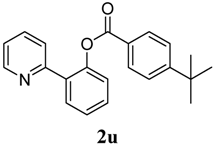
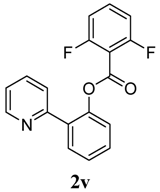
4. Conclusions
Supplementary Materials
Acknowledgments
References
- Liu, C.; Zhang, H.; Shi, W.; Lei, A. Bond formations between two nucleophiles: Transition metal catalyzed oxidative cross-coupling reactions. Chem. Rev. 2011, 111, 1780–1824. [Google Scholar] [CrossRef]
- Lyons, T.W.; Sanford, M.S. Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem. Rev. 2010, 110, 1147–1169. [Google Scholar] [CrossRef]
- Chen, X.; Engle, D.H.; Wang, D.H.; Yu, J.Q. Palladium(II)-catalyzed C–H activation/C–C cross coupling reactions: Versatility and practicality. Angew. Chem. Int. Ed. 2009, 48, 5094–5115. [Google Scholar] [CrossRef]
- Desai, L.V.; Malik, H.A.; Sanford, M.S. Oxone as an inexpensive, safe, and environmentally benign oxidant for C–H bond oxygenation. Org. Lett. 2006, 8, 1141–1144. [Google Scholar] [CrossRef]
- Yoneyama, T.; Crabtree, R.H. Pd(II) catalyzed acetoxylation of arenes with iodosyl acetate. J. Mol. Catal. A 1996, 108, 35–40. [Google Scholar] [CrossRef]
- Wang, G.W.; Yuan, T.T.; Wu, X.L. Direct ortho-acetoxylation of anilides via palladium-catalyzed sp2 C–H bond oxidative activation. J. Org. Chem. 2008, 73, 4717–4720. [Google Scholar] [CrossRef]
- Dick, A.R.; Hull, K.L.; Sanford, M.S. Palladium-catalyzed oxygenation of unactivated sp3 C–H bonds. J. Am. Chem. Soc. 2004, 126, 2300–2301. [Google Scholar] [CrossRef]
- Giri, R.; Liang, J.; Lei, J.G.; Li, J.J.; Wang, D.H.; Chen, X.; Naggar, I.C.; Guo, C.; Foxman, B.M.; Yu, J.Q. Pd-catalyzed stereoselective oxidation of methyl groups by inexpensive oxidants under mild conditions: A dual role for carboxylic anhydrides in catalytic C–H bond oxidation. Angew. Chem. Int. Ed. 2005, 44, 7420–7424. [Google Scholar] [CrossRef]
- Reddy, B.V.S.L.; Reddy, R.; Corey, E.J. Novel acetoxylation and C–C coupling reactions at unactivated positions in a-amino acid derivatives. Org. Lett. 2006, 8, 3391–3394. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Yu, J.Q. Pd(II)-catalyzed hydroxylation of arenes using 1 atm O2 or air. J. Am. Chem. Soc. 2009, 131, 14654–14655. [Google Scholar] [CrossRef]
- Chen, X.; Hao, X.S.; Goodhue, C.E.; Yu, J.Q. Cu(II)-catalyzed functionalizations of aryl C–H bonds using O2 as an oxidant. J. Am. Chem. Soc. 2006, 128, 6790–6791. [Google Scholar] [CrossRef]
- Luo, F.; Pan, C.; Cheng, J. Recent advances in transition-metal-catalyzed esterification. Synlett 2012, 23, 357–366. [Google Scholar] [CrossRef]
- James, C.A.; Snieckus, V. Combined directed metalation—cross coupling strategies. Total synthesis of the aglycones of gilvocarcin V, M and E. Tetrahedron Lett. 1997, 38, 8149–8152. [Google Scholar]
- Farr, R.N.; Kwok, D.I.; Daves, G.D., Jr. 8-Ethenyl-1-hydroxy-4-.beta.-D-ribofuranosyl-benzo[d]naphtho[1,2-b]pyran-6-one and 8-ethenyl-1-hydroxy-4-(2'-deoxy-.beta.-D-ribofurano-syl)benzo[d]naphtho[1,2-b]pyran-6-one. Synthetic C-glycosides related to the gilvocarcin, ravidomycin, and chrysomycin antibiotics. J. Org. Chem. 1992, 57, 2093–2100. [Google Scholar]
- Ye, Z.; Wang, W.; Luo, F.; Zhang, S.; Cheng, J. Rhodium-catalyzed ortho-benzoxylation of sp2 C–H bond. Org. Lett. 2009, 11, 3974–3977. [Google Scholar] [CrossRef]
- Wang, W.; Luo, F.; Zhang, S.; Cheng, J. Copper(II)-catalyzed ortho-acyloxylation of the 2-arylpyridines sp2 C–H bonds with anhydrides, using O2 as terminal oxidant. J. Org. Chem. 2010, 75, 2415–2418. [Google Scholar] [CrossRef]
