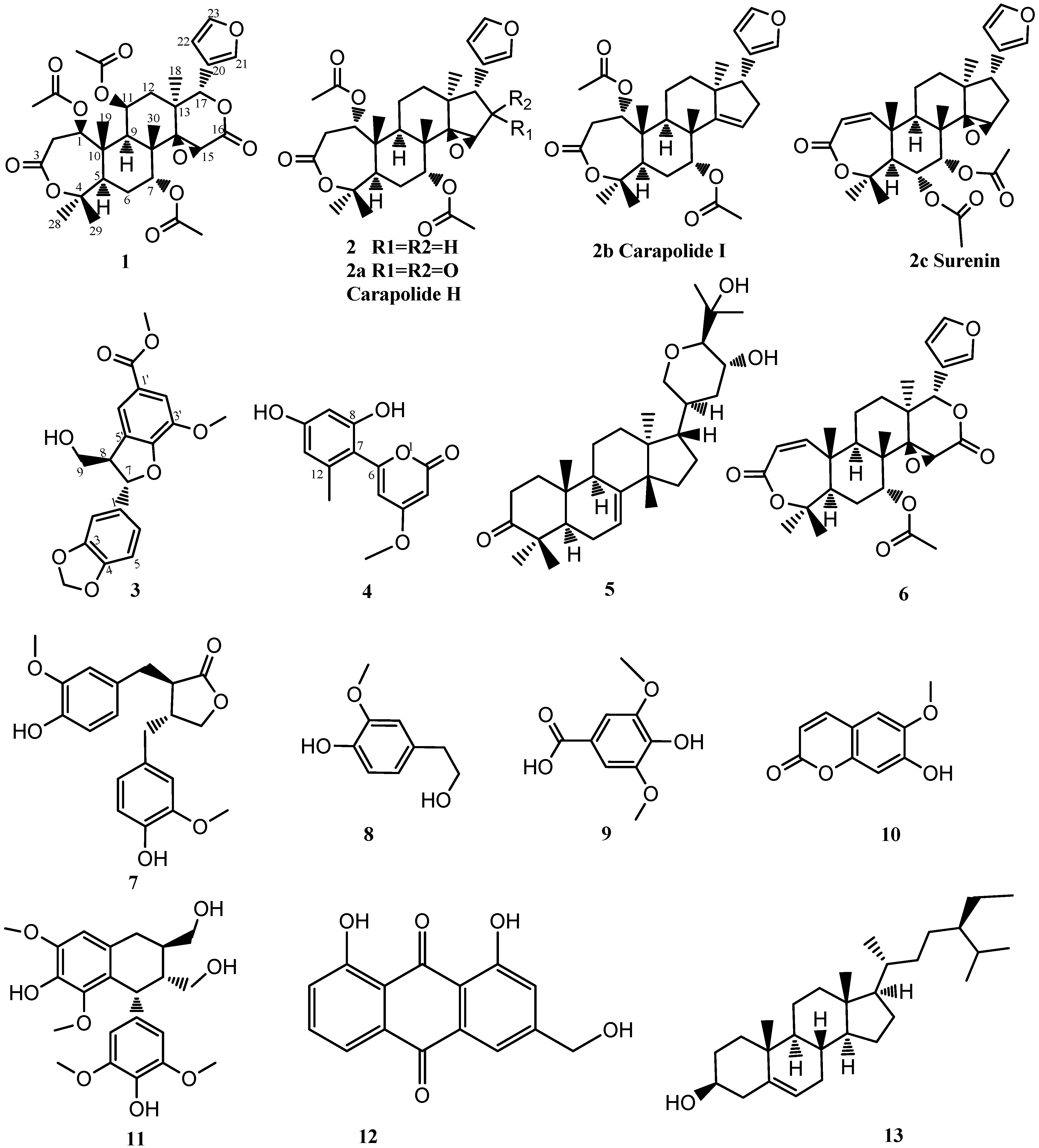
2. Results and Discussion
The EtOH extract of the roots of
T. sinensis was suspended in 70% aqueous MeOH and extracted by CHCl
3. The CHCl
3-soluble fraction was subjected to silica gel column chromatography and repeated RP-18 HPLC to yield two new limonoids
1 and
2, and one new phenylpropanoid
3, as well as ten known compounds, which were identified as 4-methoxy-6-(2′,4′-dihydroxy-6′-methylphenyl)- pyran-2-one (
4) [
9], bourjotinolone A (
5) [
10], proceranone (
6) [
11], matairesinol (
7) [
12], 4-hydroxy-3-methoxybenzeneethanol (
8) [
13], syringic acid (
9) [
14], isoscopoletin (
10) [
15], lyoniresinol (
11) [
16], aloeemodin (
12) [
17], and
β-sitosterol (
13), respectively, on the basis of their spectroscopic data and by comparison with reference data (
Figure 2).
Figure 2.
Structures of the compounds 1–13.
Figure 2.
Structures of the compounds 1–13.
Compound
1 was obtained as an amorphous white powder (CH
3OH). The molecular formula was established as C
32H
40O
12 by HRESIMS (
m/z 639.2411 [M+Na]
+, calcd. for C
32H
40O
12 + Na; 634.2860 [M+NH
4]
+ calcd. for C
32H
40O
12 + NH
4) in positive mode. Its IR bands at 1741, 1720, 1600, 1504 cm
−1 indicated the existence of carbonyl groups and olefinic carbons. Its NMR spectra (
Table 1) generally resembled those of nomilins such as 11
β,19-diacetoxy-l-deacetyl-l-epidihydronomilin [
18], 7-acetyl-11
β-acetoxy-dihydronomilin [
19] and 11β-hydroxycneorin G [
20], suggesting that
1 had a limonoid skeleton. The
1H-NMR spectrum indicated the presence of five tertiary methyls (five sharp single peaks at
δ: 1.20, 1.34, 1.37, 1.44, 1.49), three acetate methyl groups (three single peaks at
δ: 2.05, 2.08, 2.13) and three oxymethine protons (
δ: 4.83, 4.51, 5.16), and an α-oriented furan ring (
δ: 6.28, 7.37, and 7.41, 1H each). The
13C-NMR spectrum indicated the presence of five carbonyl carbons, including three acetate groups (
δ: 170.00, 169.95, 169.20) and two lactone moieties (
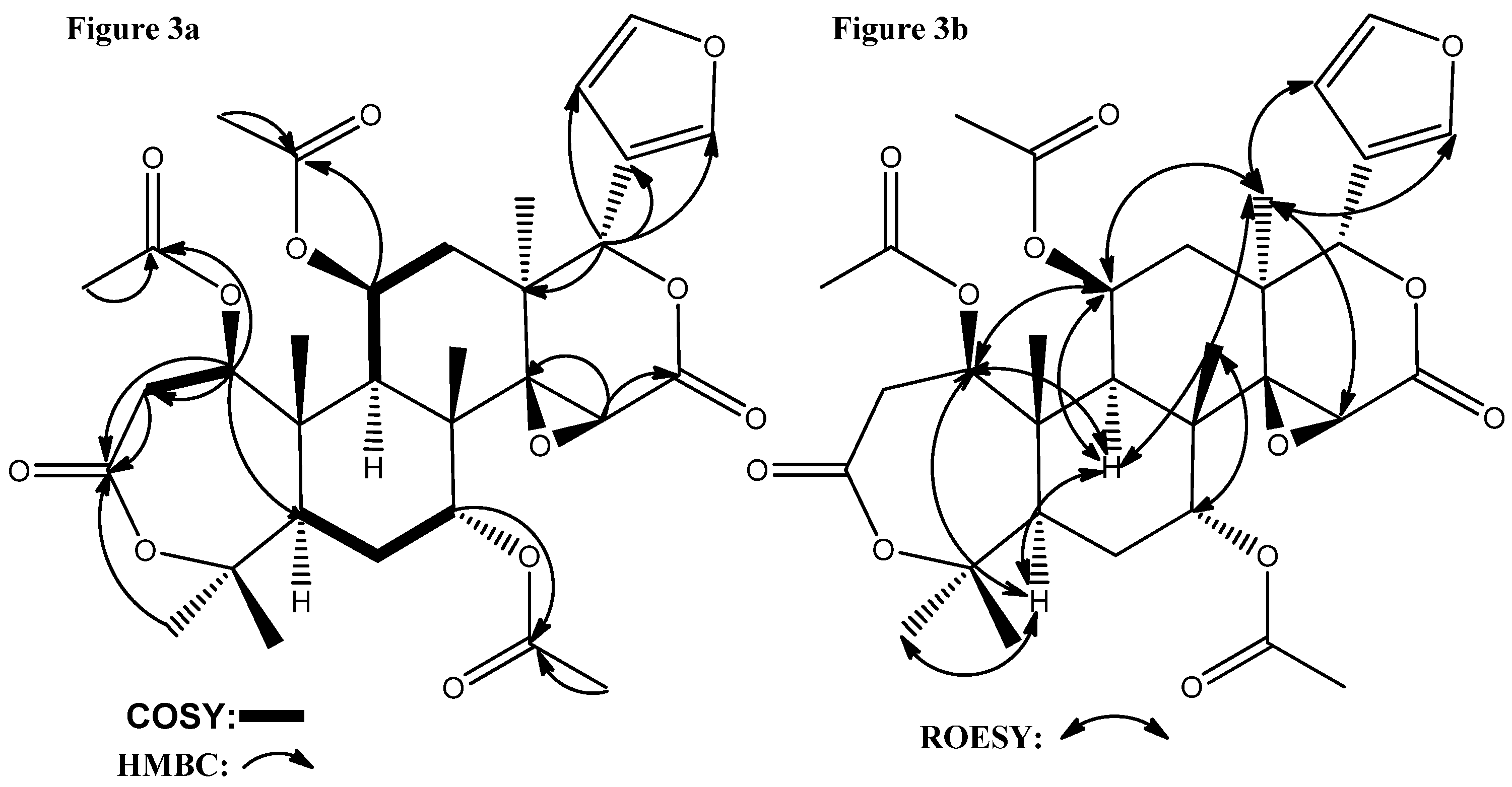
δ: 169.98, 166.58). In the HMBC spectrum (
Figure 3a), the cross-signal between
δC 170.00 and
δH 4.83 (H-1) demonstrated that the acetoxy group was attached to C-1. The HMBC data also indicated that the acetoxy group (
δC 169.95) was attached to C-7 (
δH 4.51) and the one at
δC 169.20 was attached at C-11 (
δH 5.16). The correlations of the carbonyl C-3 resonance at
δ 169.98 to the proton signals of H-2 and CH
3-28 (a four-bond correlation) suggested that the A-ring of