- Wang, W.; Pan, C.; Chen, F.; Cheng, J. Copper(II)-catalyzed ortho functionalization of 2-arylpyridines with acyl chlorides. Chem. Commun. 2011, 47, 3978–3980. [Google Scholar] [CrossRef]
- Yu, W.Y.; Sit, W.N.; Lai, K.M.; Zhou, Z.; Chan, A.S.C. Palladium-catalyzed oxidative ethoxycarbonylation of aromatic C–H bond with diethyl azodicarboxylate. J. Am. Chem. Soc. 2008, 130, 3304–3306. [Google Scholar]
- Yu, W.Y.; Sit, W.N.; Zhou, Z.; Chan, A.S.C. Palladium-catalyzed decarboxylative arylation of C–H Bonds by aryl acylperoxides. Org. Lett. 2009, 11, 3174–3177. [Google Scholar] [CrossRef]
- Chan, C.W.; Zhou, Z.; Chan, A.S.C.; Yu, W.Y. Pd-catalyzed Ortho-C-H acylation/cross coupling of aryl ketone O-methyl oximes with aldehydes using tert-butyl hydroperoxide as oxidant. Org. Lett. 2010, 12, 3926–3929. [Google Scholar] [CrossRef]
- Chan, C.W.; Zhou, Z.; Yu, W.Y. Palladium(II)-catalyzed direct ortho-C-H acylation of anilides by oxidative cross-coupling with aldehydes using tert-butyl hydroperoxide as oxidant. Adv. Synth. Catal. 2011, 353, 2999–3006. [Google Scholar] [CrossRef]
- Chan, W.W.; Lo, S.F.; Zhou, Z.; Yu, W.Y. Rh-catalyzed intermolecular carbenoid functionalization of aromatic C–H bonds by α-diazomalonates. J. Am. Chem. Soc. 2012, 134, 13565–13568. [Google Scholar] [CrossRef]
- Ng, K.H.; Chan, A.S.C.; Yu, W.Y. Pd-catalyzed intermolecular ortho-C-H amidation of anilides by N-nosyloxycarbamate. J. Am. Chem. Soc. 2010, 132, 12862–12864. [Google Scholar] [CrossRef]
- Ng, K.H.; Zhou, Z.; Yu, W.Y. Rhodium(III)-catalyzed intermolecular direct amination of aromatic C–H bonds with N-chloroamines. Org. Lett. 2012, 14, 272–275. [Google Scholar] [CrossRef]
- Ng, K.H.; Ng, F.N.; Yu, W.Y. A convenient synthesis of anthranilic acids by Pd-catalyzed direct intermolecular ortho-C-H amidation of benzoic acids. Chem. Commun. 2012, 48, 11680–11682. [Google Scholar] [CrossRef]
- Moad, G.; Solomon, D.H. The Chemistry of Free Radical Polymerization; Elsevier: Boston, MA, USA, 2006. [Google Scholar]
- Navarro, O.; Kaur, H.; Mahjoor, P.; Nolan, S.P. Cross-coupling and dehalogenation reactions catalyzed by (N-heterocyclic carbene)Pd(allyl)Cl complexes. J. Org. Chem. 2004, 69, 3173–3180. [Google Scholar] [CrossRef]
- Flowers, G.C.; Leffler, J.E. Decomposition of bis(p-methoxybenzoyl) peroxide and the carboxy-inversion product on silica.xide and the carboxy-inversion product on silica. J. Org. Chem. 1985, 50, 4406–4408. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sit, W.-N.; Chan, C.-W.; Yu, W.-Y. Palladium(II)-Catalyzed othro-C–H-Benzoxylation of 2-Arylpyridines by Oxidative Coupling with Aryl Acylperoxides. Molecules 2013, 18, 4403-4418. https://doi.org/10.3390/molecules18044403
Sit W-N, Chan C-W, Yu W-Y. Palladium(II)-Catalyzed othro-C–H-Benzoxylation of 2-Arylpyridines by Oxidative Coupling with Aryl Acylperoxides. Molecules. 2013; 18(4):4403-4418. https://doi.org/10.3390/molecules18044403
Chicago/Turabian StyleSit, Wing-Nga, Chun-Wo Chan, and Wing-Yiu Yu. 2013. "Palladium(II)-Catalyzed othro-C–H-Benzoxylation of 2-Arylpyridines by Oxidative Coupling with Aryl Acylperoxides" Molecules 18, no. 4: 4403-4418. https://doi.org/10.3390/molecules18044403
APA StyleSit, W.-N., Chan, C.-W., & Yu, W.-Y. (2013). Palladium(II)-Catalyzed othro-C–H-Benzoxylation of 2-Arylpyridines by Oxidative Coupling with Aryl Acylperoxides. Molecules, 18(4), 4403-4418. https://doi.org/10.3390/molecules18044403