1 was a lactone moiety. The cross-signals between H-15/C-14, H-15/C-16, H-17/C-13, and H17/C-20 suggested the presence of a δ-lactone moiety with a 14,15-epoxide (
δH-15 3.55s,
δC-15 5.70) in the D-ring of
1.
Figure 3.
(a) Selected HMBC and 1H-1H COSY correlations for 1; (b) Selected ROESY correlations for 1.
Figure 3.
(a) Selected HMBC and 1H-1H COSY correlations for 1; (b) Selected ROESY correlations for 1.
As regards the relative stereochemistry of
1, The ROESY correlations (
Figure 3b) detected between H-5/H-9, H-5/H3-28, H-9/H-11, H-9/H3-18, H3-18/H-11, H3-18/H-15, H3-18/H-21, H3-18/H-22, and H3-30/H-7 showed that H-5, OAc-7, H-9, H-11, Me-18, and Me-28 were in an α-orientation, whereas OAc-11, the 14,15-epoxide, H-17, Me-19, Me-29, and Me-30 were in a β-orientation, as in 11
β-hydroxycneorin G [
20]. Finally, the ROESY correlations between H-1/H-5, H-1/H-9, H-1/H-11, which showed strong similarity to the case of 11
β,19-diacetoxy-l-deacetyl-l-epidihydronomilin [
18], clearly decided the α-orientation of H-1 and the β-orientation of OAc-1. In the ROESY spectrum, the most easily confused signals are the overlapped ones of Me-19 and H-12
β, both of which are at
δ 1.44, in which case the related ROESY cross-signals cannot be used for the corresponding relative stereochemistry determination. However, when the Me-19 is oxygenated, its chemical downshifts and H-12
β is a distinct dd peak at around
δ 1.41 [
18], which can be used for the relative stereochemistry determination. Accordingly, the structure of
1 was determined to be 7-acetyl-11
β-acetoxy-l-epidihydronomilin as shown in
Figure 2, and it was named toonin A.
Table 1.
1H- and 13C-NMR spectral data (δ in ppm, J in Hz) of 1 and 2 in CDCl3 a.
Table 1.
1H- and 13C-NMR spectral data (δ in ppm, J in Hz) of 1 and 2 in CDCl3 a.
| Toonin A (1) | Toonin B (2) |
|---|
| Atom | Proton | J (Hz) | Carbon | HMBC (H-C) | Proton | J (Hz) |
| 1 | 4.83 d | 7.0 | 71.12 | C-2, 3, 5, 1-OCOCH3 | 4.94 d | 7.0 |
| 2α | 3.14 d | 17.1 | 34.58 | C-1, 3, 10 | 3.12 dd | 17.1, 7.0 |
| 2β | 3.22 dd | 17.1, 7.0 | 3.21 d | 17.1 |
| 3 | | | 169.98 | | | |
| 4 | | | 84.42 | | | |
| 5 | 2.43 brd | 13.5 | 44.51 | C-4, 6, 10, 19, 29 | 2.52 dd | 13.5, 2.0 |
| 6α | 2.12 m | 13.5 | 26.08 | | 1.99 brd | 13.5 |
| 6β | 1.88 brt | 1.84 m |
| 7 | 4.51 brs | | 74.39 | C-5, 9, 24 | 4.70 brs | |
| 8 | | | 41.65 | | | |
| 9 | 2.88 d | 3.0 | 40.00 | C-8, 10, 19, 30 | 2.91 dd | 11.5, 2.0 |
| 10 | | | 45.75 | | | |
| 11 | 5.16 m | | 67.24 | | 1.55, 1.62 m | |
| 12α | 2.29 dd | 15.7, 11.5 | 37.02 | C-9, 14 | 1.84, 1.62 m | |
| 12β | 1.44 m |
| 13 | | | 37.87 | | | |
| 14 | | | 68.61 | | | |
| 15 | 3.55 s | | 54.70 | C-14, 16 | 3.42 s | |
| 16 | | | 166.58 | | | |
| 17 | 5.57 s | | 78.32 | C-12, 14, 18, 21, 22, 23 | 2.64 m | |
| 18 | 1.20 s | | 16.88 | C-12, 13, 14, 17 | 0.94 s | |
| 19 | 1.44 s | | 17.10 | C-1, 5, 9 | 1.18 s | |
| 30 | 1.34 s | | 20.00 | C-7, 8, 9, 14 | 1.11 s | |
| 28 | 1.49 s | | 34.53 | C-4, 5, 29 | 1.51 s | |
| 29 | 1.37 s | | 23.41 | C-4, 5, 28 | 1.40 s | |
| 7-OCOCH3 | | | 169.95 | | | |
| 7-OCOCH3 | 2.08 | | 21.57 | 7-OCOCH3 | 2.07 s | |
| 1-OCOCH3 | | | 170.00 | | | |
| 1-OCOCH3 | 2.13 | | 21.03 | 1-OCOCH3 | 2.16 s | |
| 11-OCOCH3 | | | 169.20 | | | |
| 11-OCOCH3 | 2.05 | | 20.63 | 11-OCOCH3 | | |
| 21 | 7.37 | | 140.80 | C-20, 22, 23 | 7.11 s | |
| 20 | | | 120.00 | | | |
| 22 | 6.28 | | 109.82 | C-21, 20, 23 | 6.15 s | |
| 23 | 7.41 | | 142.92 | C-21, 20 | 7.38 s | |
Compound
2 was obtained as an amorphous white powder (CH
3OH). The molecular formula was established as C
30H
40O
8 by HR-ESIMS at
m/z 551.26166 [M+Na]
+ (calcd. for C
30H
40O
8 + Na), 567.23561 [M+K]
+ (calcd. for C
30H
40O
8 + K) in positive mode. Its NMR spectra generally resembled compound
1 and showed strong similarity to the evodulane type nomilins carapolide H and carapolide I (
2a,
2b,
Figure 2) [
21]. The
1H-NMR spectrum revealed resonances for five tertiary methyl groups, two acetoxy groups, and an epoxide proton (s,
δ: 3.42, H-15). In addition to the characteristic α-orientation furan signals, two oxymethine resonances were clearly discernable at
δ: 4.94 (d,
J = 7.0 Hz, H-l) and 4.70 (brs, H-7). The HMBC cross-signals can deduce the OAc groups were attached at C-1 and C-7. The correlations of the carbonyl C-3 resonance at
δ 170.00 to the proton signals of H-1 and CH
3-28 suggested the existence of a lactone moiety at A-ring of
2. Compared with carapolide H (
2a,
Figure 2), the D-ring of compound
2 has the epoxide signal (s,
δH-15: 3.42s;
δC-15: 56.73), however, it is a CH
2 signal of C-16 (
δH-16: 1.65, 2.71,
δC-16: 31.54) instead of a ketone carbon. Through further literature research, the NMR data of the D-ring was found to be very similar to the D-ring of surenin (
2c,
Figure 2) [
22] whose C-16 is also a methylene
. The correlations at H2-16/C-13, H2-16/C-17, H-17/C-13, H-17/C-18, H-17/C-20, decided all the D-ring signals (
δH-16: 1.65, 2.71,
δC-16: 31.54;
δH-17: 2.64,
δC-17: 39.16). And H-17/C-20 (
δC-20: 123.03), H-17/C-21(
δC-21: 138.84) revealed the furan ring [7.11 (1H, s, H-21), 6.15 (1H, s, H-22), 7.38 (1H, s, H-23); 123.03 (C-20), 138.84(C-21), 110.92 (C-22), 142.71 (C-23)] was attached at C-17. Therefore, structure
2 was attributed to 14,15-epoxycarapolide I and named toonin B.
Compound
3 was obtained as an amorphous white powder (CH
3OH). The molecular formula C
19H
18O
7 was established by HR-ESIMS
+:
m/z 359.1125 [M+H]
+ (calcd. for C
19H
18O
7 + H), 381.0945 [M+Na]
+, 739.1997 [2M+Na]
+ in positive mode. Its IR spectrum showed characteristic absorptions for hydroxyl group (3445 cm
−1), carbonyl group (1712 cm
−1), and phenyl group (1613, 1599, 1492 cm
−1). The NMR data was very similar to that of the known compound cedralin A [
6]. The only difference, one more methoxyl group at [
δH 3.81 (3H, s, OCO
CH3-1′),
δC 51.92 (OC
OCH3-1′)] of
3, suggested methyl esterification of the carboxylic acid group at C-1′ of cedralin A [
6] leading to production of
3, so compound
3 was identified as a (7
S,8
R)-7,8-dihydro-8-(hydroxymethyl)-3′-methoxy-7-[3,4-(methylenedioxy) phenyl] benzofuran-1′-carboxylic acid methyl ester) and named toonin C (
Figure 2).
Compound
4, 4-methoxy-6-(2′,4′-dihydroxy-6′-methylphenyl)-pyran-2-one, had previously been reported [
9,
23,
24,
25] and its structure had been confirmed by X-ray analysis [
24] and
1H-NMR spectra reported by Conner in 1987 [
25]. However, its
13C-NMR spectrum has never been provided, so this paper gives for the first time a detailed description of its
13C-NMR spectrum.
The DPPH assay showed that the EtOH extract of the root was more active than that of the leaves (
Table 2). The CHCl
3 fraction from the roots was also very active.
Table 2.
Free Radical Scavenging Capacity of the extracts.
Table 2.
Free Radical Scavenging Capacity of the extracts.
| Tested samples | Ascorbic acid | EtOH extract of leaves | EtOH extract of roots | CHCl3 fraction from roots |
|---|
| (0.05 mM) | (0.25 mg/mL) | (0.25 mg/mL) | (0.25 mg/mL) |
|---|
| Clearing ratio | 59.5 ± 0.8 | 86.5 ± 1.6 | 91.1 ± 1.2 | 80.5 ± 1.4 |
Our research showed that the extract of T. sinensis had certain antioxidant capacity, which gave substantial support for its historical use as a healthy food. Our continuing chemical investigation on the free radical scavenging active fraction of the roots of T. sinensis yielded three new and ten known compounds, among which eight known phenols (3, 4, 7–12) and four Meliaceae limonoids (1, 2, 5, 6) were found.
Limonoids
1 and
6 belong to the ring A,D-seco group, and compound
2 is a ring A-seco group compound, while compound
5 without a seco-ring is a protolimonoid [
26]. The ring A,D-seco and the protolimonoid groups have been isolated from the leaves, seeds, stems, or cortex of
T. sinensis. The ring A,D-seco group with a functional oxygen group at position 11 (C-11) has been used as the distinguishing feature for species of Meliaceae [
20]. However, besides the C-11 oxygenated ones, our research showed that C-11 non-oxygenated limonoids also exist in this plant. Compound
2 belongs to the evodulone type of limonoids, featuring a ring A lactone. These types of limonoids are examples of an A ring opened before the D ring, which are not common in limonoids from the Meliaceae family.
3. Experimental
3.1. General
All solvents used in the extraction and isolation processes were redistilled prior to use. IR spectra (KBr) were obtained on a Perkin Elmer Spectrum 2000 instrument. All NMR spectra were recorded on a Bruker DRX-500 instrument in CDCl3 or DMSO-d6. Chemical shifts are reported in ppm using TMS as internal standard. High-resolution ESI mass spectra were obtained on a JEOL-LC Mate LCMS system. LC-MS analyses were carried out using an Esquire 3000 instrument equipped with autosampler and DAD. Bruker DataAnalysis 3.1 software was used for data acquisition and processing. A Lichrocart cartridge, set 55-2, Merck 1.5024 was used as separation column, flow rate was 0.5 mL/min, time 15.0 min. Gradient elution was performed with water/0.05% formic acid (solvent A) and acetonitrile/0.05% formic acid (solvent B) at a constant flow rate of 0.5 mL/min. An increasing linear gradient (v/v) of solvent B was used [t (min), %B]: 0, 0; 9.5, 100; 12.2 100; 12.3, 0; 15.0, 0. Detection was carried out at 200 nm, with peak scanning between 195-600.2 nm. All of the analyses were carried out using a Turbo Ionspray source in the negative mode with the following settings: capillary exit, 100 V; nebulizer, curtain, collision, and drying gas (N2). Full scan acquisition was performed by scanning from m/z 100 to 1,750 U in profile at a cycle time of 2 s with a step size of 0.1 U and a pause between each scan of 2 ms. MS/MS product ions were produced by collision-activated dissociation (CAD) of the selected precursor ions in the collision cell of the triple quadrupole mass spectrometer and analyzed using the second analyzer of the instrument. In all of the experiments, quadrupoles (Q1 and Q3) were operated at unit resolution. Different MS/MS experiments, neutral loss scan, product ion scan, and precursor ion scan of selected molecules were carried out to confirm the structure of the compounds previously identified by full scan mode. Analytical HPLC was performed on a Purospher STAR RP-18 column, 3 μm, 55 mm length × 4 mm ID, Gradient elution was performed with water/0.01% TFA (solvent A) and acetonitrile/0.01% TFA (solvent B) at a constant flow rate of 1.5 mL/min. An increasing linear gradient (v/v) of solvent B was used [t (min), %B]: 0, 10; 7.5, 100; 10, 100; 10.5, 10; 13.0, 10. Detection was carried out at 200 and 220 nm, with peak scanning between 195-600.2 nm. Millennium software was used for data acquisition and processing. An Agilent ChemicalStation was used off-line for library building and RT-UV library search. Preparative HPLC (Varian) for fractions 18 and 6 was performed on a Purospher RP-18 column, 12 μm, 250 mm length × 50 mm ID, Gradient elution was performed with water (solvent A) and acetonitrile (solvent B) at a constant flow rate of 40 mL/min. An increasing linear gradient (v/v) of solvent B was used [t (min), %B]: 0, 20; 65, 85; 80, 100; 95, 100. Detection was carried out at 210 nm. Preparative HPLC (Varian) for the 16th fraction: Purospher RP-18 column, 12 μm, 250 mm length × 25 mm ID, Gradient elution was performed with water (solvent A) and acetonitrile (solvent B) at a constant flow rate of 10 mL/min. An increasing linear gradient (v/v) of solvent B was used [t (min), %B]: 0, 15; 60, 45; 90, 100; 111, 100. Detection was carried out at 270 nm. Preparative HPLC (Varian) for the 30–39th fractions: Purospher RP-18 column, 12 μm, 250 mm length × 25 mm ID, Gradient elution was performed with water (solvent A) and acetonitrile (solvent B) at a constant flow rate of 10 mL/min. An increasing linear gradient (v/v) of solvent B was used [t (min), %B]: 0, 30; 60, 62; 90, 100; 111, 100. Detection was carried out at 250 nm.
Plant Material. The roots of T. sinensis were initially collected in Tonglin County of Anhui Province in October, 2011, and identified by one of the authors (G.-H.B.). A voucher specimen (No. AHAU2011102-1) was deposited in the herbarium of Anhui Agricultural University.
3.2. Compound Isolation
The air-dried and powdered roots (15 kg) were extracted with 95% EtOH (3 × 30 L) at room temperature. After removal of the solvent by evaporation under vacuum, the residue was diluted with 70% aqueous methanol (4 L) and then extracted successively with CHCl3 (4 × 4 L), EtOAc (3 × 4 L), and n-BuOH (4 × 4 L). The CHCl3 extract (355 g) was fractionated on a silica gel (100–200 mesh) column (11 × 100 cm) and gradually eluted by hexane-dichloromethane-methanol to yield 18 fractions. All of the 18 fractions were examined by TLC and LC-PAD-MS and some interesting constituents were found in fractions 6 (2.0 g), 14 (7.9 g), 15 (7.1 g), 16 (8.3 g) and fraction 18 (5 g). These five fractions were further fractionated by repeated preparative HPLC. Further preparative HPLC of Fraction 15 gave three limonoids: the new compounds toonin A (1, 14.5 mg, Rt 6.588 min), toonin B (2, 5 mg, Rt 7.256 min), and the known one proceranone (6, 10 mg, Rt 7.080 min). The new compound toonin C (3, 6.39 mg, Rt 7.256 min) and the known compound lyoniresinol (11, 10.3 mg, Rt 3.931 min) were obtained from Fraction 6. Further HPLC purification of fraction 18 yielded 4-methoxy-6-(2′,4′-dihydroxy-6′-methylphenyl)-pyran-2-one (4, 1.96 mg, Rt 4.577 min) and syringic acid (9, 5.68 mg, Rt 1.565 min). Fraction 14 was subjected to repeated preparative HPLC yielding bourjotinolone A (5, 82.6 mg, Rt 9.073 min) and matairesinol (7, 13.95 mg, Rt 5.368 min). 4-Hydroxy-3-methoxybenzeneethanol (8, 5.5 mg, Rt 0.974 min), isoscopoletin (10, 6.0 mg, Rt 3.716 min), aloeemodin (12), and β-sitosterol (13) were obtained by repeated HPLC purification of Fraction 16.
3.3. Isolates
Toonin A (
1). White amorphous powder, [α]
D25 −25.3 (c 0.8, CHCl
3). IR (KBr) ν
max (cm
−1): 2987, 1741, 1720, 1508, 1444, 1370, 1221, 1123, 1024, 876, 774. ESIMS
−:
m/z 615.3 [M−H]
− (6), 677.2 [M+HCO
3]
− (38), 156.9 (100) in negative mode, ESIMS
+:
m/z 639.2 [M+Na]
+ (100) in positive mode; HRESIMS
+:
m/z 639.2411 [M+Na]
+ (calcd. for C
32H
40O
12 + Na), 634.2860 [M+NH
4]
+ in positive mode.
1H and
13C-NMR data (CDCl
3), see
Table 1.
Toonin B (
2). White powder, [α]
D25 −43.7 (c 0.8, CHCl
3). IR (KBr) ν
max (cm
−1): 2984, 2948, 1802, 1731, 1600, 1504, 1459, 1435, 1374, 1235, 1124, 1027, 874, 789 cm
−1; EIMS:
m/z 528 [M]
+ (16), 468 (5), 410 (7), 350 (8), 208 (24), 107 (100), 79 (13), 43 (51); ESIMS
−:
m/z 527.2 [M−H]
− (94) in negative mode; ESIMS
+:
m/z 529.2 [M+H]
+ (30), 546.2 [M+NH
4]
+ (100), 1074.1 [2M+NH4]
+ (15) in positive mode; HRESIMS
+:
m/z 551.26166 [M+Na]
+ (calcd. for C
30H
40O
8 + Na), 567.23561 [M+K]
+ in positive mode.
1H-NMR (CDCl
3), see
Table 1.
13C-NMR (CDCl
3)
δ: 70.48 (C-1), 34.08 (C-2), 170.00 (C-3), 85.24 (C-4), 44.08 (C-5), 26.47 (C-6), 73.22 (C-7), 41.73 (C-8), 37.05 (C-9), 44.25 (C-10), 15.80 (C-11), 29.42 (C-12), 41.46 (C-13), 72.48 (C-14), 56.73 (C-15), 31.54 (C-16), 39.16 (C-17), 21.02 (C-18), 15.82 (C-19), 123.03 (C-20), 138.84(C-21), 110.92 (C-22), 142.71 (C-23), 34.03 (C-28), 23.01 (C-29), 18.85 (C-30), 169.96 (O
COCH
3-1), 21.07 (OCO
CH3-1), 169.54 (O
COCH
3-7), 20.60 (OCO
CH3-7).
Toonin C (3). white powder (CH3OH); [α]D25 −22.6 (c 0.1, CH3OH); IR (KBr) νmax (cm−1): 3445, 2948, 1712, 1613, 1599, 1492, 1435, 1331, 1250, 1108, 1038, 936, 769 cm−1; ESIMS+: m/z 359.2 [M+H]+ (52), 381.1 [M+Na]+ (100), 739.0 [2M+Na]+ (26); in positive mode; HRESIMS+: m/z 359.1125 [M+H]+ (calcd. for C19H18O7 + H), 381.0945 [M+Na]+, 739.1997 [2M+Na]+ in positive mode. 1H-NMR (DMSO-d6) δ: 6.88 (1H, d, J = 1.5 Hz, H-2), 6.91 (1H, d, J = 8.0 Hz, H-5), 6.84 (1H, dd, J = 8.0, 1.5 Hz, H-6), 5.60 (1H, d, J = 6.5 Hz, H-7β), 3.49 (1H, dd, J = 6.5, 5.5 Hz, H-8α), 3.69 (2H, m, H-9), 5.11 (1H, t, J = 7.0 Hz, HO-9), 7.44 (1H, s, H-2′), 7.55 (1H, s, H-6′), 6.01 (2H, s, OCH2O), 3.81 (3H, s, OCOCH3-1′), 3.83 (3H, s, OCH3-3′). 13C-NMR (DMSO-d6) δ: 134.94 (C-1), 106.17 (C-2), 146.99 (C-3), 148.36 (C-4), 108.36 (C-5), 119.30 (C-6), 86.67 (C-7), 52.45 (C-8), 62.58 (C-9), 121.94 (C-1′), 113.35 (C-2′), 142.66 (C-3′), 151.72 (C-4′), 129.44 (C-5′), 118.93 (C-6′), 100.98 (OCH2O), 166.30 (OCOCH3-1′), 51.92 (OCOCH3-1′), 56.08 (OCH3-3′).
4-Methoxy-6-(2′,4′-dihydroxy-6′-methylphenyl)-pyran-2-one (4). White powder (CH3OH); IR (KBr) νmax (cm−1): 3335, 2942, 1680, 1610 1558, 1514, 1454, 1406, 1336, 1269, 1222, 1161, 840. ESIMS−: m/z 247.1 [M−H]− (100), 495.1 1 [2M−H]− (5) in negative mode; ESIMS+: m/z 249.1 [M+H]+ (100), 271.1 [M+Na]+ (12), 497.1 [2M+H]+ (3) in positive mode; HRESIMS−: m/z 247.06118 [M−H]− (calcd. for C13H12O5-H) in negative mode. 1H-NMR (DMSO-d6) δ: 5.60 (1H, d, J = 2.2 Hz, H-3), 6.12 (1H, d, J = 2.2 Hz, H-5), 6.22 (1H, d, J = 1.5 Hz, H-3′), 6.15 (1H, d, J = 1.5 Hz, H-5′), 3.83 (3H, s, OCH3-4), 9.55, 9.66 (2H, brs, HO-4, 5), 2.08 (3H, s, CH3-6′). 13C-NMR (DMSO-d6) δ: 164.90 (C-2), 88.62 (C-3), 171.74 (C-4), 105.34 (C-5), 159.30 (C-6), 111.57 (C-1′), 157.68 (C-2′), 99.96 (C-3′), 159.91 (C-4′), 109.24 (C-5′), 139.12 (C-6′), 56.28 (OCH3-4), 19.75 (CH3-6′).
The assignments were confirmed by a combination of 1H-1H COSY, HSQC, HMBC, and ROESY spectra.